Green Solvents as an Alternative to DMF in ZIF-90 Synthesis
Abstract
1. Introduction
2. Results
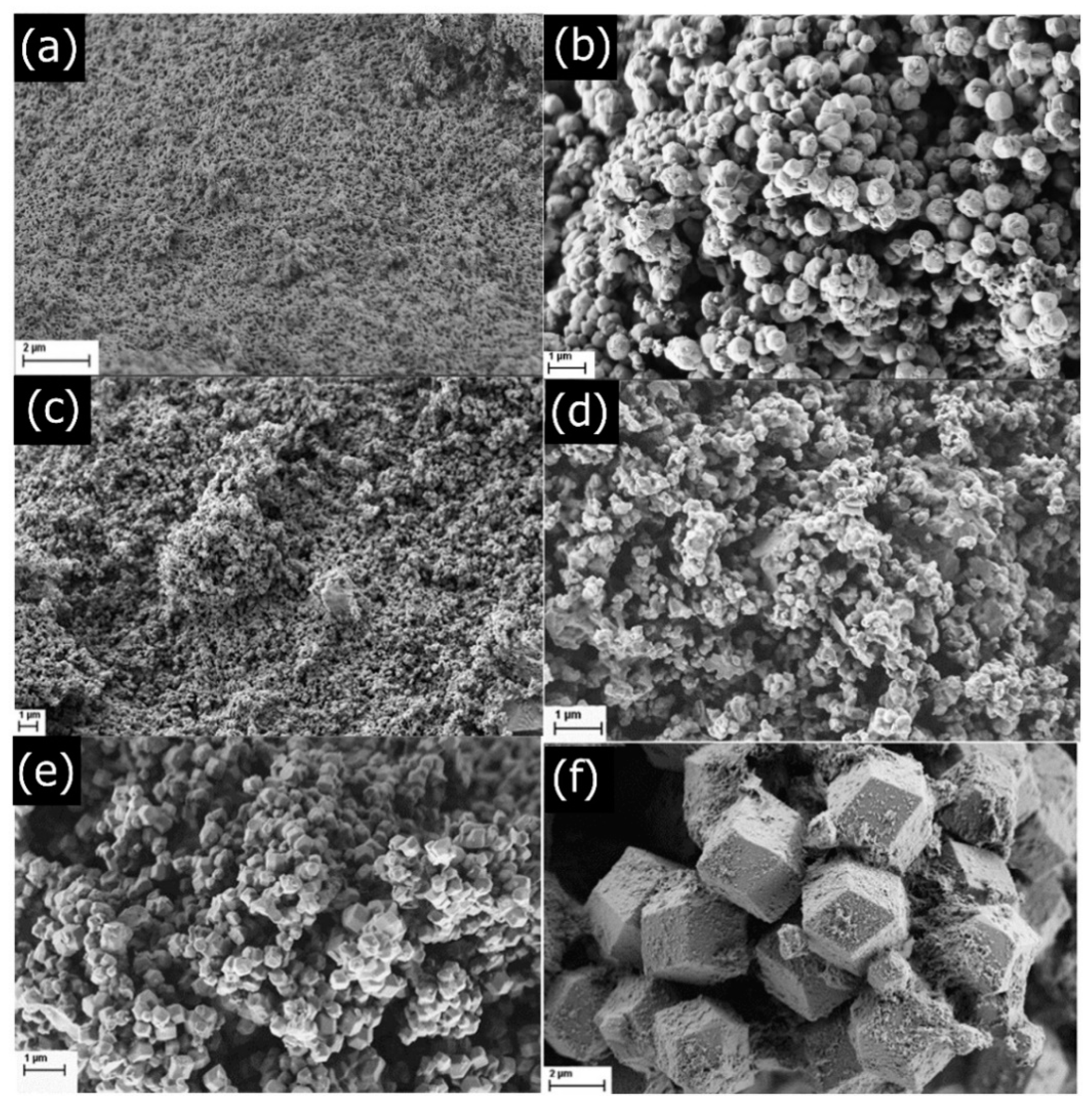
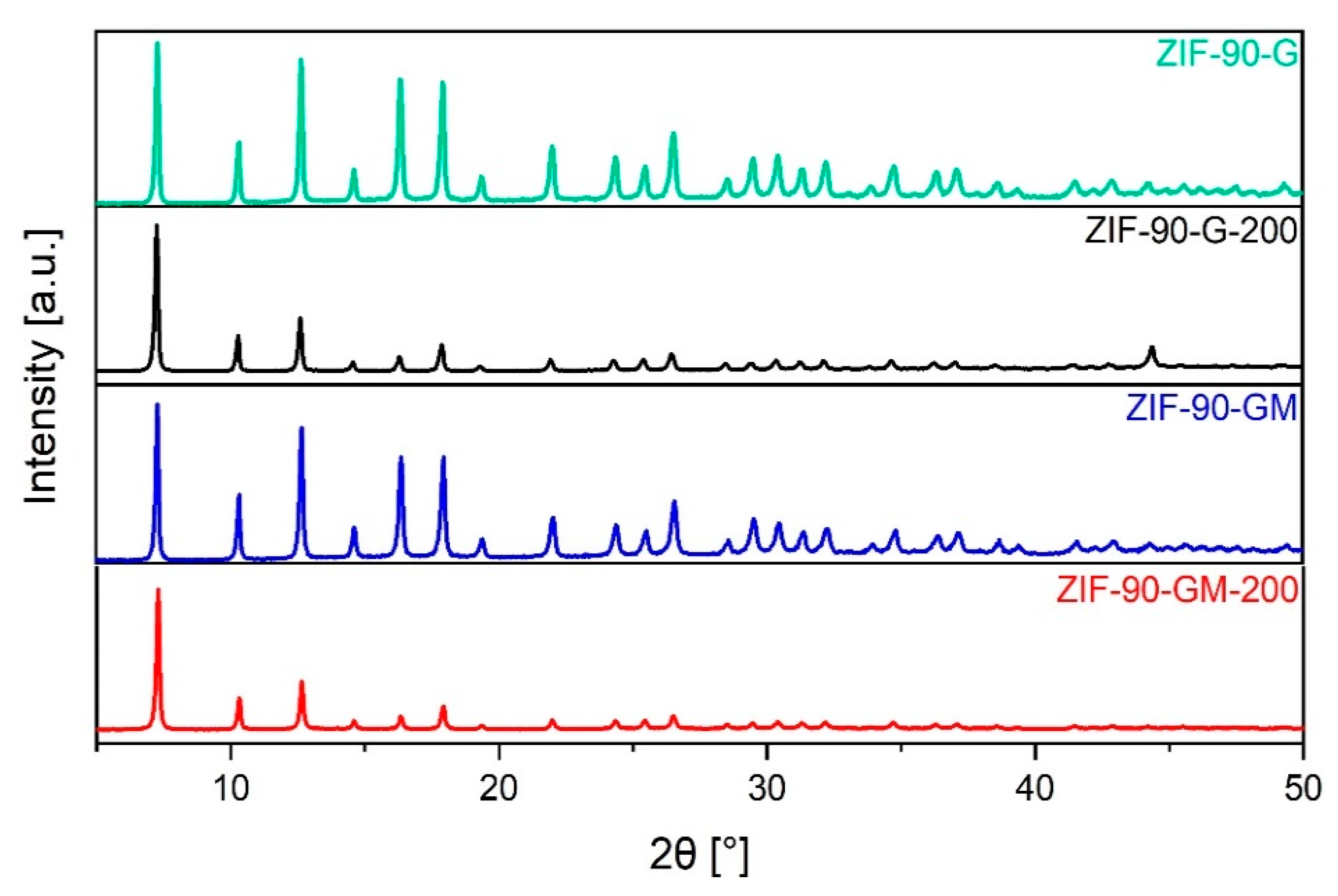
2.1. XRD, SEM, and TG Analyses of As-Synthesised ZIF-90
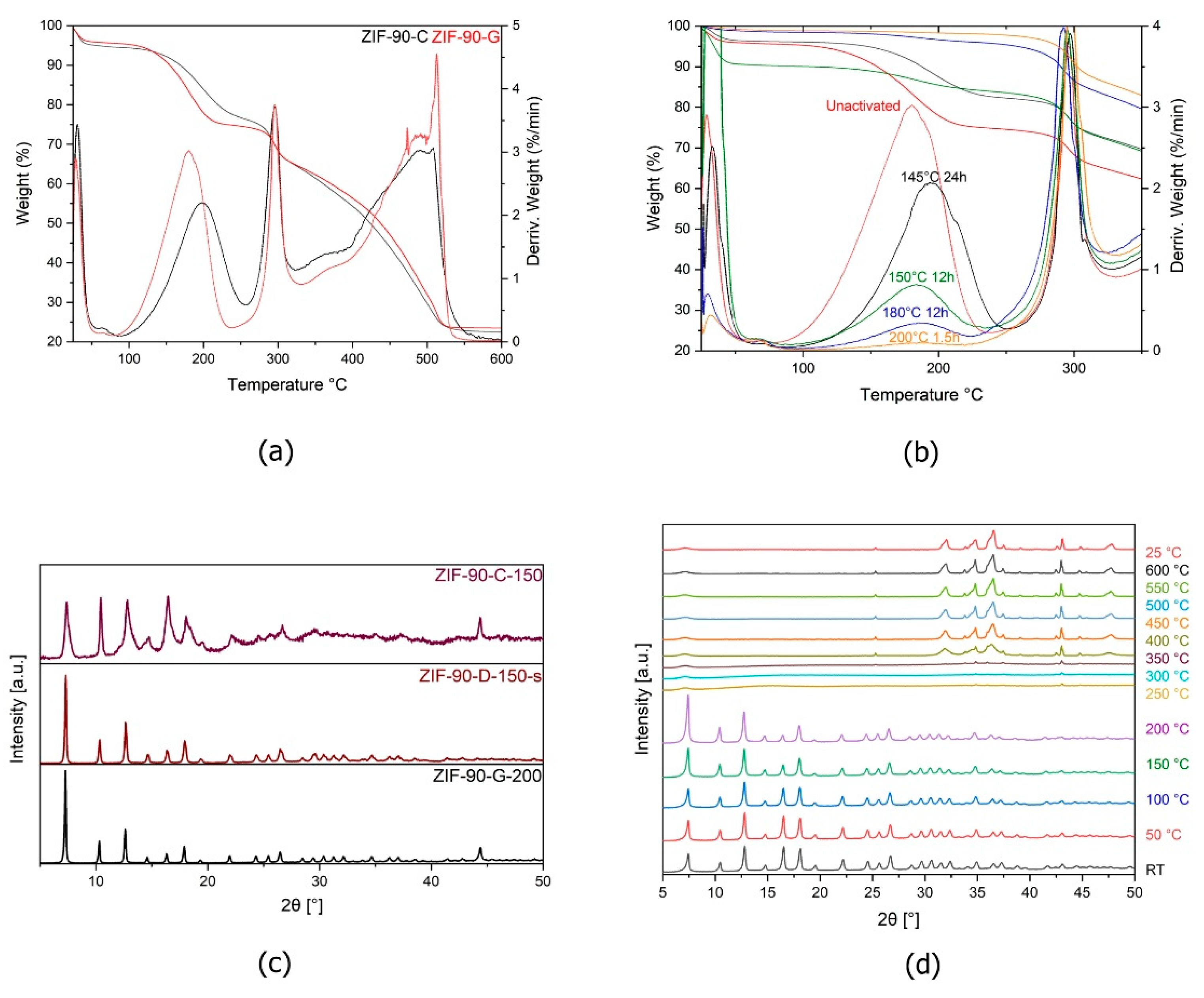
2.2. TGA and Activation
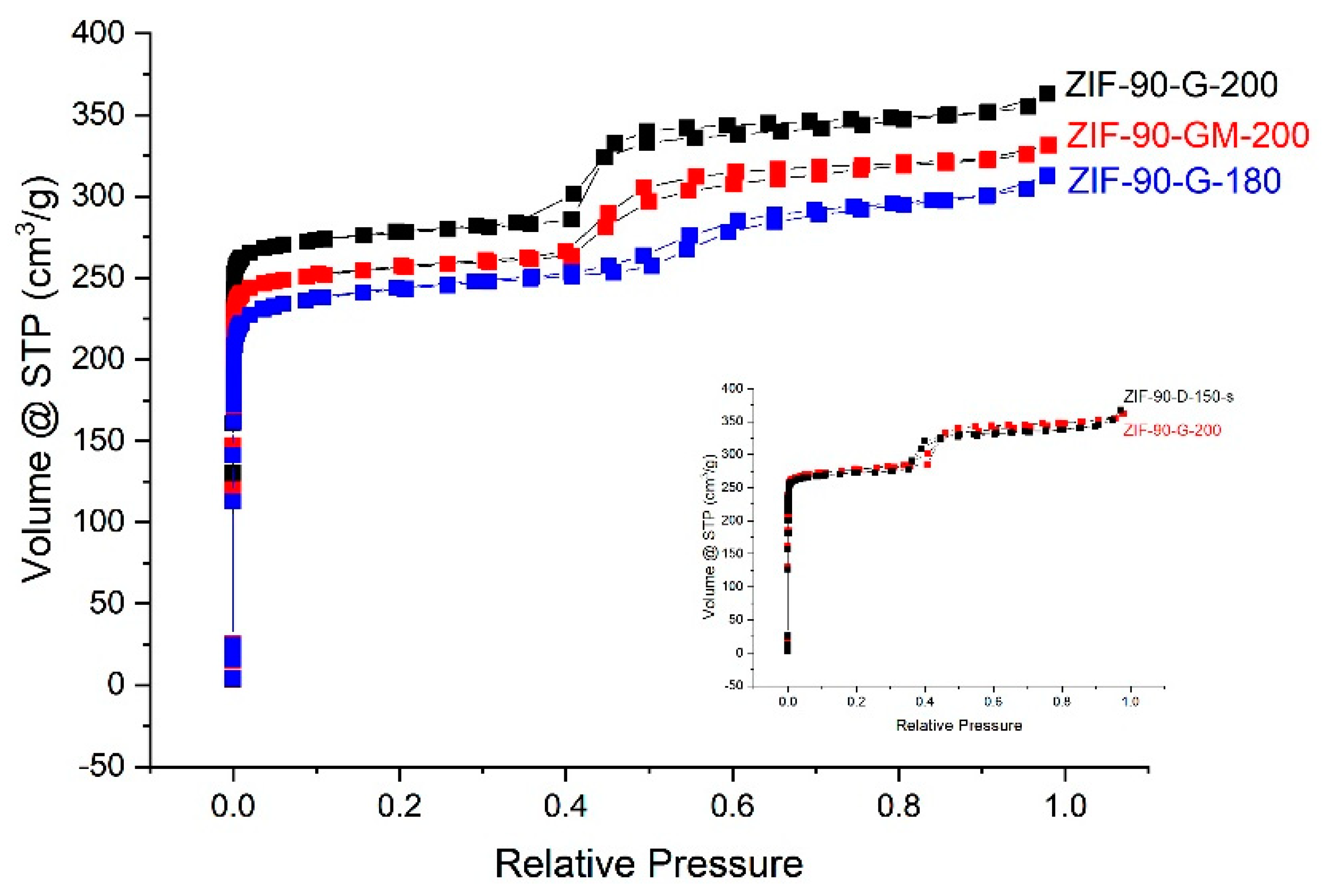
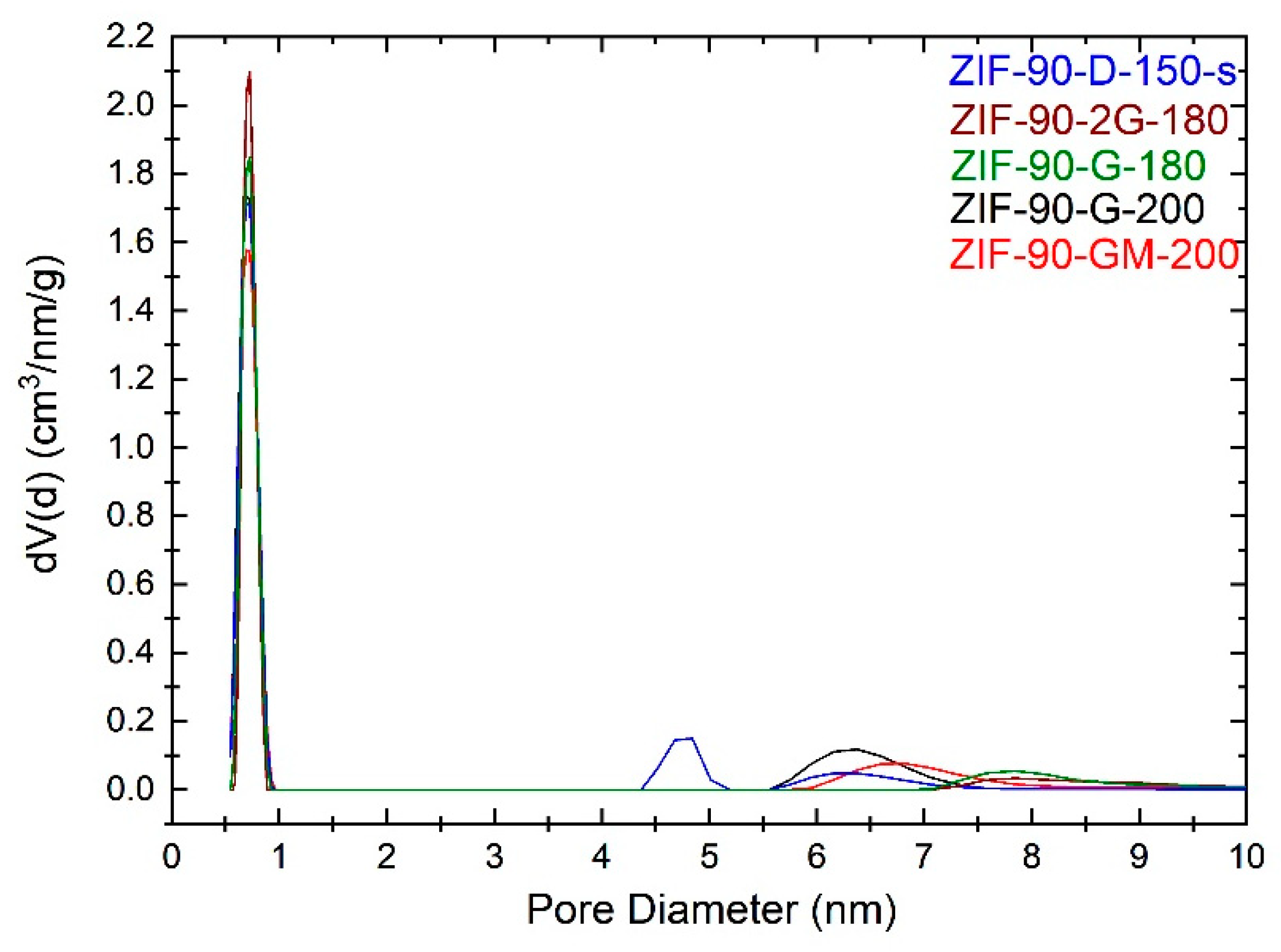
2.3. N2 Adsorption Isotherm Measurements
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. ZIF-90 Synthesis
4.2.1. ZIF-90-D Synthesis
4.2.2. Green Solvent Based ZIF-90 Syntheses
4.3. Activation of ZIFs
4.3.1. Solvent Exchange Activation
4.3.2. Thermal Burst Activation
4.4. Characterisation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bose, R.; Ethiraj, J.; Sridhar, P.; Varghese, J.J.; Kaisare, N.S.; Selvam, P. Adsorption of hydrogen and carbon dioxide in zeolitic imidazolate framework structure with SOD topology: Experimental and modelling studies. Adsorption 2020, 1, 3. [Google Scholar] [CrossRef]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; Okeeffe, M.; Yaghi, O.M. Synthesis, structure, and carbon dioxide capture properties of zeolitic imidazolate frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef]
- Akbari Beni, F.; Niknam Shahrak, M. Alkali metals-promoted capacity of ZIF-8 and ZIF-90 for carbon capturing: A molecular simulation study. Polyhedron 2020, 178, 114338. [Google Scholar] [CrossRef]
- Ghahramaninezhad, M.; Mohajer, F.; Niknam Shahrak, M. Improved CO2 capture performances of ZIF-90 through sequential reduction and lithiation reactions to form a hard/hard structure. Front. Chem. Sci. Eng. 2020, 14, 425–435. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, S.; Weidman, J.; Guo, R. Surface modification of ZIF-90 with triptycene for enhanced interfacial interaction in mixed-matrix membranes for gas separation. J. Polym. Sci. 2020, 58, 2675–2687. [Google Scholar] [CrossRef]
- Xiang, W.; Sun, Z.; Wu, Y.; He, L.N.; Liu, C.J. Enhanced cycloaddition of CO2 to epichlorohydrin over zeolitic imidazolate frameworks with mixed linkers under solventless and co-catalyst-free condition. Catal. Today 2020, 339, 337–343. [Google Scholar] [CrossRef]
- Hashemian, S.; Sedrpoushan, A.; Eshbala, F.H. Co-Zeolite Imidazolate Frameworks (ZIF-9@Zeolite) as Heterogen Catalyst for Alcohols Oxidation. Catal. Lett. 2017, 147, 196–203. [Google Scholar] [CrossRef]
- García-Palacín, M.; Martínez, J.I.; Paseta, L.; Deacon, A.; Johnson, T.; Malankowska, M.; Téllez, C.; Coronas, J. Sized-Controlled ZIF-8 Nanoparticle Synthesis from Recycled Mother Liquors: Environmental Impact Assessment. ACS Sustain. Chem. Eng. 2020, 8, 2973–2980. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Kim, K.H. Green synthesis of metal–organic frameworks: A state-of-the-art review of potential environmental and medical applications. Coord. Chem. Rev. 2020, 420, 213407. [Google Scholar] [CrossRef]
- Hu, L.; Chen, L.; Peng, X.; Zhang, J.; Mo, X.; Liu, Y.; Yan, Z. Bifunctional metal-doped ZIF-8: A highly efficient catalyst for the synthesis of cyclic carbonates from CO2 cycloaddition. Microporous Mesoporous Mater. 2020, 299, 110123. [Google Scholar] [CrossRef]
- Chang, C.W.; Kao, Y.H.; Shen, P.H.; Kang, P.C.; Wang, C.Y. Nanoconfinement of metal oxide MgO and ZnO in zeolitic imidazolate framework ZIF-8 for CO2 adsorption and regeneration. J. Hazard. Mater. 2020, 400, 122974. [Google Scholar] [CrossRef] [PubMed]
- Hardian, R.; Liang, Z.; Zhang, X.; Szekely, G. Artificial intelligence: The silver bullet for sustainable materials development. Green Chem. 2020, 22, 7521–7528. [Google Scholar] [CrossRef]
- Beldon, P.J.; Fábián, L.; Stein, R.S.; Thirumurugan, A.; Cheetham, A.K.; Friščić, T. Rapid Room-Temperature Synthesis of Zeolitic Imidazolate Frameworks by Using Mechanochemistry. Angew. Chem. Int. Ed. 2010, 49, 9640–9643. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-B.; Lin, R.-B.; Cheng, X.-N.; Zhang, J.-P.; Chen, X.-M. Solvent/additive-free synthesis of porous/zeolitic metal azolate frameworks from metal oxide/hydroxidew. Chem. Commun. 2011, 47, 9185–9187. [Google Scholar] [CrossRef]
- Hua, D.; Ong, Y.K.; Wang, Y.; Yang, T.; Chung, T.S. ZIF-90/P84 mixed matrix membranes for pervaporation dehydration of isopropanol. J. Membr. Sci. 2014, 453, 155–167. [Google Scholar] [CrossRef]
- Duan, C.; Li, F.; Luo, S.; Xiao, J.; Li, L.; Xi, H. Facile synthesis of hierarchical porous metal-organic frameworks with enhanced catalytic activity. Chem. Eng. J. 2018, 334, 1477–1483. [Google Scholar] [CrossRef]
- Zhang, H.; Duan, C.; Li, F.; Yan, X.; Xi, H. Green and rapid synthesis of hierarchical porous zeolitic imidazolate frameworks for enhanced CO2 capture. Inorg. Chim. Acta 2018, 482, 358–363. [Google Scholar] [CrossRef]
- Duan, C.; Li, F.; Xiao, J.; Liu, Z.; Li, C.; Xi, H. Rapid room-temperature synthesis of hierarchical porous zeolitic imidazolate frameworks with high space-time yield. Sci. China Mater. 2017, 60, 1205–1214. [Google Scholar] [CrossRef]
- Brown, A.J.; Johnson, J.R.; Lydon, M.E.; Koros, W.J.; Jones, C.W.; Nair, S. Continuous polycrystalline zeolitic imidazolate framework-90 membranes on polymeric hollow fibers. Angew. Chem. Int. Ed. 2012, 51, 10615–10618. [Google Scholar] [CrossRef]
- Shieh, F.K.; Wang, S.C.; Leo, S.Y.; Wu, K.C.W. Water-based synthesis of zeolitic imidazolate framework-90 (ZIF-90) with a controllable particle size. Chem. Eur. J. 2013, 19, 11139–11142. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shen, K.; Li, Y. Greening the Processes of Metal-Organic Framework Synthesis and their Use in Sustainable Catalysis. ChemSusChem 2017, 10, 3165–3187. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.J.; Tu, W.C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Gao, F.; Bai, R.; Ferlin, F.; Vaccaro, L.; Li, M.; Gu, Y. Replacement strategies for non-green dipolar aprotic solvents. Green Chem. 2020, 22, 6240–6257. [Google Scholar] [CrossRef]
- Zhang, J.; White, G.B.; Ryan, M.D.; Hunt, A.J.; Katz, M.J. Dihydrolevoglucosenone (Cyrene) As a Green Alternative to N,N-Dimethylformamide (DMF) in MOF Synthesis. ACS Sustain. Chem. Eng. 2016, 4, 7186–7192. [Google Scholar] [CrossRef]
- Wong, C.Y.Y.; Choi, A.W.T.; Lui, M.Y.; Fridrich, B.; Horváth, A.K.; Mika, L.T.; Horváth, I.T. Stability of gamma-valerolactone under neutral, acidic, and basic conditions. Struct. Chem. 2017, 28, 423–429. [Google Scholar] [CrossRef]
- Anastasiou, I.; Van Velthoven, N.; Tomarelli, E.; Lombi, A.; Lanari, D.; Liu, P.; Bals, S.; De Vos, D.E.; Vaccaro, L. C2–H Arylation of Indoles Catalyzed by Palladium-Containing Metal-Organic-Framework in γ-Valerolactone. ChemSusChem 2020, 13, 2786–2791. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.; Ursino, C.; Avruscio, E.; Desiderio, G.; Perrone, A.; Santoro, S.; Galiano, F.; Figoli, A. Innovative Poly (Vinylidene Fluoride) (PVDF) Electrospun Nanofiber Membrane Preparation Using DMSO as a Low Toxicity Solvent. Membranes 2020, 10, 36. [Google Scholar] [CrossRef]
- Marino, T.; Russo, F.; Criscuoli, A.; Figoli, A. TamiSolve® NxG as novel solvent for polymeric membrane preparation. J. Membr. Sci. 2017, 542, 418–429. [Google Scholar] [CrossRef]
- Cseri, L.; Szekely, G. Towards cleaner PolarClean: Efficient synthesis and extended applications of the polar aprotic solvent methyl 5-(dimethylamino)-2-methyl-5-oxopentanoate. Green Chem. 2019, 21, 4178–4188. [Google Scholar] [CrossRef]
- Gao, M.; Wang, J.; Rong, Z.; Shi, Q.; Dong, J. A combined experimental-computational investigation on water adsorption in various ZIFs with the SOD and RHO topologies. RSC Adv. 2018, 8, 39627–39634. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, A.; Hou, K.; Liu, M.; Wang, Y.; Song, C.; Zhang, G.; Guo, X. Size- and morphology-controlled NH2-MIL-53(Al) prepared in DMF-water mixed solvents. Dalton Trans. 2013, 42, 13698–13705. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.D.R.; Baker, T.L.; Dao, B.; Kwon, C.; Tian, F. Exploring the Aggregative Growth of Nanoporous Zeolitic Imidazolate Framework ZIF-8. Cryst. Growth Des. 2020, 20, 2305–2312. [Google Scholar] [CrossRef]
- Li, X.; Tang, B.; Huang, W.; Yu, H. Effect from Mechanical Stirring Time and Speed on Adsorption Performance of ZIF-90 for n -Hexane. Z. Anorg. Allg. Chem. 2019, 645, 73–78. [Google Scholar] [CrossRef]

| Product | Linker Solvent | Metal Precursor Solvent |
|---|---|---|
| ZIF-90-D | DMF (645 mmol) * | MeOH (1250 mmol) |
| ZIF-90-C | CyreneTM (481 mmol) | MeOH (1250 mmol) |
| ZIF-90-G | GVL (525 mmol) | MeOH (1250 mmol) |
| ZIF-90-2G | GVL (263 mmol) + MeOH (625 mmol) | GVL (263 mmol) + MeOH (625 mmol) |
| ZIF-90-GM | GVL (263 mmol) + MeOH (625 mmol) | MeOH (1250 mmol) |
| ZIF-90-G2M | GVL (179 mmol) + MeOH (825 mmol) | MeOH (1250 mmol) |
| ZIF | Activation | SBET 2 (m2/g) | Vtotal 3 (cm3/g) | Vmicro 4 (cm3/g) |
|---|---|---|---|---|
| ZIF-90-D-150-s | 150 °C/12 h 1 | 1119 | 0.514 | 0.390 |
| ZIF-90-2G-180 | 180 °C/12 h | 929 | 0.420 | 0.392 |
| ZIF-90-G-180 | 180 °C/12 h | 977 | 0.437 | 0.331 |
| ZIF-90-G-200 | 200 °C/1.5 h | 1136 | 0.508 | 0.311 |
| ZIF-90-GM-200 | 200 °C/1.5 h | 1044 | 0.465 | 0.358 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Škrjanc, A.; Byrne, C.; Zabukovec Logar, N. Green Solvents as an Alternative to DMF in ZIF-90 Synthesis. Molecules 2021, 26, 1573. https://doi.org/10.3390/molecules26061573
Škrjanc A, Byrne C, Zabukovec Logar N. Green Solvents as an Alternative to DMF in ZIF-90 Synthesis. Molecules. 2021; 26(6):1573. https://doi.org/10.3390/molecules26061573
Chicago/Turabian StyleŠkrjanc, Aljaž, Ciara Byrne, and Nataša Zabukovec Logar. 2021. "Green Solvents as an Alternative to DMF in ZIF-90 Synthesis" Molecules 26, no. 6: 1573. https://doi.org/10.3390/molecules26061573
APA StyleŠkrjanc, A., Byrne, C., & Zabukovec Logar, N. (2021). Green Solvents as an Alternative to DMF in ZIF-90 Synthesis. Molecules, 26(6), 1573. https://doi.org/10.3390/molecules26061573






