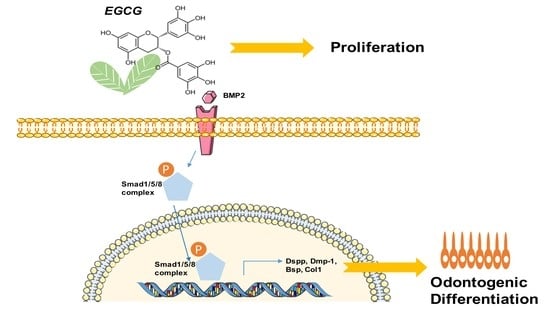
Epigallocatechin-3-Gallate Promotes Osteo-/Odontogenic Differentiation of Stem Cells from the Apical Papilla through Activating the BMP–Smad Signaling Pathway
Abstract
1. Introduction
2. Results
2.1. In Vitro Characterization of SCAPs
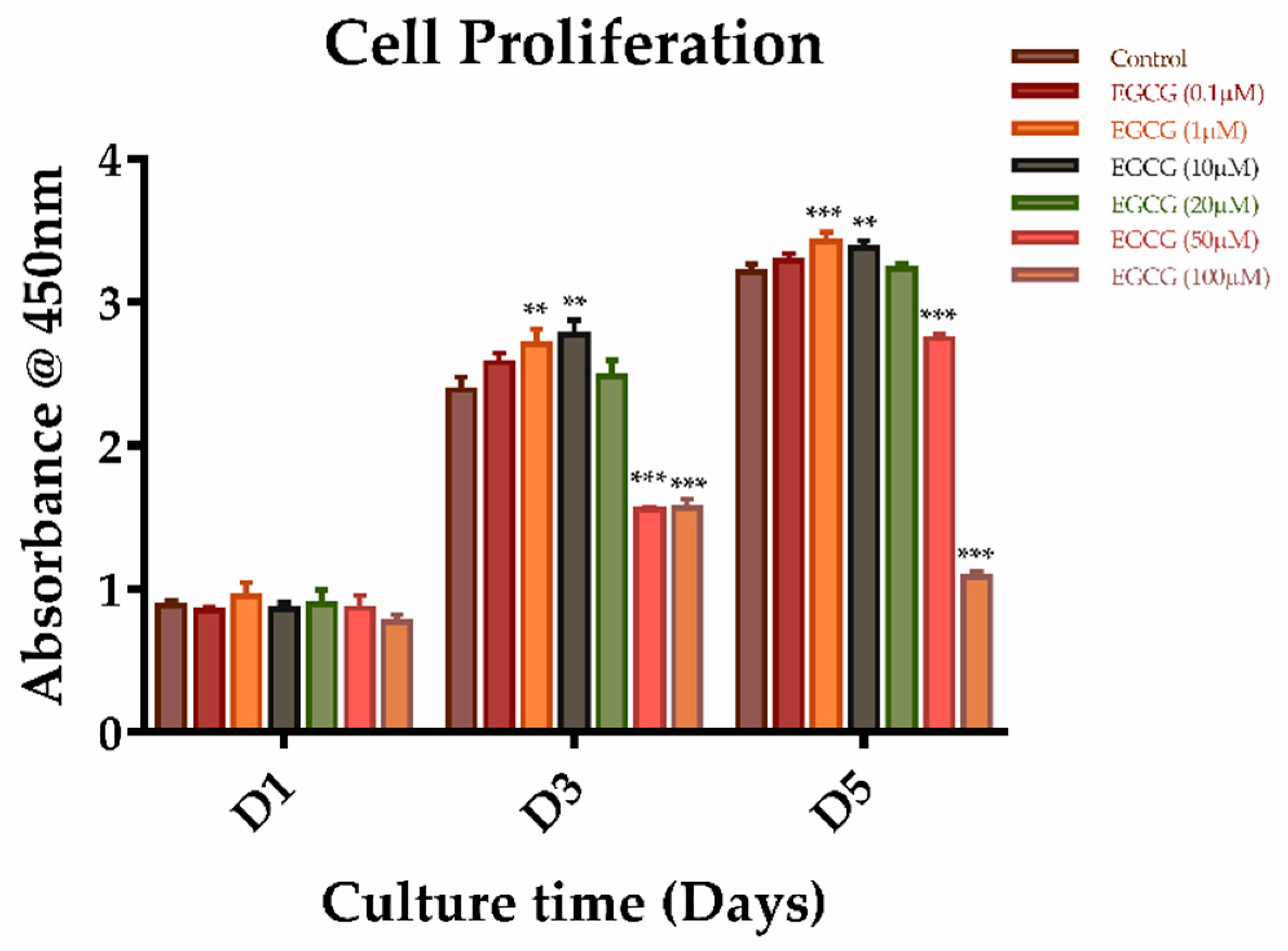
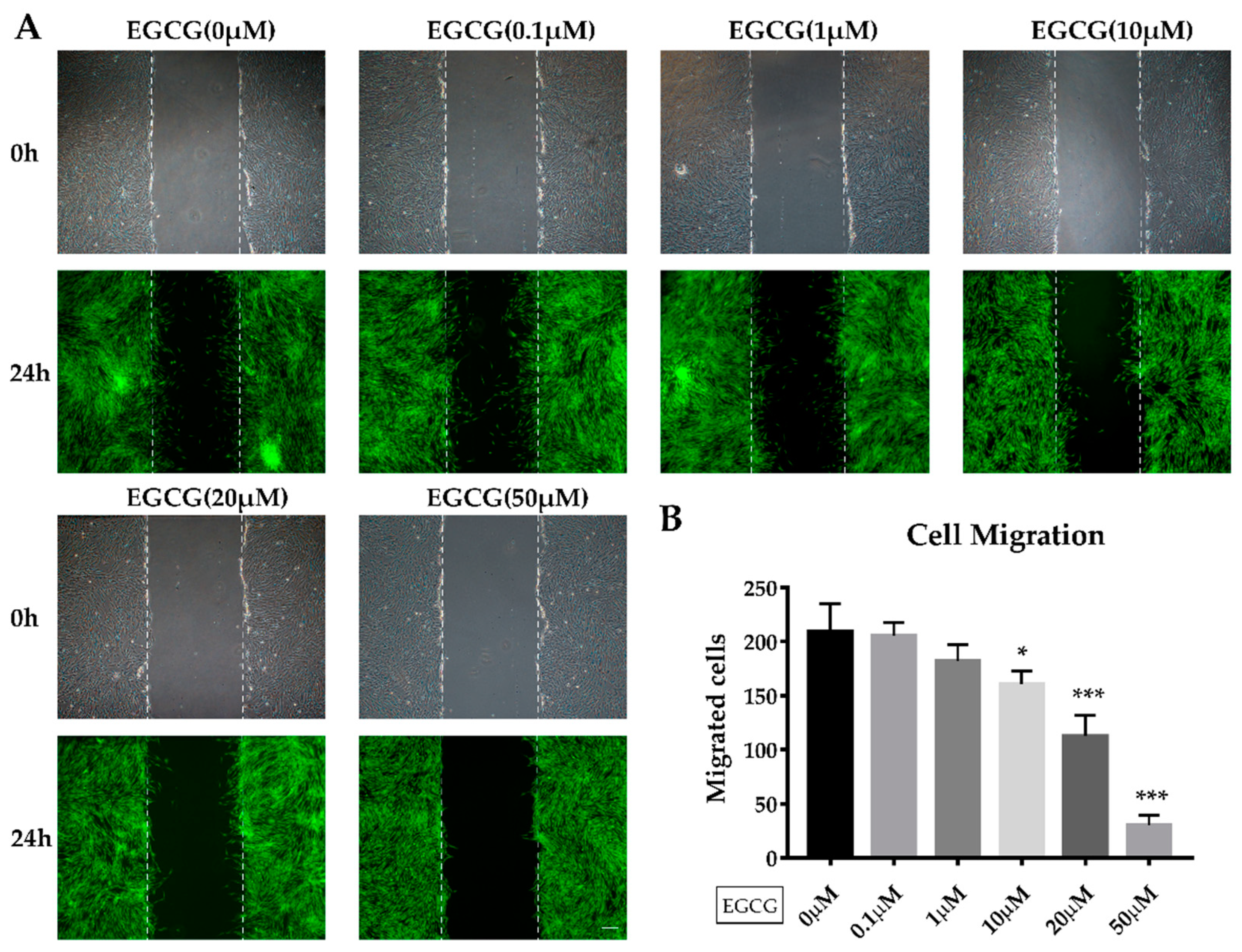
2.2. The Effects of EGCG on Proliferation and Migration of SCAPs
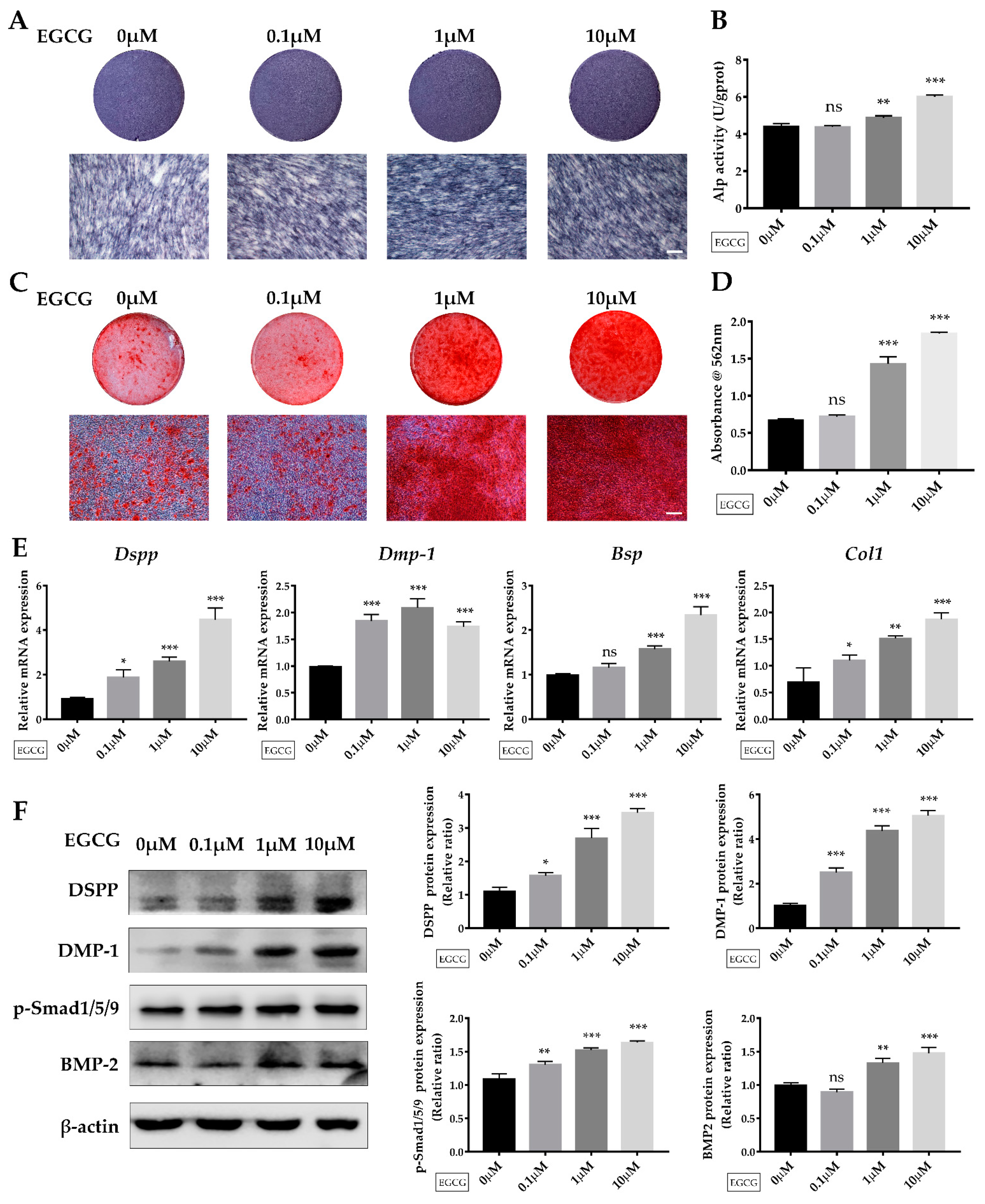
2.3. EGCG Promotes Osteo-/Odontogenic Differentiation and Mineralization of SCAPs
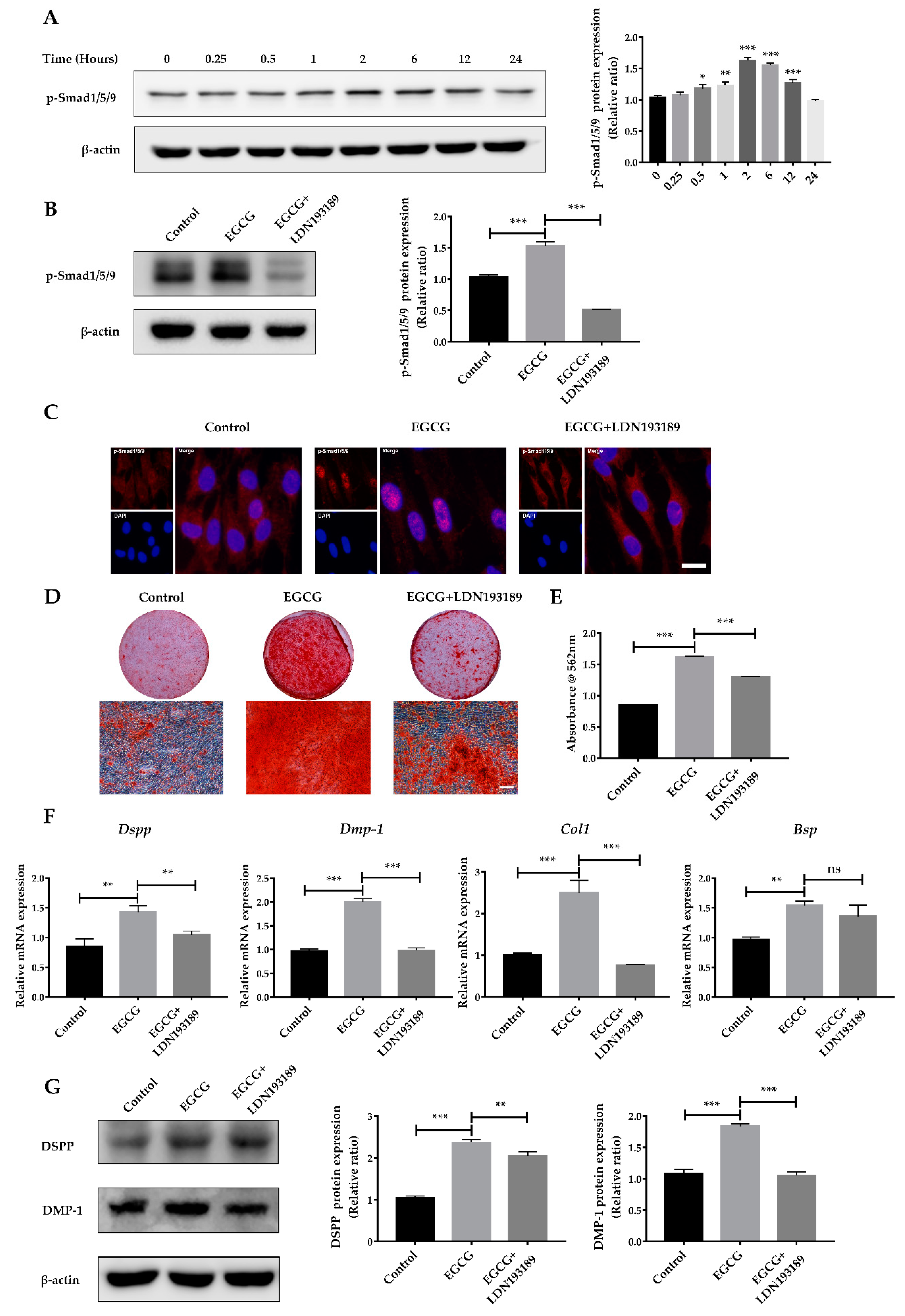
2.4. Suppression of the BMP–Smad Signaling Pathway Reverses EGCG-Induced Osteo-/Odontogenic Differentiation of SCAPs
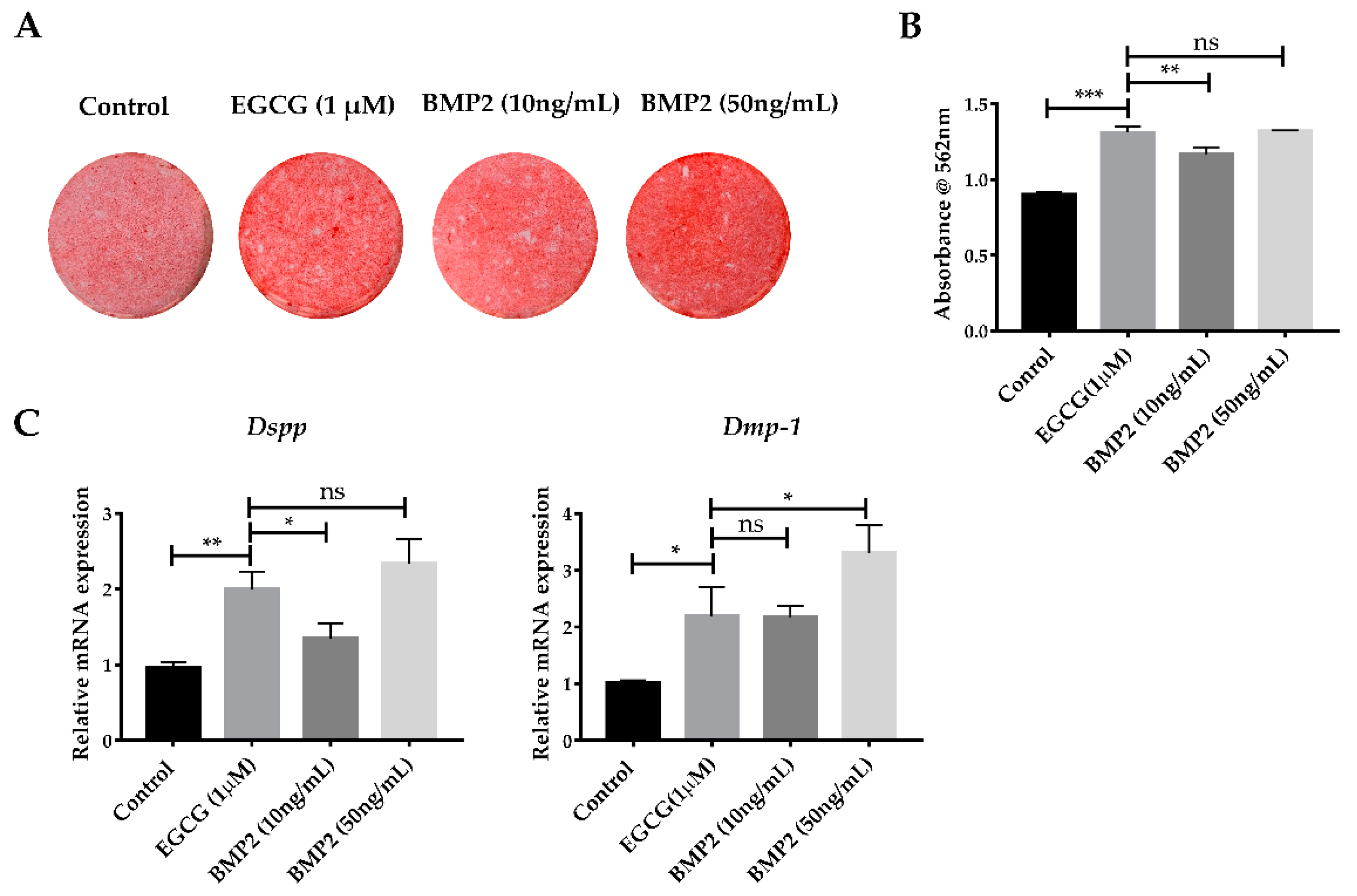
2.5. EGCG Shows the Comparable Ability to Promote Mineralization with Recombinant BMP2
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Colony-Forming Assay
4.3. Flow Cytometric Analysis
4.4. Immunofluorescence Staining
4.5. Multiple Lineage Differentiation
4.6. CCK-8 Assay
4.7. Wound Healing Assay
4.8. Alkaline Phosphatase (ALP) Staining and Alizarin Red S (ARS) Staining
4.9. Quantitative Reverse Transcriptase Polymerase Chain Reaction (qRT-PCR) Analysis
4.10. Western Blotting
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L.; et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.J.; Ramos, J.C.; Martins, J.B.; Diogenes, A.; Figueiredo, M.H.; Ferreira, P.; Viegas, C.; Santos, J.M. Histologic Evaluation of Regenerative Endodontic Procedures with the Use of Chitosan Scaffolds in Immature Dog Teeth with Apical Periodontitis. J. Endod. 2017, 43, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.J.M.J.; Diogo, P.; Sequeira, D.; Ramos, J.C.; Diogenes, A.; Santos, J.M. Does Apical Papilla Survive and Develop in Apical Periodontitis Presence after Regenerative Endodontic Procedures? Appl. Sci. 2019, 9, 3942. [Google Scholar] [CrossRef]
- Li, J.; Parada, C.; Chai, Y. Cellular and molecular mechanisms of tooth root development. Development 2017, 144, 374–384. [Google Scholar] [CrossRef]
- Huang, G.T.; Sonoyama, W.; Liu, Y.; Liu, H.; Wang, S.; Shi, S. The hidden treasure in apical papilla: The potential role in pulp/dentin regeneration and bioroot engineering. J. Endod. 2008, 34, 645–651. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.Y.; Wang, S.; et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef]
- Dong, R.; Yao, R.; Du, J.; Wang, S.; Fan, Z. Depletion of histone demethylase KDM2A enhanced the adipogenic and chondrogenic differentiation potentials of stem cells from apical papilla. Exp. Cell Res. 2013, 319, 2874–2882. [Google Scholar] [CrossRef]
- Sequeira, D.B.; Seabra, C.M.; Palma, P.J.; Cardoso, A.L.; Peça, J.; Santos, J.M. Effects of a New Bioceramic Material on Human Apical Papilla Cells. J. Funct. Biomater. 2018, 9, 74. [Google Scholar] [CrossRef]
- Chowdhury, A.; Sarkar, J.; Chakraborti, T.; Pramanik, P.K.; Chakraborti, S. Protective role of epigallocatechin-3-gallate in health and disease: A perspective. Biomed. Pharmacother. 2016, 78, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Kang, L.; Wang, C.Z.; Huang, H.H.; Cheng, T.L.; Huang, H.T.; Lee, M.J.; Lin, Y.S.; Ho, M.L.; Wang, G.J.; et al. (-)-Epigallocatechin-3-Gallate (EGCG) Enhances Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells. Molecules 2018, 23, 3221. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, Y.; Liu, J.; Jin, C.; Meng, Y.; Pei, D. Influence of epigallocatechin-3-gallate in promoting proliferation and osteogenic differentiation of human periodontal ligament cells. BMC Oral Health 2019, 19, 73. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, K.; Xu, T.; Wu, J.; Li, P.; Wang, H.; Wu, H.; Wu, G. Epigallocatechin-3-gallate enhances the osteoblastogenic differentiation of human adipose-derived stem cells. Drug Des. Dev. Ther. 2019, 13, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Sasayama, S.; Hara, T.; Tanaka, T.; Honda, Y.; Baba, S. Osteogenesis of Multipotent Progenitor Cells using the Epigallocatechin Gallate-Modified Gelatin Sponge Scaffold in the Rat Congenital Cleft-Jaw Model. Int. J. Mol. Sci. 2018, 19, 3803. [Google Scholar] [CrossRef]
- Shin, Y.S.; Seo, J.Y.; Oh, S.H.; Kim, J.H.; Kim, S.T.; Park, Y.B.; Moon, H.S. The effects of ErhBMP-2-/EGCG-coated BCP bone substitute on dehiscence around dental implants in dogs. Oral Dis. 2014, 20, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.S.; Kim, H.J.; Hwang, Y.C.; Rosa, V.; Yu, M.K.; Min, K.S. Effects of Epigallocatechin Gallate, an Antibacterial Cross-linking Agent, on Proliferation and Differentiation of Human Dental Pulp Cells Cultured in Collagen Scaffolds. J. Endod. 2017, 43, 289–296. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Xu, S.; Wang, F.; Wang, B.; Han, K.; Sun, D.; Li, L. Epigallocatechin-3-gallate Protects against Hydrogen Peroxide-Induced Inhibition of Osteogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 7532798. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Q.; Dong, J.; Jia, Z.; Hao, F.; Shan, C. Effects of epigallocatechin-3-gallate on proliferation and differentiation of mouse cochlear neural stem cells: Involvement of PI3K/Akt signaling pathway. Eur. J. Pharm Sci. 2016, 88, 267–273. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Xu, X.; Song, M.; Tao, H.; Bai, Y. Green tea epigallocatechin-3-gallate (EGCG) promotes neural progenitor cell proliferation and sonic hedgehog pathway activation during adult hippocampal neurogenesis. Mol. Nutr. Food Res. 2012, 56, 1292–1303. [Google Scholar] [CrossRef]
- Yamashiro, T.; Tummers, M.; Thesleff, I. Expression of bone morphogenetic proteins and Msx genes during root formation. J. Dent. Res. 2003, 82, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Dang, M.; Zhang, Z.; Hu, J.; Eyster, T.W.; Ni, L.; Ma, P.X. Dentin regeneration by stem cells of apical papilla on injectable nanofibrous microspheres and stimulated by controlled BMP-2 release. Acta Biomater. 2016, 36, 63–72. [Google Scholar] [CrossRef]
- Qin, W.; Yang, F.; Deng, R.; Li, D.; Song, Z.; Tian, Y.; Wang, R.; Ling, J.; Lin, Z. Smad 1/5 is involved in bone morphogenetic protein-2-induced odontoblastic differentiation in human dental pulp cells. J. Endod. 2012, 38, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Jing, J.; Li, J.; Zhao, H.; Punj, V.; Zhang, T.; Xu, J.; Chai, Y. BMP signaling orchestrates a transcriptional network to control the fate of mesenchymal stem cells in mice. Development 2017, 144, 2560–2569. [Google Scholar] [CrossRef]
- Malik, Z.; Alexiou, M.; Hallgrimsson, B.; Economides, A.N.; Luder, H.U.; Graf, D. Bone Morphogenetic Protein 2 Coordinates Early Tooth Mineralization. J. Dent. Res. 2018, 97, 835–843. [Google Scholar] [CrossRef]
- Aguilar, P.; Lertchirakarn, V. Comparison of stem cell behaviors between indigenous high and low-CD24 percentage expressing cells of stem cells from apical papilla (SCAPs). Tissue Cell 2016, 48, 397–406. [Google Scholar] [CrossRef]
- Gosau, M.; Götz, W.; Felthaus, O.; Ettl, T.; Jäger, A.; Morsczeck, C. Comparison of the differentiation potential of neural crest derived progenitor cells from apical papilla (dNC-PCs) and stem cells from exfoliated deciduous teeth (SHED) into mineralising cells. Arch. Oral Biol. 2013, 58, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Bakopoulou, A.; Leyhausen, G.; Volk, J.; Tsiftsoglou, A.; Garefis, P.; Koidis, P.; Geurtsen, W. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch. Oral Biol. 2011, 56, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.Y.; Li, H.B.; Sui, Z.Q.; Corke, H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Crit. Rev. Food Sci. Nutr. 2018, 58, 924–941. [Google Scholar] [CrossRef]
- Irimie, A.I.; Braicu, C.; Zanoaga, O.; Pileczki, V.; Gherman, C.; Berindan-Neagoe, I.; Campian, R.S. Epigallocatechin-3-gallate suppresses cell proliferation and promotes apoptosis and autophagy in oral cancer SSC-4 cells. Onco Targets Ther. 2015, 8, 461–470. [Google Scholar] [CrossRef]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef]
- Mah, Y.J.; Song, J.S.; Kim, S.O.; Lee, J.H.; Jeon, M.; Jung, U.W.; Moon, S.J.; Kim, J.H.; Choi, H.J. The effect of epigallocatechin-3-gallate (EGCG) on human alveolar bone cells both in vitro and in vivo. Arch. Oral Biol. 2014, 59, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Vali, B.; Rao, L.G.; El-Sohemy, A. Epigallocatechin-3-gallate increases the formation of mineralized bone nodules by human osteoblast-like cells. J. Nutr. Biochem. 2007, 18, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Liu, L.; Wang, Y.; Yang, R.; Hu, C.; Rung, S.; Man, Y.; Qu, Y. Evaluation of epigallocatechin-3-gallate (EGCG)-modified scaffold determines macrophage recruitment. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Ortega, M.M.; Montaño-Figueroa, A.G.; Rodríguez-Félix, D.E.; Prado-Villegas, G.; Pino-Ocaño, K.P.; Valencia-Córdova, M.J.; Quiroz-Castillo, J.M.; Herrera-Franco, P.J. Preparation by coaxial electrospinning and characterization of membranes releasing (-) epicatechin as scaffold for tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 46, 184–189. [Google Scholar] [CrossRef]
- Chu, C.; Deng, J.; Xiang, L.; Wu, Y.; Wei, X.; Qu, Y.; Man, Y. Evaluation of epigallocatechin-3-gallate (EGCG) cross-linked collagen membranes and concerns on osteoblasts. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Zhang, Y.F.; Wan, C.Y.; Sun, Z.Y.; Nie, S.; Jian, S.J.; Zhang, L.; Song, G.T.; Chen, Z. Autophagy in SDF-1alpha-mediated DPSC migration and pulp regeneration. Biomaterials 2015, 44, 11–23. [Google Scholar] [CrossRef]
- Li, S.; Lin, C.; Zhang, J.; Tao, H.; Liu, H.; Yuan, G.; Chen, Z. Quaking promotes the odontoblastic differentiation of human dental pulp stem cells. J. Cell Physiol. 2018, 233, 7292–7304. [Google Scholar] [CrossRef] [PubMed]

| Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Gapdh | TCATGGGTGTGAACCATGAGAA | GGCATGGACTGTGGTCATGAG |
| Dspp | TGCTGGAGCCACAAAC | AAACCCTATGCAACCTTC |
| Dmp-1 | ACAGGCAAATGAAGACCC | TTCACTGGCTTGTATGG |
| Bsp | CGAAGCAGAAGTGGATGAAA | TGCCTCTGTGCTGTTGGTACTG |
| Col1 | GCGGCTCCCCATTTTTATACC | GCTCTCCTCCCATGTTAAATAGCAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Lin, Y.; Fang, X.; Yang, J.; Chen, Z. Epigallocatechin-3-Gallate Promotes Osteo-/Odontogenic Differentiation of Stem Cells from the Apical Papilla through Activating the BMP–Smad Signaling Pathway. Molecules 2021, 26, 1580. https://doi.org/10.3390/molecules26061580
Liu Z, Lin Y, Fang X, Yang J, Chen Z. Epigallocatechin-3-Gallate Promotes Osteo-/Odontogenic Differentiation of Stem Cells from the Apical Papilla through Activating the BMP–Smad Signaling Pathway. Molecules. 2021; 26(6):1580. https://doi.org/10.3390/molecules26061580
Chicago/Turabian StyleLiu, Zeni, Yuxiu Lin, Xiaolin Fang, Jingwen Yang, and Zhi Chen. 2021. "Epigallocatechin-3-Gallate Promotes Osteo-/Odontogenic Differentiation of Stem Cells from the Apical Papilla through Activating the BMP–Smad Signaling Pathway" Molecules 26, no. 6: 1580. https://doi.org/10.3390/molecules26061580
APA StyleLiu, Z., Lin, Y., Fang, X., Yang, J., & Chen, Z. (2021). Epigallocatechin-3-Gallate Promotes Osteo-/Odontogenic Differentiation of Stem Cells from the Apical Papilla through Activating the BMP–Smad Signaling Pathway. Molecules, 26(6), 1580. https://doi.org/10.3390/molecules26061580