The Oral Administration of Sanguisorba officinalis Extract Improves Physical Performance through LDHA Modulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of the Roots of S. officinalis
2.3. High-Performance Liquid Chromatography Analysis
2.4. Mice
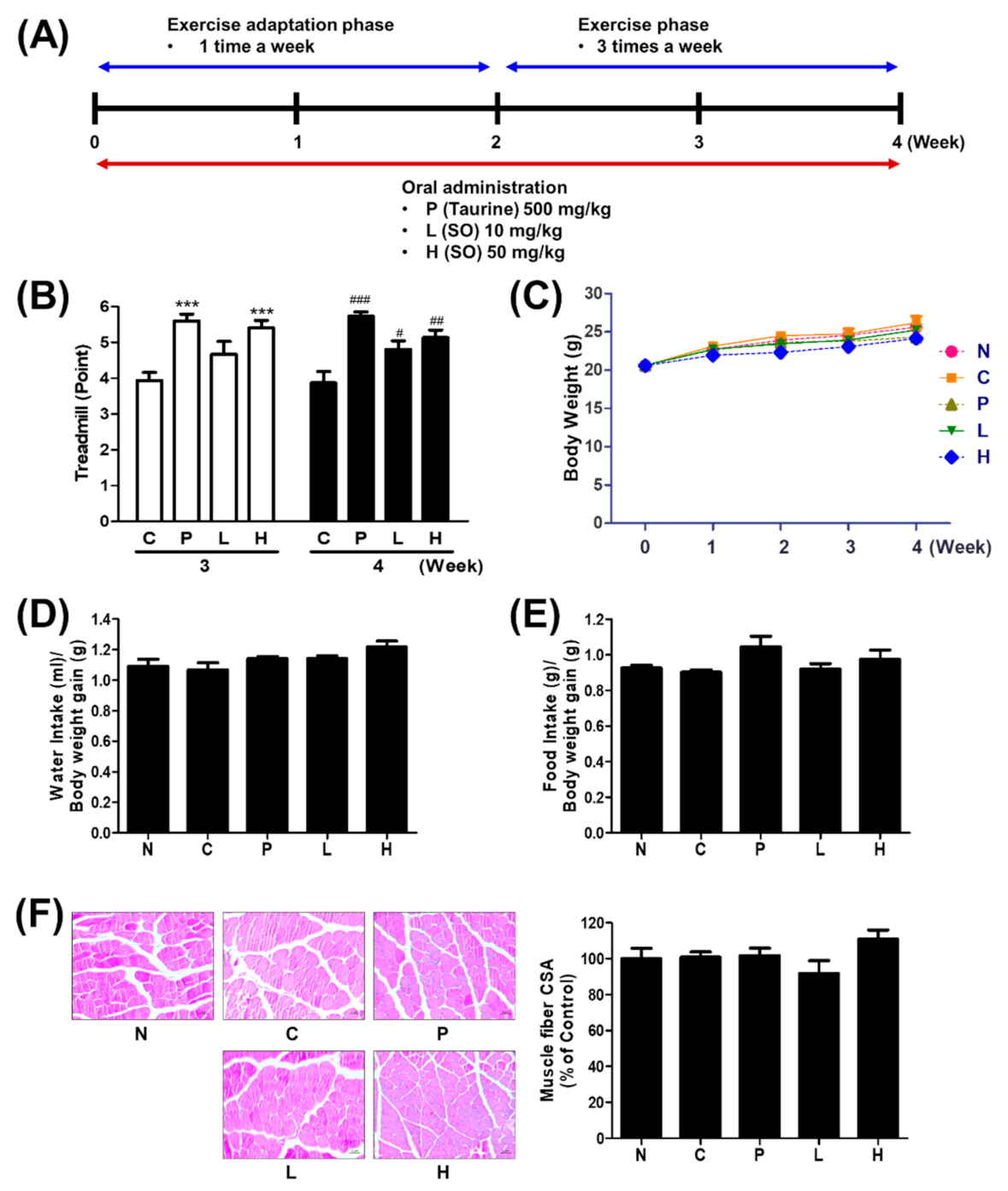
2.5. Physical Activity Protocols
2.5.1. Endurance Test
2.5.2. Experimental Mice Sacrifice and Measured Tissue Weight
2.6. Tissue Histology
2.7. Assessment of Glycolysis Related Factors
2.8. Cell Culture
2.9. Western Blot Analysis
2.10. LDHA Activity Assay
2.11. Cellular Metabolic Analysis
2.12. Statistical Analysis
3. Results
3.1. The HPLC Analysis of S. officinalis
3.2. The Effect of S. officinalis Extract on Endurance Performance in Mice
3.3. The Effect of S. officinalis Extract on the Blood Biochemistry
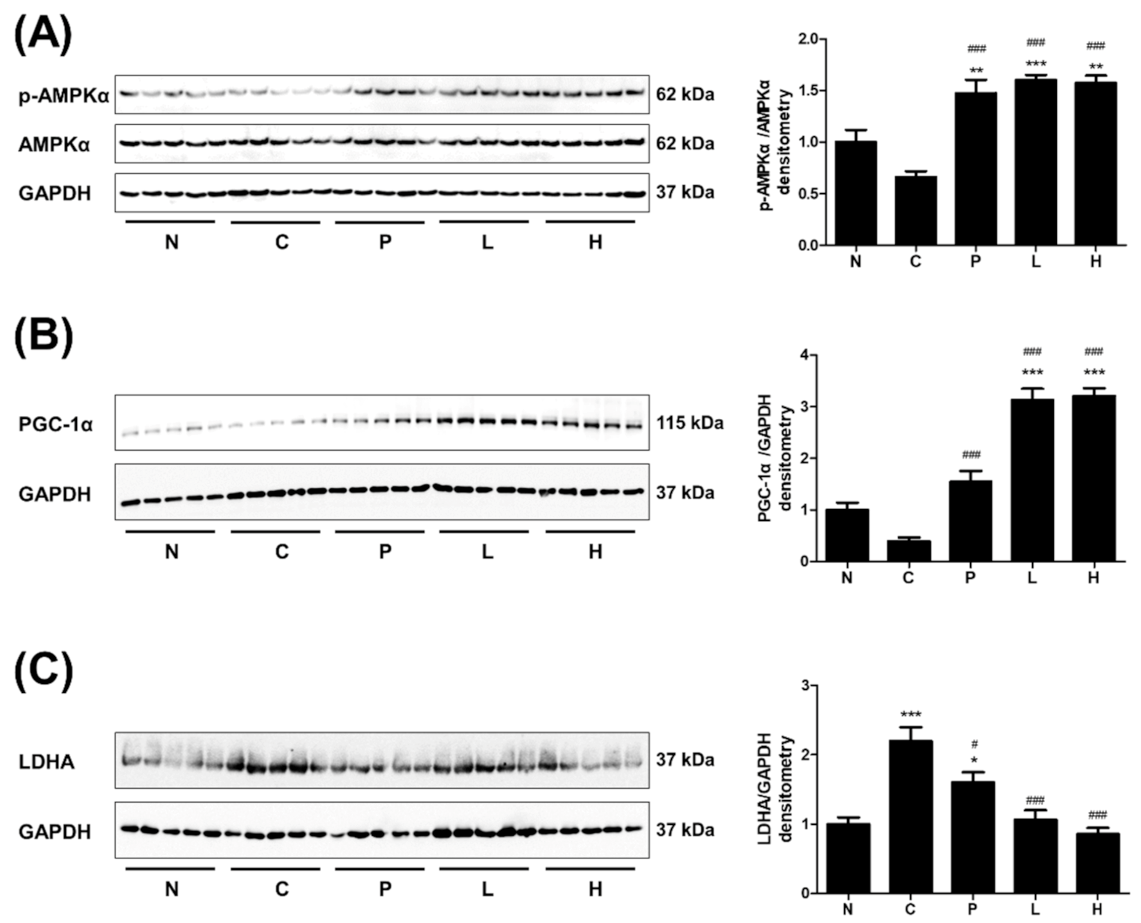
3.4. The Effect of S. officinalis Extract on AMPK, PGC-1α, and LDHA in Skeletal Muscle
3.5. The Effect of S. Officinalis Extract on LDHA Activity and Lactate Metabolism in Myocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Todd, J.J. Lactate: Valuable for Physical Performance and Maintenance of Brain Function During Exercise. Biosci. Horiz. Int. J. Stud. Res. 2014, 7. [Google Scholar] [CrossRef]
- Bassett, D.R.; Howley, E.T. Limiting Factors for Maximum Oxygen Uptake and Determinants of Endurance Performance. Med. Sci. Sports Exerc. 2000, 32, 70–84. [Google Scholar] [CrossRef]
- Astrup, A. Healthy Lifestyles in Europe: Prevention of Obesity and Type II Diabetes by Diet and Physical Activity. Public Health Nutr. 2001, 4, 499–515. [Google Scholar] [CrossRef]
- Rolland, Y.; Lauwers-Cances, V.; Cesari, M.; Vellas, B.; Pahor, M.; Grandjean, H. Physical Performance Measures as Predictors of Mortality in a Cohort of Community-dwelling Older French Women. Eur. J. Epidemiol. 2006, 21, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Porporato, P.E. Understanding Cachexia as a Cancer Metabolism Syndrome. Oncogenesis 2016, 5. [Google Scholar] [CrossRef]
- Fan, J.; Hitosugi, T.; Chung, T.-W.; Xie, J.; Ge, Q.; Gu, T.-L.; Polakiewicz, R.D.; Chen, G.Z.; Boggon, T.J.; Lonial, S. Tyrosine Phosphorylation of Lactate Dehydrogenase A is Important for NADH/NAD+ Redox Homeostasis in Cancer Cells. Mol. Cell. Biol. 2011, 31, 4938–4950. [Google Scholar] [CrossRef] [PubMed]
- Cairns, S.P. Lactic Acid and Exercise Performance. Sports Med. 2006, 36, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Coombes, J.; McNaughton, L. Effects of Branched-chain Amino Acid Supplementation on Serum Creatine Kinase and Lactate Dehydrogenase After Prolonged Exercise. J. Sports Med. Phys. Fit. 2000, 40, 240. [Google Scholar]
- Rodrigues, B.M.; Dantas, E.; de Salles, B.F.; Miranda, H.; Koch, A.J.; Willardson, J.M.; Simão, R. Creatine Kinase and Lactate Dehydrogenase Responses After Upper-body Resistance Exercise with Different Rest Intervals. J. Strength Cond. Res. 2010, 24, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Collomp, K.; Ahmaidi, S.; Chatard, J.C.; Audran, M.; Prefaut, C. Benefits of Caffeine Ingestion on Sprint Performance in Trained and Untrained Swimmers. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 64, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, K.S.; Chang, M.J.; Sung, J.H. Effects of Panax Ginseng Extract on Exercise-induced Oxidative Stress. J. Sports Med. Phys. Fit. 2005, 45, 178–182. [Google Scholar]
- Zhao, Z.; He, X.; Zhang, Q.; Wei, X.; Huang, L.; Fang, J.C.; Wang, X.; Zhao, M.; Bai, Y.; Zheng, X. Traditional Uses, Chemical Constituents and Biological Activities of Plants from the Genus Sanguisorba L. Am. J. Chin. Med. 2017, 45, 199–224. [Google Scholar] [CrossRef]
- Jang, E.; Inn, K.S.; Jang, Y.P.; Lee, K.T.; Lee, J.H. Phytotherapeutic Activities of Sanguisorba Officinalis and Its Chemical Constituents: A Review. Am. J. Chin. Med. 2018, 46, 299–318. [Google Scholar] [CrossRef] [PubMed]
- Ranfa, A.; Maurizi, A.; Romano, B.; Bodesmo, M. The importance of Traditional Uses and Nutraceutical Aspects of Some Edible Wild Plants in Human Nutrition: The Case of Umbria (Central Italy). Plant. Biosyst. 2014, 148, 297–306. [Google Scholar] [CrossRef]
- Im, S.H.; Wang, Z.; Lim, S.S.; Lee, O.H.; Kang, I.J. Bioactivity-guided Isolation and Identification of Anti-adipogenic Compounds from Sanguisorba Officinalis. Pharm. Biol. 2017, 55, 2057–2064. [Google Scholar] [CrossRef]
- Yasueda, A.; Kayama, H.; Murohashi, M.; Nishimura, J.; Wakame, K.; Komatsu, K.I.; Ogino, T.; Miyoshi, N.; Takahashi, H.; Uemura, M.; et al. Sanguisorba Officinalis L. Derived from Herbal Medicine Prevents Intestinal Inflammation by Inducing Autophagy in Macrophages. Sci. Rep. 2020, 10, 9972. [Google Scholar] [CrossRef]
- Chung, T.-W.; Kim, E.-Y.; Han, C.W.; Park, S.Y.; Jeong, M.S.; Yoon, D.; Choi, H.-J.; Jin, L.; Park, M.-J.; Kwon, Y.J. Machilin A Inhibits Tumor Growth and Macrophage M2 Polarization Through the Reduction of Lactic Acid. Cancers 2019, 11, 963. [Google Scholar] [CrossRef]
- Kim, E.-Y.; Choi, H.-J.; Park, M.-J.; Jung, Y.-S.; Lee, S.-O.; Kim, K.-J.; Choi, J.-H.; Chung, T.-W.; Ha, K.-T. Myristica Fragrans Suppresses Tumor Growth and Metabolism by Inhibiting Lactate Dehydrogenase A. Am. J. Chin. Med. 2016, 44, 1063–1079. [Google Scholar] [CrossRef] [PubMed]
- Su, X.D.; Ali, I.; Arooj, M.; Koh, Y.S.; Yang, S.Y.; Kim, Y.H. Chemical Constituents from Sanguisorba Officinalis L. and Their Inhibitory Effects on LPS-stimulated Pro-inflammatory Cytokine Production in Bone Marrow-derived Dendritic Cells. Arch. Pharmacal Res. 2018, 41, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Cardiff, R.D.; Miller, C.H.; Munn, R.J. Manual Hematoxylin and Eosin Staining of Mouse Tissue Sections. Cold Spring Harb. Protoc. 2014, 2014, pdb-prot073411. [Google Scholar] [CrossRef] [PubMed]
- Lechner, C.; Zahalka, M.A.; Giot, J.-F.; Møller, N.; Ullrich, A. ERK6, A Mitogen-activated Protein Kinase Involved in C2C12 Myoblast Differentiation. Proc. Natl. Acad. Sci. USA 1996, 93, 4355–4359. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Haramizu, S.; Ota, N.; Hase, T. Tea Catechin Ingestion Combined with Habitual Exercise Suppresses the Aging-associated Decline in Physical Performance in Senescence-accelerated Mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R281–R289. [Google Scholar] [CrossRef]
- Iglesias Neira, J.; Pazos, M.; Maestre, R.; Torres, J.L.; Medina, I. Galloylated Polyphenols as Inhibitors of Hemoglobin-catalyzed Lipid Oxidation in Fish Muscle. J. Agric. Food Chem. 2011, 59, 5684–5691. [Google Scholar] [CrossRef]
- Swamy, M.S.; Sivanna, N.; Tamatam, A.; Khanum, F. Effect of Poly Phenols in Enhancing the Swimming Capacity of Rats. Funct. Foods Health Dis. 2011, 1, 482–491. [Google Scholar] [CrossRef]
- Jones, J.F.; Maloney, E.M.; Boneva, R.S.; Jones, A.-B.; Reeves, W.C. Complementary and Alternative Medical Therapy Utilization by People with Chronic Fatiguing Illnesses in the United States. BMC Complementary Altern. Med. 2007, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Barroso, G.C.; Thiele, E.S. Muscle Injuries in Athletes. Rev. Bras. Ortop. 2011, 46, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Blannin, A.K.; Walsh, N.P.; Field, C.N.; Pritchard, J.C. Effect of Exercise-induced Muscle Damage on the Blood Lactate Response to Incremental Exercise in Humans. Eur. J. Appl. Physiol. Occup. Physiol. 1998, 77, 292–295. [Google Scholar] [CrossRef]
- Theofilidis, G.; Bogdanis, G.C.; Koutedakis, Y.; Karatzaferi, C. Monitoring Exercise-induced Muscle Fatigue and Adaptations: Making Sense of Popular or Emerging Indices and Biomarkers. Sports 2018, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Pinto, H.D.; Vanin, A.A.; Miranda, E.F.; Tomazoni, S.S.; Johnson, D.S.; Albuquerque-Pontes, G.M.; Ivo de O Aleixo, J.; Grandinetti, V.d.S.; Casalechi, H.L.; Paulo de Tarso, C. Photobiomodulation Therapy Improves Performance and Accelerates Recovery of High-level Rugby Players in Field Test: A Randomized, Crossover, Double-blind, Placebo-controlled Clinical Study. J. Strength Cond. Res. 2016, 30, 3329–3338. [Google Scholar] [CrossRef] [PubMed]
- Özen, T.; Korkmaz, H. Modulatory Effect of Urtica dioica L. (Urticaceae) Leaf Extract on Biotransformation Enzyme Systems, Antioxidant Enzymes, Lactate Dehydrogenase and Lipid Peroxidation in Mice. Phytomedicine 2003, 10, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, S.; Li, S.; Yang, X.; Qin, Y.; Zhang, Y.; Liu, C. Screening and Isolation of Potential Lactate Dehydrogenase Inhibitors from Five Chinese Medicinal Herbs: Soybean, Radix Pueraria, Flos Pueraria, Rhizoma Belamcandae, and Radix Astragali. J. Sep. Sci. 2016, 39, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Akbari-Fakhrabadi, M.; Najafi, M.; Mortazavian, S.; Rasouli, M.; Memari, A.H.; Shidfar, F. Effect of Saffron (Crocus sativus L.) and Endurance Training on Mitochondrial Biogenesis, Endurance Capacity, Inflammation, Antioxidant, and Metabolic Biomarkers in Wistar Rats. J. Food Biochem. 2019, 43, e12946. [Google Scholar] [CrossRef]
- Jiang, R.; Wang, M.; Shi, L.; Zhou, J.; Ma, R.; Feng, K.; Chen, X.; Xu, X.; Li, X.; Li, T. Panax Ginseng Total Protein Facilitates Recovery from Dexamethasone-Induced Muscle Atrophy through the Activation of Glucose Consumption in C2C12 Myotubes. BioMed Res. Int. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhang, Y.; Gao, J.; Ding, X.; Gao, S. The Anti-fatigue Effect of 20(R)-ginsenoside Rg3 in Mice by Intranasally Administration. Biol. Pharm. Bull. 2008, 31, 2024–2027. [Google Scholar] [CrossRef]
- Pratiwi, Y.S.; Lesmana, R.; Goenawan, H.; Sylviana, N.; Setiawan, I.; Tarawan, V.M.; Lestari, K.; Abdulah, R.; Dwipa, L.; Purba, A. Nutmeg Extract Increases Skeletal Muscle Mass in Aging Rats Partly via IGF1-AKT-mTOR Pathway and Inhibition of Autophagy. Evid. Based Complementary Altern. Med. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Woodford, M.R.; Chen, V.Z.; Backe, S.J.; Bratslavsky, G.; Mollapour, M. Structural and Functional Regulation of Lactate Dehydrogenase-A in Cancer. Future Med. Chem. 2020, 12, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.N.; Lin, S.Y.; Liao, Y.H.; Li, Z.J.; Wong, A.M. Late-onset Caloric Restriction Alters Skeletal Muscle Metabolism by Modulating Pyruvate Metabolism. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E942–949. [Google Scholar] [CrossRef] [PubMed]
- Kane, D.A. Lactate Oxidation at the Mitochondria: A Lactate-malate-aspartate Shuttle at Work. Front. Neurosci. 2014, 8, 366. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.-j.; Qin, Z.; Wang, P.-y.; Sun, Y.; Liu, X. Muscle Fatigue: General Understanding and Treatment. Exp. Mol. Med. 2017, 49, e384–e384. [Google Scholar] [CrossRef]
- Egan, B.; Carson, B.P.; Garcia-Roves, P.M.; Chibalin, A.V.; Sarsfield, F.M.; Barron, N.; McCaffrey, N.; Moyna, N.M.; Zierath, J.R.; O’Gorman, D.J. Exercise Intensity-dependent Regulation of Peroxisome Proliferator-activated Receptor γ Coactivator-1α mRNA Abundance is Associated with Differential Activation of Upstream Signalling Kinases in Human Skeletal Muscle. J. Physiol. 2010, 588, 1779–1790. [Google Scholar] [CrossRef]
- Thirupathi, A.; De Souza, C.T. Multi-regulatory Network of ROS: The Interconnection of ROS, PGC-1 alpha, and AMPK-SIRT1 During Exercise. J. Physiol. Biochem. 2017, 73, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal Muscle Energy Metabolism During Exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S.; Handschin, C.; St.-Pierre, J.; Spiegelman, B.M. AMP-activated Protein Kinase (AMPK) Action in Skeletal Muscle via Direct Phosphorylation of PGC-1α. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef] [PubMed]
- Irrcher, I.; Ljubicic, V.; Hood, D.A. Interactions between ROS and AMP Kinase Activity in the Regulation of PGC-1 Alpha transcription in Skeletal Muscle Cells. Am. J. Physiol. Cell. Physiol. 2009, 296, C116–C123. [Google Scholar] [CrossRef]
- Adamovich, Y.; Shlomai, A.; Tsvetkov, P.; Umansky, K.B.; Reuven, N.; Estall, J.L.; Spiegelman, B.M.; Shaul, Y. The Protein Level of PGC-1 alpha, a Key Metabolic Regulator, Is Controlled by NADH-NQO1. Mol. Cell. Biol. 2013, 33, 2603–2613. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Li Ji, L. Role of PGC-1alpha Signaling in Skeletal Muscle Health and Disease. Ann. N. Y. Acad. Sci. 2012, 1271, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Summermatter, S.; Santos, G.; Perez-Schindler, J.; Handschin, C. Skeletal Muscle PGC-1 Alpha Controls Whole-body Lactate Homeostasis Through Estrogen-related Receptor Alpha-dependent Activation of LDH B and Repression of LDH A. Proc. Natl. Acad. Sci. USA 2013, 110, 8738–8743. [Google Scholar] [CrossRef]
- Theret, M.; Gsaier, L.; Schaffer, B.; Juban, G.; Ben Larbi, S.; Weiss-Gayet, M.; Bultot, L.; Collodet, C.; Foretz, M.; Desplanches, D.; et al. AMPK Alpha 1-LDH Pathway Regulates Muscle Stem Cell Self-renewal by Controlling Metabolic Homeostasis. Embo. J. 2017, 36, 1946–1962. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, Y.; Gu, Y.; Xu, C. Anti-inflammatory Mechanism of Taurine Against Ischemic Stroke is Related to Down-regulation of PARP and NF-κB. Amino Acids 2012, 42, 1735–1747. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; You, J.-S.; Zhao, X.; Park, J.-Y.; Kim, S.-H.; Chang, K.-J. Antiobesity and Hypolipidemic Effects of Lotus Leaf Hot Water Extract with Taurine Supplementation in Rats Fed a High Fat Diet. J. Biomed. Sci. 2010, 17, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Roshan, V.D.; Aslani, E.; Stannard, S.R. Taurine Supplementation Has Anti-atherogenic and Anti-inflammatory Effects Before and After Incremental Exercise in Heart Failure. Ther. Adv. Cardiovasc. Dis. 2017, 11, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jin, H.; Nguyen, M.; Carr, J.; Lee, Y.; Hsu, C.; Faiman, M.; Schloss, J.; Wu, J. Role of Taurine in Regulation of Intracellular Calcium Level and Neuroprotective Function in Cultured Neurons. J. Neurosci. Res. 2001, 66, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Miyazaki, N.; Schaffer, S.; Azuma, J. Potential Anti-aging Role of Taurine via Proper Protein Folding: A Study from Taurine Transporter Knockout Mouse. In Taurine 9; Springer: Cham, Switzerland, 2015; pp. 481–487. [Google Scholar]
- Ripps, H.; Shen, W. Review: Taurine: A “Very Essential” Amino Acid. Mol. Vis. 2012, 18, 2673–2686. [Google Scholar] [PubMed]
- Dawson, R., Jr.; Biasetti, M.; Messina, S.; Dominy, J. The Cytoprotective Role of Taurine in Exercise-induced Muscle Injury. Amino Acids 2002, 22, 309–324. [Google Scholar] [CrossRef]
- Silva, L.A.; Silveira, P.C.; Ronsani, M.M.; Souza, P.S.; Scheffer, D.; Vieira, L.C.; Benetti, M.; De Souza, C.T.; Pinho, R.A. Taurine Supplementation Decreases Oxidative Stress in Skeletal Muscle After Eccentric Exercise. Cell Biochem. Funct. 2011, 29, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Kaferstein, F.; Abdussalam, M. Food Safety in the 21st Century. Bull. World Health Organ. 1999, 77, 347–351. [Google Scholar] [PubMed]
- Bauhammer, I.; Sacha, M.; Haltner, E. Validation and Stability Analysis of a Modified Lactate Dehydrogenase (LDH) Test Method to be Employed for an In Vitro Viable Skin Model. Heliyon 2019, 5, e01618. [Google Scholar] [CrossRef] [PubMed]
- Drent, M.; Cobben, N.; Henderson, R.; Wouters, E.; van Dieijen-Visser, M. Usefulness of Lactate Dehydrogenase and Its Isoenzymes as Indicators of Lung Damage or Inflammation. Eur. Respir. J. 1996, 9, 1736–1742. [Google Scholar] [CrossRef]
- Shikov, A.N.; Pozharitskaya, O.N.; Faustova, N.M.; Kosman, V.M.; Makarov, V.G.; Razzazi-Fazeli, E.; Novak, J. Pharmacokinetic Study of Bioactive Glycopeptide from Strongylocentrotus Droebachiensis After Intranasal Administration to Rats Using Biomarker Approach. Mar. Drugs 2019, 17, 577. [Google Scholar] [CrossRef] [PubMed]
- Shikov, A.N.; Narkevich, I.A.; Flisyuk, E.V.; Luzhanin, V.G.; Pozharitskaya, O.N. Medicinal Plants from the 14th Edition of the Russian Pharmacopoeia, Recent Updates. J. Ethnopharmacol. 2020, 268, 113685. [Google Scholar] [CrossRef] [PubMed]
- Karkanis, A.; Vellios, E.; Thomaidis, T.; Bilalis, D.; Efthimiadou, A.; Travlos, I. Phytochemistry and Biological Properties of Burnet Weed (Sanguisorba spp.): A Review. Not. Sci. Biol. 2014, 6, 395–398. [Google Scholar] [CrossRef]
- Yokozawa, T.; Chen, C.P.; Tanaka, T.; Kitani, K. A Study on the Nitric Oxide Production-suppressing Activity of Sanguisorbae Radix Components. Biol. Pharm. Bull. 2000, 23, 717–722. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seo, C.-S.; Jeong, S.-J.; Yoo, S.-R.; Lee, N.-R.; Shin, H.-K. Quantitative Analysis and In Vitro Anti-inflammatory Effects of Gallic Acid, Ellagic Acid, and Quercetin from Radix Sanguisorbae. Pharmacogn. Mag. 2016, 12, 104. [Google Scholar]
- Seo, J.; Do, C.; Jung, M.; Jung, S.; Kang, S. In Vivo Antiviral Activitiy of Sanguisorba Officinalis Roots Against Viral Hemorrhagic Septicemia Virus in Olive Flounder Paralichthys Olivaceus. Planta Med. 2015, 81, PW–45. [Google Scholar] [CrossRef]
- Chen, X.; Shang, F.; Meng, Y.; Li, L.; Cui, Y.; Zhang, M.; Qi, K.; Xue, T. Ethanol Extract of Sanguisorba Officinalis L. Inhibits Biofilm Formation of Methicillin-resistant Staphylococcus Aureus in an Ica-dependent Manner. J. Dairy Sci. 2015, 98, 8486–8491. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhang, Z.-L.; Liu, X.; Zhang, S.; He, L.; Wang, Z.; Wang, G.-S. Terpene Glycosides from the Roots of Sanguisorba Officinalis L. and Their Hemostatic Activities. Molecules 2012, 17, 7629–7636. [Google Scholar] [CrossRef] [PubMed]
- Janovska, D.; Kubikova, K.; Kokoska, L. Screening for Antimicrobial Activity of Some Medicinal Plants Species of Traditional Chinese Medicine. Czech J. Food Sci. 2003, 21, 107–110. [Google Scholar] [CrossRef]
- Shin, J.; Kim, J.-S.; Kwon, K.-H.; Nam, J.-S.; Jung, J.Y.; Cho, N.-P.; Cho, S.-D. Apoptotic Effect of Hot Water Extract of Sanguisorba officinalis L. in Human Oral Cancer Cells. Oncol. Lett. 2012, 4, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, D.; Herrmann, F.; Wink, M. Extracts from Traditional Chinese Medical Plants Inhibit Glycogen Synthase Kinase 3β Activity, a Potential Alzheimer Target. Z. Phytother. 2009, 30, V16. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, J.-Z.; Liu, Y.; Wang, K.; Ding, W.; Wang, H.; Liu, X.; Zhou, S.; Lu, X.-C.; Yang, H.-B. Nuclear Lactate Dehydrogenase A Senses ROS to Produce α-hydroxybutyrate for HPV-induced Cervical Tumor Growth. Nat. Commun. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.J.; Kim, A.; Kim, G.T.; Yu, H.Y.; Lee, E.S.; Park, M.J.; Kim, Y.J.; Shim, S.M.; Park, T.S. Inhibition of Lactate Dehydrogenase A Suppresses Inflammatory Response in RAW 264.7 Macrophages. Mol. Med. Rep. 2019, 19, 629–637. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Loo, T.Y.; Shen, J.-G.; Wang, N.; Wang, D.-M.; Yang, D.-P.; Mo, S.-L.; Guan, X.-Y.; Chen, J.-P. LDH-A Silencing Suppresses Breast Cancer Tumorigenicity Through Induction of Oxidative Stress Mediated Mitochondrial Pathway Apoptosis. Breast Cancer Res. Treat. 2012, 131, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.-S.; Chan, K.-H.; Hsu, M.-C.; Liu, J.-F. Supplementation with Soybean Peptides, Taurine, Pueraria isoflavone, and Ginseng Saponin Complex Improves Endurance Exercise Capacity in Humans. J. Med. Food 2011, 14, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, A.J. Effect of Vitamin C Supplements on Physical Performance. Curr. Sports Med. Rep. 2012, 11, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.d.R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Alvarez-Parrilla, E.; González-Aguilar, G.A.; De la Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-chemistry, Foodomics and Health Effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef] [PubMed]
- Hajimoradi, M.; Fazilati, M.; Gharib-Naseri, M.K.; Sarkaki, A. Gallic Acid and Exercise Training Improve Motor Function, Nerve Conduction Velocity but not Pain Sense Reflex After Experimental Sciatic Nerve Crush in Male Rats. Avicenna J. Phytomedicine 2015, 5, 288. [Google Scholar]
- Maki, K.C.; Reeves, M.S.; Farmer, M.; Yasunaga, K.; Matsuo, N.; Katsuragi, Y.; Komikado, M.; Tokimitsu, I.; Wilder, D.; Jones, F. Green Tea Catechin Consumption Enhances Exercise-induced Abdominal Fat Loss in Overweight and Obese Adults. J. Nutr. 2009, 139, 264–270. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.H.; Kim, M.; Choi, H.-J.; Jin, J.S.; Lee, S.-O.; Bae, S.-J.; Ryu, D.; Ha, K.-T. The Oral Administration of Sanguisorba officinalis Extract Improves Physical Performance through LDHA Modulation. Molecules 2021, 26, 1579. https://doi.org/10.3390/molecules26061579
Han JH, Kim M, Choi H-J, Jin JS, Lee S-O, Bae S-J, Ryu D, Ha K-T. The Oral Administration of Sanguisorba officinalis Extract Improves Physical Performance through LDHA Modulation. Molecules. 2021; 26(6):1579. https://doi.org/10.3390/molecules26061579
Chicago/Turabian StyleHan, Jung Ho, MinJeong Kim, Hee-Jin Choi, Jung Sook Jin, Syng-Ook Lee, Sung-Jin Bae, Dongryeol Ryu, and Ki-Tae Ha. 2021. "The Oral Administration of Sanguisorba officinalis Extract Improves Physical Performance through LDHA Modulation" Molecules 26, no. 6: 1579. https://doi.org/10.3390/molecules26061579
APA StyleHan, J. H., Kim, M., Choi, H.-J., Jin, J. S., Lee, S.-O., Bae, S.-J., Ryu, D., & Ha, K.-T. (2021). The Oral Administration of Sanguisorba officinalis Extract Improves Physical Performance through LDHA Modulation. Molecules, 26(6), 1579. https://doi.org/10.3390/molecules26061579






