Poly(Pyridinium Salt)s Containing 2,7-Diamino-9,9′-Dioctylfluorene Moieties with Various Organic Counterions Exhibiting Both Lyotropic Liquid-Crystalline and Light-Emitting Properties
Abstract
1. Introduction
2. Results and Discussion
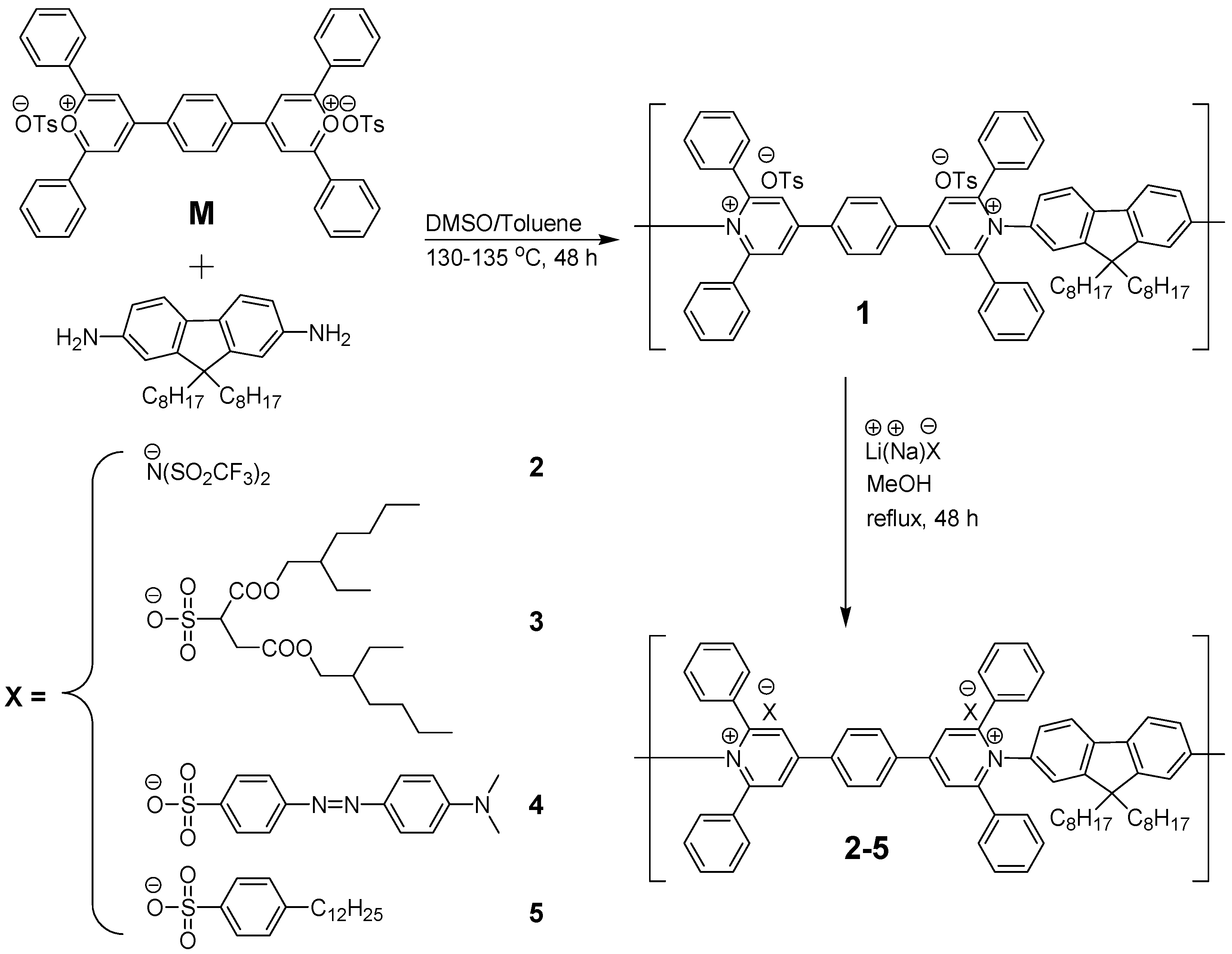
2.1. Chemical Structures of Polymers 1–5
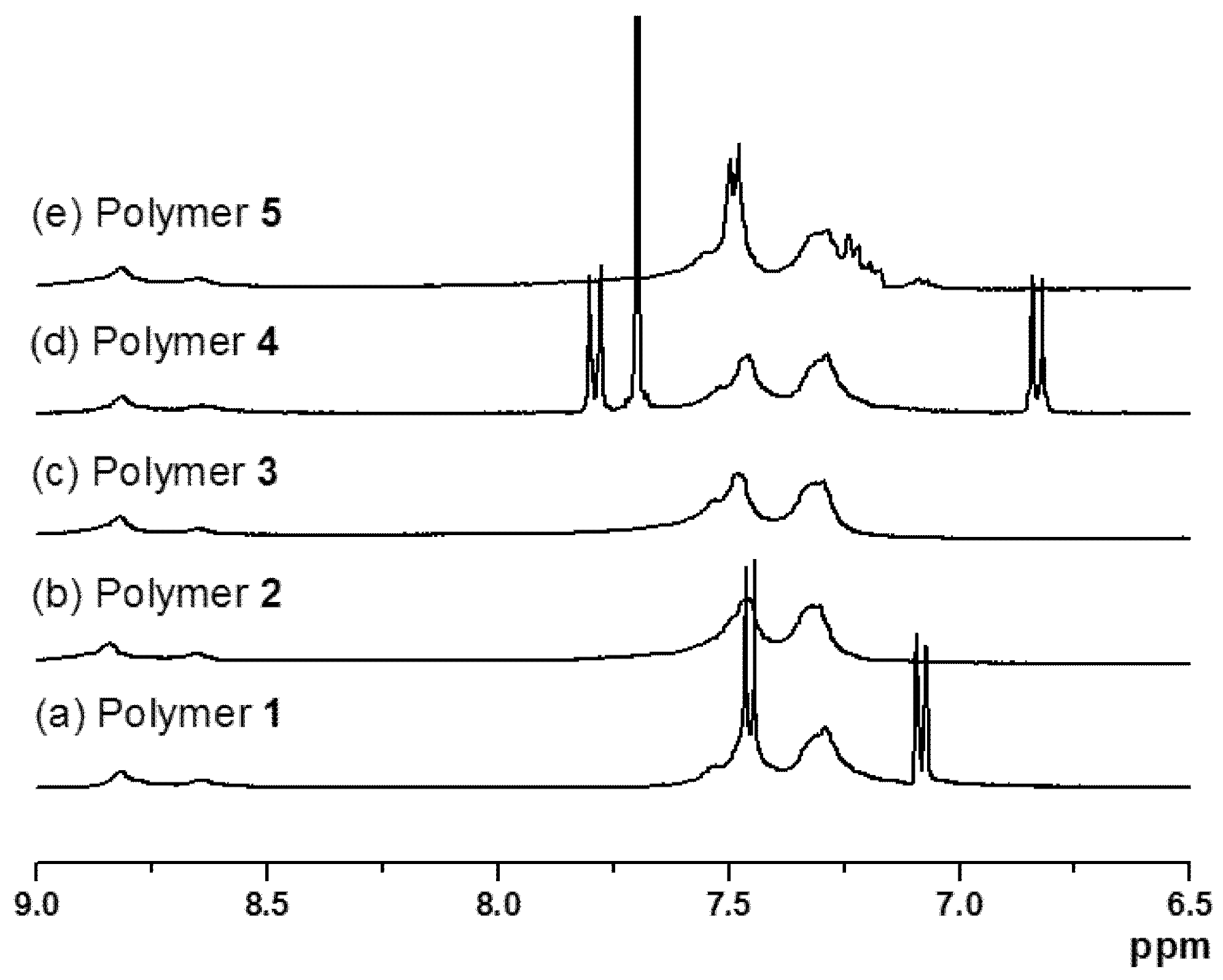
2.2. Molecular Weights of Polymers 1–5
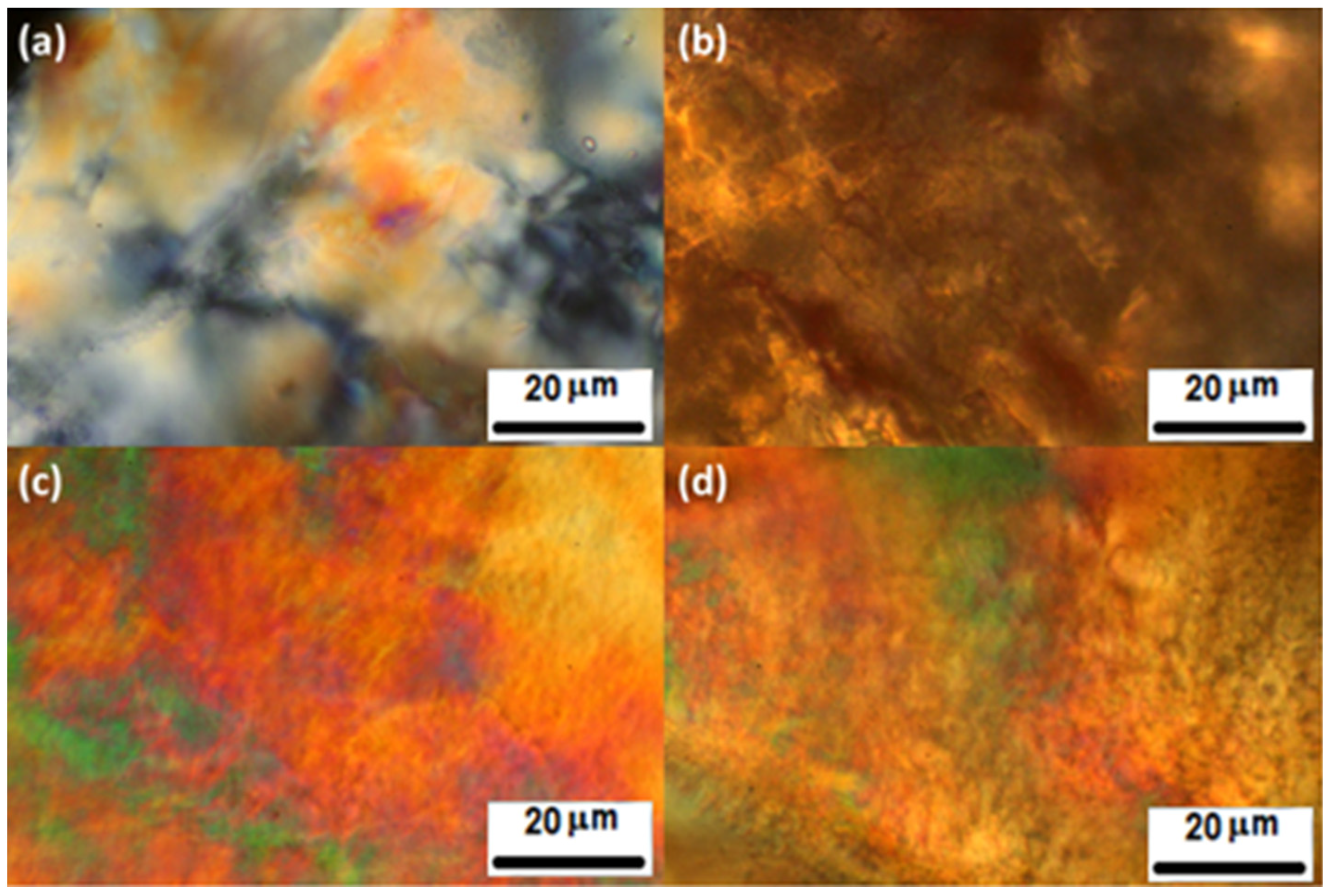
2.3. Solution Properties of 1–5 by Polarized Optical Microscopy
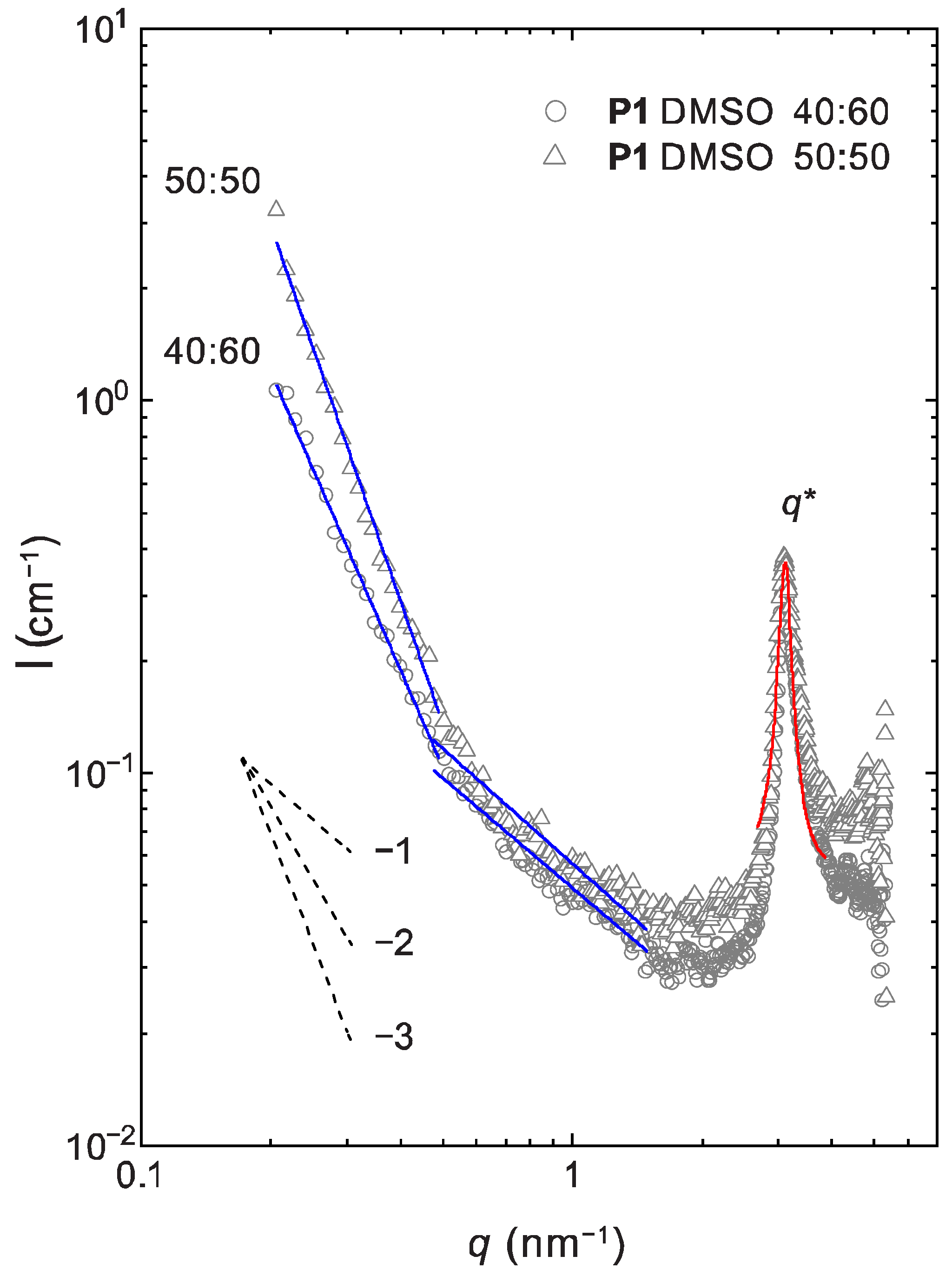
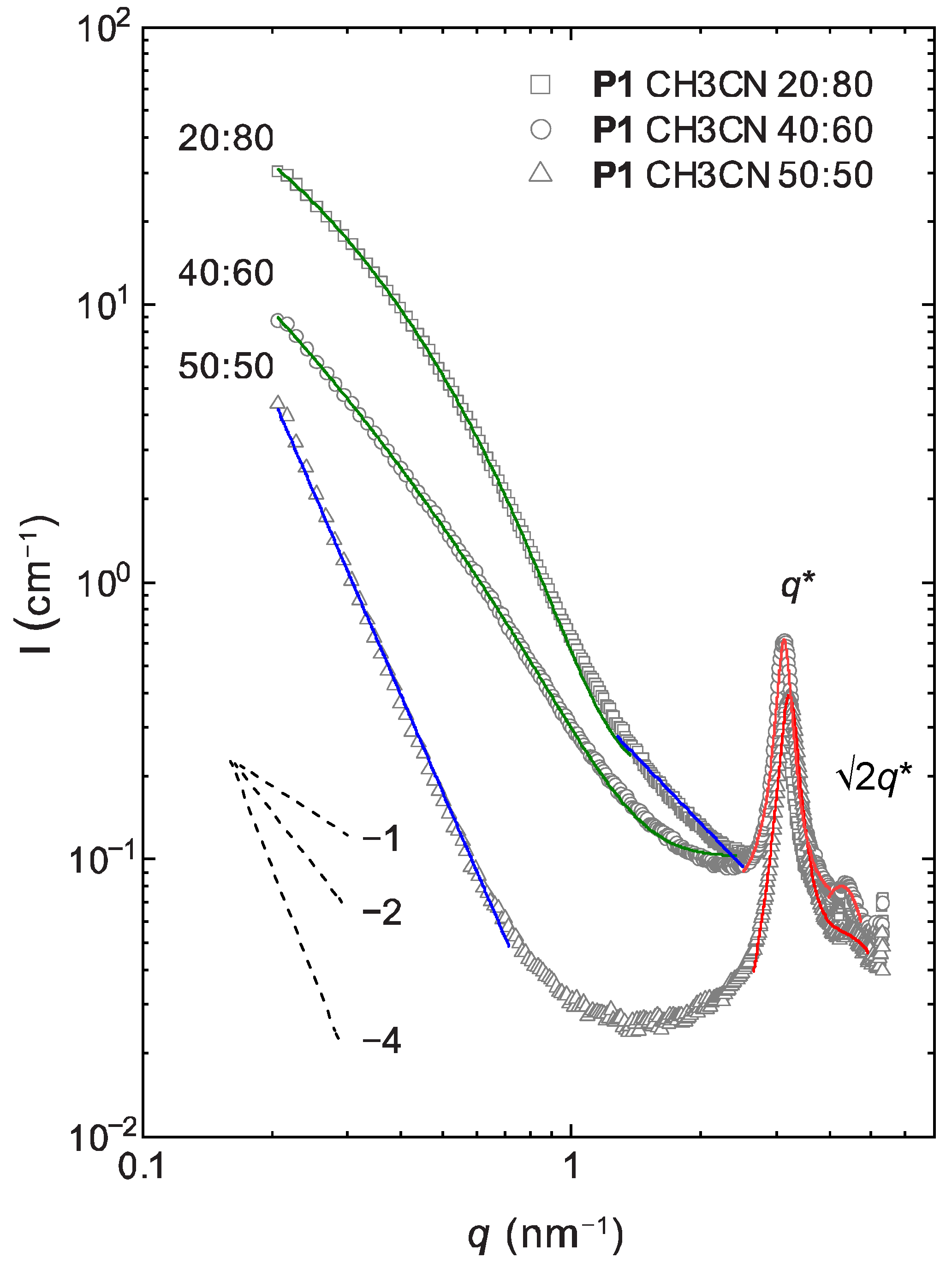
2.4. Lyotropic Properties of Polymer 1 by Small-Angle X-ray Scattering (SAXS)
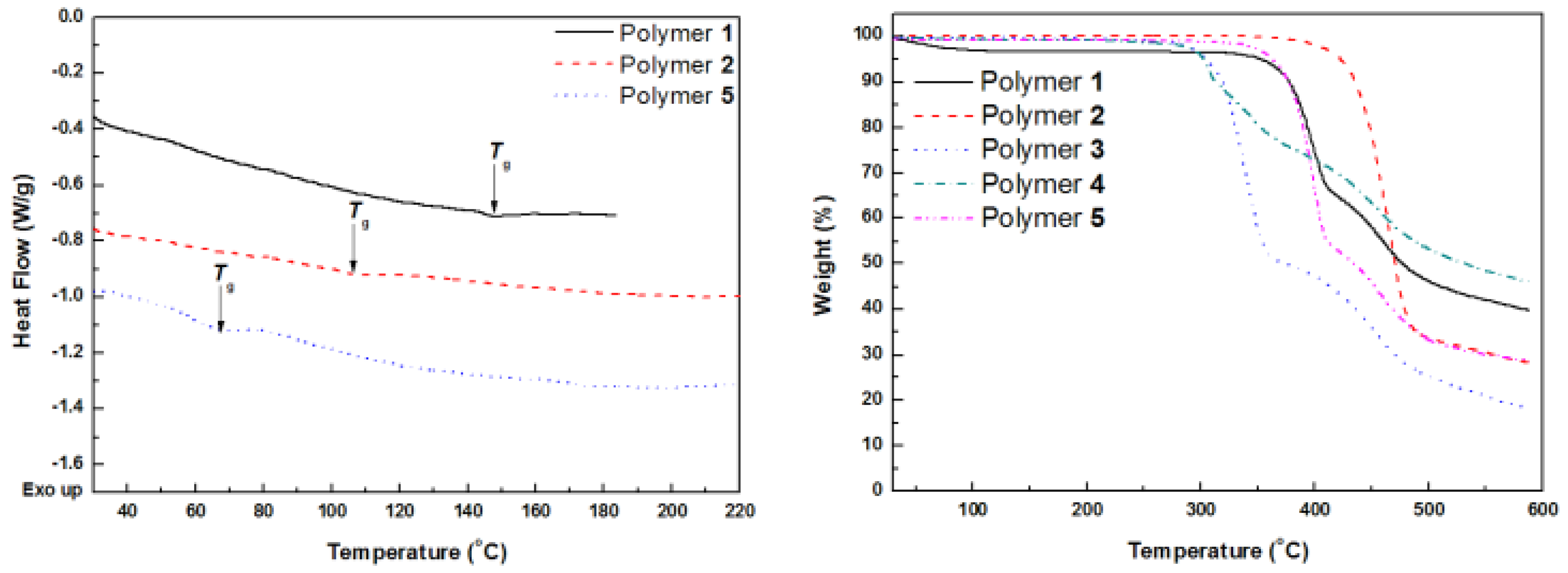
2.5. Thermal Properties of Polymers 1–5
2.6. Optical Properties of Polymers 1–5
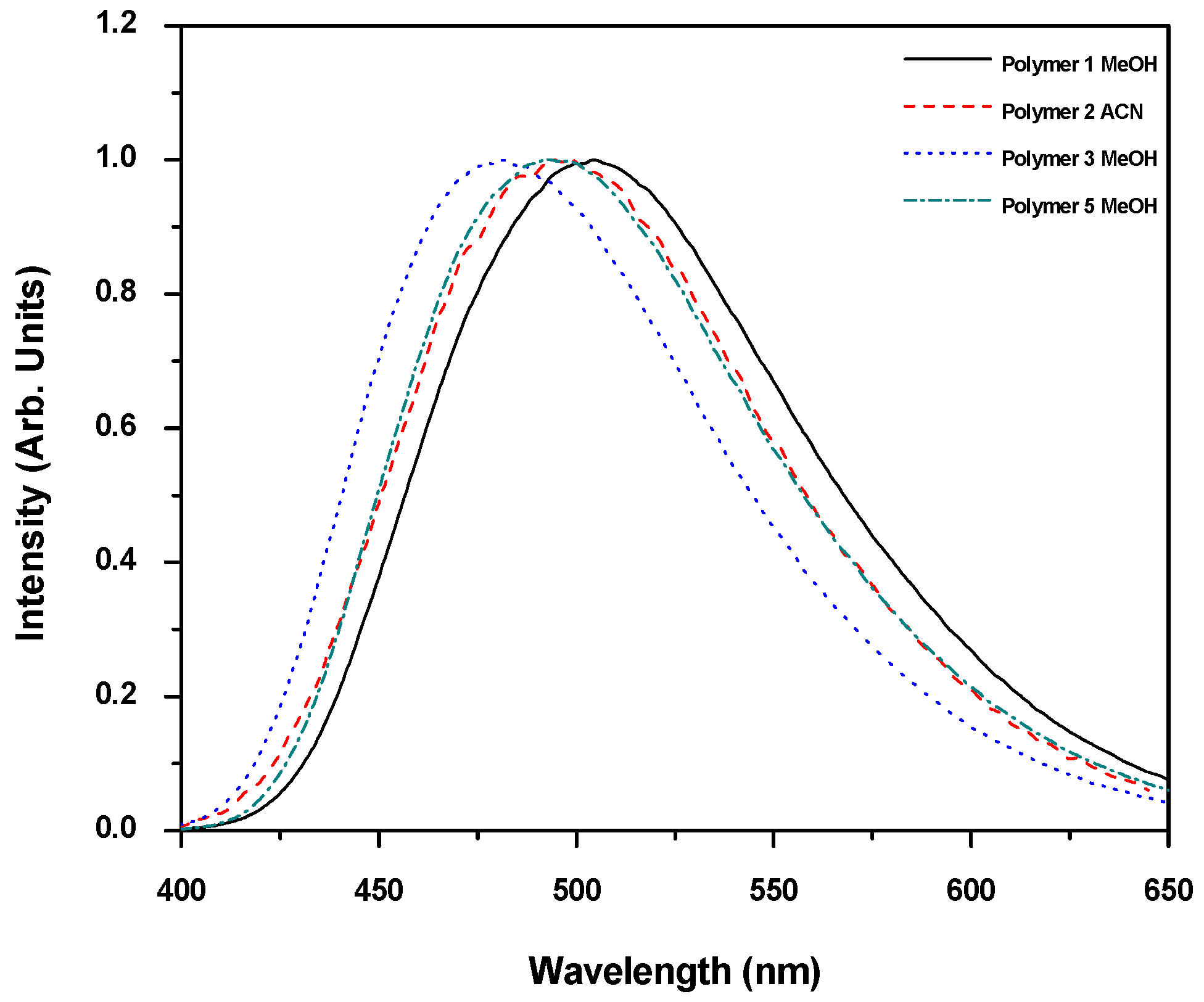
2.6.1. UV-Vis Absorption and Emission Spectra of Polymers 1–5 in Organic Solvents
2.6.2. Emission Properties of Polymers 1–5 in the Solid State (Film and Powdered)
2.6.3. Emission Properties Polymers 1, 2, and 5 in Organic Solvents-Water Mixture to Study Aggregation Behaviour
3. Materials and Methods
3.1. Instrumentation
3.2. Materials
3.3. Synthesis of Polymer 1
3.4. Synthesis of Polymers 2–5
3.5. X-ray Scattering Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Katritzky, A.R.; Tarr, R.D.; Heilman, S.M.; Rasmussen, J.K.; Krepski, L.R. Polymers by the reaction of bis(pyrylium salts) with diamines: A novel approach to ionene polymers. J. Polym. Sci. Part A Polym. Chem. 1988, 26, 3323–3336. [Google Scholar] [CrossRef]
- Harris, F.W.; Chuang, K.C.; Huang, S.A.X.; Janimak, J.J.; Cheng, S.Z.D. Aromatic poly(pyridinium salt)s: Synthesis and structures of organo-soluble, rigid-rod poly(pyridinium tetrafluoroborate)s. Polymer 1994, 35, 4940–4948. [Google Scholar] [CrossRef]
- Huang, S.A.X.; Chuang, K.C.; Cheng, S.Z.D.; Harris, F.W. Aromatic poly(pyridinium salt)s part 2. Synthesis and properties of organo-soluble, rigid-rod poly(pyridinium triflate)s. Polymer 2000, 41, 5001–5009. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Burchett, R.A.; Han, H.; Cebe, J.J. Synthesis and characterization of poly(pyridinium salt)s with organic counterion exhibiting both lyotropic liquid-crystalline and light-emitting properties. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 2710–2715. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Burchett, R.A.; Han, H.; Cebe, J.J. Synthesis and characterization of poly(pyridinium salt)s with organic counterion exhibiting both lyotropic liquid-crystalline and light-emitting properties. Macromolecules 2001, 34, 7579–7581. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Han, H.; Cebe, J.J.; Nedeltchev, I.K.; Kang, S.-W.; Kumar, S. Synthesis and characterization of poly(pyridinium salt)s with organic counterions exhibiting both thermotropic liquid-crystalline and light-emitting properties. Macromolecules 2004, 37, 2688–2694. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Han, H.; Nedeltchev, A.K. Synthesis and characterization of poly(pyridinium salt)s with organic counterions exhibiting both thermotropic liquid-crystalline and light-emitting properties. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1028–1041. [Google Scholar] [CrossRef]
- Jo, T.S.; Han, H.; Bhowmik, P.K.; Heinrich, B.; Donnio, B. Thermotropic liquid-crystalline and light-emitting properties of poly(pyridinium) salts containing various diamine connectors and hydrophilic macrocounterions. Polymers 2019, 11, 851. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, P.K.; Kamatam, S.; Han, H.; Nedeltchev, A.K. Synthesis and characterization of poly(pyridinium salt)s with oxyalkylene units exhibiting amphotropic liquid-crystalline and photoluminescence properties. Polymer 2008, 49, 1748–1760. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Han, H.; Nedeltchev, A.K.; Mandal, H.D.; Jimenez-Hernandez, J.A.; McGannon, P.M. Poly(pyridinium salt)s with organic counterions derived from an aromatic diamine containing oxyethylene unit exhibiting amphotropic liquid-crystalline and photoluminescence properties. Polymer 2009, 50, 3128–3135. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Han, H.; Nedeltchev, A.K.; Mandal, H.D.; Jimenez-Hernandez, J.A.; McGannon, P.M. Poly(pyridinium salt)s with organic counterions derived from an aromatic diamine containing tetraoxyethylene units exhibiting amphotropic liquid-crystalline and photoluminescence properties. J. Appl. Polym. Sci. 2010, 116, 1197–1206. [Google Scholar] [CrossRef]
- Knaapila, M.; Stepanyan, R.; Horsburgh, L.E.; Monkman, A.P.; Serimaa, R.; Ikkala, O.; Subbotin, A.; Torkkeli, M.; ten Brinke, G. Structure and phase equilibria of polyelectrolytic hairy-rod supramolecules in the melt state. J. Phys. Chem. B 2003, 107, 14199–14203. [Google Scholar] [CrossRef][Green Version]
- Zhang, S.-J.; Pfefferle, L.D.; Osuji, C.O. Lyotropic hexagonal ordering in aqueous media by conjugated hairy-rod supramolecules. Macromolecules 2010, 43, 7549–7555. [Google Scholar] [CrossRef]
- Subbotin, A.; Stepanyan, R.; Knaapila, M.; Ikkala, O.; ten Brinke, G. Phase behaviour and structure formation of hairy-rod supramolecules. Eur. Phys. J. E 2003, 12, 333–345. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Burchett, R.A.; Han, H.; Cebe, J.J. Synthesis and characterization of poly(pyridinium salt)s with organic counterion exhibiting both lyotropic liquid-crystalline and light-emitting properties. Polymer 2002, 43, 1953–1958. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Han, H.; Nedeltchev, A.K. Synthesis and characterization of poly(pyridinium salt)s with anthracene moieties exhibiting both lyotropic liquid-crystalline and UV light-emitting properties. Polymer 2006, 47, 8281–8288. [Google Scholar] [CrossRef]
- Nedeltchev, A.K.; Han, H.; Bhowmik, P.K. Solution, thermal and optical properties of new poly(pyridinium salt)s derived from bisquinoline diamines. Polym. Chem. 2010, 1, 908–915. [Google Scholar] [CrossRef]
- Nedeltchev, A.K.; Han, H.; Bhowmik, P.K. Solution, thermal and optical properties of novel poly(pyridinium salt)s derived from conjugated pyridine diamines. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4408–4418. [Google Scholar] [CrossRef]
- Nedeltchev, A.K.; Han, H.; Bhowmik, P.K. Solution, thermal, and optical properties of poly(pyridinium salt)s derived from an ambipolar diamine consisting of diphenylquinoline and triphenyl amine moieties. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4611–4620. [Google Scholar] [CrossRef]
- Nedeltchev, A.K.; Han, H.; Bhowmik, P.K.; Ma, L. Solution, thermal and optical properties of new poly(pyridinium salt)s derived from conjugated quinoline diamines. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 1907–1918. [Google Scholar] [CrossRef]
- Jo, T.S.; Nedeltchev, A.K.; Biswas, B.; Han, H.; Bhowmik, P.K. Synthesis and characterization of poly(pyridinium salt)s derived from various aromatic diamines. Polymer 2012, 53, 1063–1071. [Google Scholar] [CrossRef]
- Jose, R.; Truong, D.; Nguyen, V.; Han, H.; Bhowmik, P.K. Poly(pyridinium salt)s with organic counterions derived from 3,3′-dimethylnaphthidine. J. Polym. Res. 2015, 22, 14. [Google Scholar] [CrossRef]
- Delozier, D.M.; Tigelaar, D.M.; Watson, K.A.; Smith, J.G., Jr.; Klein, D.J.; Lillehei, P.T.; Connell, J.W. Investigation of ionomers as dispersants for single wall carbon nanotubes. Polymer 2005, 46, 2506–2521. [Google Scholar] [CrossRef]
- Jo, T.S.; Han, H.; Bhowmik, P.K.; Ma, L. Dispersion of single-walled carbon nanotubes with poly(pyridinium salt)s. Polym. Chem. 2011, 2, 1953–1955. [Google Scholar] [CrossRef]
- Jo, T.S.; Han, H.; Bhowmik, P.K.; Ma, L. Dispersion of single-walled carbon nanotubes with poly(pyridinium salt)s containing various rigid aromatic moieties. Macromol. Chem. Phys. 2012, 213, 1378–1384. [Google Scholar] [CrossRef]
- Alam, M.M.; Biswas, B.; Nedeltchev, A.K.; Han, H.; Ranasinghe, A.D.; Bhowmik, P.K.; Goswami, K. Phosphine oxide containing poly(pyridinium salt)s as fire retardant materials. Polymers 2019, 11, 1141. [Google Scholar] [CrossRef]
- Frolov, D.G.; Makhaeva, E.E.; Keshtov, M.L. Electrochromic behavior of films and «smart windows» prototypes based on π-conjugated and non-conjugated poly(pyridinium salt)s. Synth. Met. 2019, 248, 14–19. [Google Scholar] [CrossRef]
- Frolov, D.G.; Petrov, M.M.; Makheva, E.E.; Keshtov, M.L.; Khokhlov, A.R. Electrochromic behavior of poly(pyridinium triflates) films: Electrolyte ions influence. Synth. Met. 2018, 239, 29–35. [Google Scholar] [CrossRef]
- Keshtov, M.L.; Udum, Y.A.; Toppare, L.; Kochurov, V.S.; Khokhlov, A.R. Synthesis of aromatic poly(pyridinium salt)s and their electrochromic properties. Mater. Chem. Phys. 2013, 139, 936–943. [Google Scholar] [CrossRef]
- Tigelaar, D.M.; Klein, D.J.; Xu, T.-B.; Su, J.; Bryant, R.G. Synthesis and characterization of poly(pyridinium triflate)s with alkyl and aromatic spacer groups for potential use as nonlinear optic materials. High Perform. Polym. 2005, 17, 515–531. [Google Scholar] [CrossRef]
- Lu, Y.; Xiao, C.; Yu, Z.; Zeng, X.; Ren, Y.; Li, C. Poly(pyridinium) salts containing calix[4]arene segments in the main chain as potential biosensors. J. Mater. Chem. 2009, 19, 8796–8802. [Google Scholar] [CrossRef]
- Han, F.; Lu, Y.; Zhang, Q.; Sun, J.; Zeng, X.; Li, C. Homogeneous and sensitive DNA detection based on polyelectrolyte complexes of cationic conjugated poly(pyridinium salts) and DNA. J. Mater. Chem. 2012, 22, 4106–4112. [Google Scholar] [CrossRef]
- Sun, J.; Lu, Y.; Wang, L.; Cheng, D.; Sun, Y.; Zeng, X. Fluorescence turn-on detection of DNA based on the aggregation-induced emission of conjugated poly(pyridinium salt)s. Polym. Chem. 2013, 4, 4045–4051. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Sun, J.; Lu, Y.; Sun, Y.; Cheng, D.; Li, C. Conjugated poly(pyridinium salt)s as fluorescence light-up probes for heparin sensing. J. Appl. Polym. Sci. 2014, 131, 40933. [Google Scholar] [CrossRef]
- Chang, Y.; Jin, L.; Duan, J.; Zhang, Q.; Wang, J.; Lu, Y. New conjugated poly(pyridinium salt) derivative: AIE characteristics, the interaction with DNA and selective fluorescence enhancement induced by dsDNA. RSC Adv. 2015, 5, 103358–103364. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Jin, L.; Chang, Y.; Duan, J.; Lu, Y. Anew conjugated poly(pyridinium salt) derived from phenanthridine diamine: Its synthesis, optical properties and interaction with calf thymus DNA. Polym. J. 2015, 47, 753–959. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Choi, K.; Zentel, R. Multilayer thin films by layer-by-layer assembly of hole- and electron-transport polyelectrolytes: Optical and electrochemical properties. Macromol. Chem. Phys. 2006, 207, 1870–1879. [Google Scholar] [CrossRef]
- Richardson, J.J.; Cui, J.; Björnmalm, M.; Braunger, J.A.; Ejima, H.; Caruso, F. Innovation in layer-by-layer assembly. Chem. Rev. 2016, 116, 14828–14867. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, M. Polyfluorenes: Twenty years of progress. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 2867–2873. [Google Scholar] [CrossRef]
- Neher, D. Polyfluorenes homopolymers: Conjugated liquid-crystalline polymers for bright blue emissions and polarized electroluminescence. Macromol. Rapid Commun. 2001, 22, 1365–1385. [Google Scholar] [CrossRef]
- Scherf, U.; List, E.J.W. Semiconducting polyfluorenes—Towards reliable structure–property relationships. Adv. Mater. 2002, 14, 477–487. [Google Scholar] [CrossRef]
- Akcelrud, L. Electroluminescent polymers. Prog. Polym. Sci. 2003, 28, 875–962. [Google Scholar] [CrossRef]
- Scherf, U.; Neher, D. (Eds.) Polyfluorenes. In Advanced in Polymers Science; Springer: Berlin, Germany, 2008; Volume 212, pp. 1–322. [Google Scholar]
- Sakamoto, J.; Rehahn, M.; Wegner, G.; Schlüter, A.D. Suzuki polycondensation: Polyarylenes à la Carte. Macromol. Rapid Commun. 2009, 30, 653–683. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.-H.; Yin, C.-R.; Lai, W.-Y.; Huang, W. Polyfluorene-based semiconductors combined with various periodic table elements for organic electronics. Prog. Polym. Sci. 2012, 37, 1192–1264. [Google Scholar] [CrossRef]
- Guo, X.; Baumgarten, M.; Müllen, K. Designing π-conjugated polymers for organic electronics. Prog. Polym. Sci. 2013, 38, 1832–1908. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, D.; Wen, D.; Wang, C.; Bai, X.; Wang, S. Novel conjugate side-chain fluorinated polymers based on fluorene for light-emitting and ternary flash memory devices. ChemistryOpen 2019, 8, 1267–1275. [Google Scholar] [CrossRef]
- Gopalan, A.-I.; Komathi, S.; Muthuchamy, N.; Lee, K.-P.; Whitcombe, M.J.; Dhana, L.; Sai-Anand, G. Functionalized conjugated polymers for sensing and molecular imprinting applications. Prog. Polym. Sci. 2019, 88, 1–129. [Google Scholar]
- Yamamoto, T. Electrically conducting and thermally stable π-conjugated poly(arylene)s prepared by organometallic processes. Prog. Polym. Sci. 1992, 17, 1153–1205. [Google Scholar] [CrossRef]
- Huang, F.; Wu, H.; Wang, D.; Yang, W.; Cao, Y. Novel electroluminescent conjugated polyelectrolytes based on polyfluorene. Chem. Mater. 2004, 16, 708–716. [Google Scholar] [CrossRef]
- Wang, H.; Lu, P.; Wang, B.; Qiu, S.; Liu, M.; Hanif, M.; Cheng, G.; Liu, S.; Ma, Y. A water-soluble π-conjugated polymer with up to 100 mg·mL−1 solubility. Macromol. Rapid Commun. 2007, 28, 1645–1650. [Google Scholar] [CrossRef]
- Huang, F.; Wu, H.; Peng, J.; Yang, W.; Cao, Y. Polyfluorene Polyelectrolytes and Their Precursors Processable from Environment-Friendly Solvents (Alcohol or Water) for PLED Applications. Curr. Org. Chem. 2007, 11, 1207–1219. [Google Scholar] [CrossRef]
- Abbel, R.; Schnning, A.P.H.J.; Meijer, E.W. Fluorene-based materials and their supramolecular properties. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 4215–4233. [Google Scholar] [CrossRef]
- Wågberg, T.; Liu, B.; Orädd, G.; Eliasson, B.; Edman, L. Cationic polyfluorene: Conformation and aggregation in a “good” solvent. Eur. Polym. J. 2009, 45, 3230–3235. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B. Cationic water-soluble polyfluorene homopolymers and copolymers: Synthesis, characterization and their applications in DNA sensing. Curr. Org. Chem. 2011, 15, 446–464. [Google Scholar] [CrossRef]
- Liu, X.; Fan, Q.; Huang, W. DNA biosensors based on water-soluble conjugated polymers. Biosens. Bioelectron. 2011, 26, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Kim, E.H.; Wei, A.; Negishi, E. Pd- and Ni-catalyzed cross-coupling reactions in the synthesis of organic electronic materials. Sci. Technol. Adv. Mater. 2014, 15, 44201. [Google Scholar] [CrossRef]
- Liu, B.; Bazan, G.C. Homogeneous fluorescence-based DNA detection with water-soluble conjugated polymers. Chem. Mater. 2004, 16, 4467–4476. [Google Scholar] [CrossRef]
- Bazan, G.C. Novel organic materials through control of multichromophore interactions. J. Org. Chem. 2007, 72, 8615–8635. [Google Scholar] [CrossRef]
- Liu, B.; Bazan, G.C. Tetradydrofuran activates fluorescence resonant energy transfer from a cationic conjugated polyelectrolyte to fluorescein-labeled DNA in aqueous media. Chem. Asian J. 2007, 2, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.Y.; Vak, D.; Korystov, D.; Mikhailovsky, A.; Bazan, G.C.; Kim, D.-Y. Cationic conjugated polyelectrolytes with molecular spacers for efficient fluorescence energy transfer to dye-labeled DNA. Adv. Funct. Mater. 2007, 17, 290–295. [Google Scholar] [CrossRef]
- Liu, B.; Bazan, G.C. (Eds.) Conjugated Polyelectrolytes: Fundamentals and Applications; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Zhou, L.; Lv, F.; Liu, L.; Wang, S. Water-soluble conjugated organic molecules as optical and electrochemical materials for interdisciplinary biological applications. Acc. Chem. Res. 2019, 52, 3211–3222. [Google Scholar] [CrossRef] [PubMed]
- Preston, J. Rigid chain polymers. Angew. Makromol. Chem. 1982, 109, 1–19. [Google Scholar] [CrossRef]
- Lin, J.; Sherrington, D.C.; Nield, E.; Richards, W.W. Novel wholly aromatic lyotropic liquid crystalline polyesters. Synthesis, characterization, and solution properties. Macromolecules 1992, 25, 7107–7113. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Han, H. Lyotropic liquid crystalline main-chain viologen polymers. J. Polym. Sci. Part A Polym. Chem. 1995, 33, 1745–1749. [Google Scholar] [CrossRef]
- Han, H.; Bhowmik, P.K. Liquid crystalline main-chain viologen polymers. Trends Polym. Sci. 1995, 3, 199–206. [Google Scholar]
- Bhowmik, P.K.; Molla, A.H.; Han, H.; Gangoda, M.E.; Bose, R.N. Lyotropic liquid crystalline main-chain viologen polymers: Homopolymer of 4,4′-bipyridyl with the ditosylate of trans-1,4-cyclohexanedimethanol and its copolymers with the ditosylate of 1,8-octanediol. Macromolecules 1998, 31, 621–630. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Han, H.; Nedeltchev, I.K. Main-chain viologen polymers with triflimide counterion exhibiting lyotropic liquid-crystalline properties in polar organic solvents. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 2015–2024. [Google Scholar] [CrossRef]
- Viale, S.; Jager, W.F.; Picken, S.J. Synthesis and characterization of a water-soluble rigid-rod polymer. Polymer 2003, 44, 7843–7850. [Google Scholar] [CrossRef]
- Viale, S.; Best, A.S.; Mendes, E.; Jager, W.F.; Picken, S.J. A supramolecular nematic phase in sulfonated polyaramides. Chem. Commun. 2004, 14, 1596–1597. [Google Scholar] [CrossRef]
- Viale, S.; Best, A.S.; Mendes, E.; Jager, W.F.; Picken, S.J. Formation of aqueous molecular nematic liquid crystal phase in poly(p-sulfophenylene sulfoterephthalamide). Chem. Commun. 2005, 12, 1528–1530. [Google Scholar] [CrossRef]
- Viale, S.; Li, N.; Schotman, A.H.M.; Best, A.S.; Picken, S.J. Synthesis and formation of a supramolecular nematic liquid crystal in poly(p-phenylene–sulfoterephthalamide)–H2O. Macromolecules 2005, 38, 3647–3652. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Protic ionic liquids: Properties and applications. Chem. Rev. 2008, 108, 206–237. [Google Scholar] [CrossRef]
- Makowski, M.P.; Mattice, W.L. Fluorecence and conformation of a rigid rod poly(pyridinium salt). Polym. Prepr. Am. Chem. Soc. Div. Polym. Chem. 1992, 33, 833–834. [Google Scholar]
- Makowski, M.P.; Mattice, W.L. Characterization of rigid rod poly(pyridinium salt)s by conformation analysis, molecular dynamics, and steady-state and time resolved fluorescence. Polymer 1993, 34, 1606–1612. [Google Scholar] [CrossRef]
- Spiliopoulos, J.I.; Mikroyannidis, J.A. Poly(pyridinium salt)s with stilbene or distyrylbenzene chromophores. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 2454–2462. [Google Scholar] [CrossRef]
- Chen, L.; McBranch, D.W.; Wang, H.-L.; Helgeson, R.; Wudl, F.; Whitten, D.G. Highly sensitive biological and chemical sensors based on reversible fluorescence quenching in a conjugated polymer. Proc. Natl. Acad. Sci. USA 1999, 96, 12287–12292. [Google Scholar] [CrossRef]
- Pinto, M.R.; Schanze, K.S. Conjugated polyelectrolytes: Synthesis and applications. Synthesis 2002, 9, 1293–1309. [Google Scholar]
- Gettinger, C.L.; Heeger, A.J.; Drake, J.M.; Pine, D.J. A photoluminescence study of poly(phenylene vinylene) derivatives: The effect of intrinsic persistence length. J. Chem. Phys. 1994, 101, 1673–1678. [Google Scholar] [CrossRef][Green Version]
- Li, X.-C.; Sirringhaus, H.; Garnier, F.; Holmes, A.B.; Moratti, S.C.; Feeder, N.; Clegg, W.; Teat, S.J.; Friend, R.H. A highly π-stacked organic semiconductor for thin film transistors based on fused thiophenes. J. Am. Chem. Soc. 1998, 120, 2206–2207. [Google Scholar] [CrossRef]
- Sarker, A.M.; Strehmel, B.; Neckers, D.C. Synthesis, characterization, and optical properties of copolymers containing fluorine-substituted distyrylbenzene and nonconjugated spacers. Macromolecules 1999, 32, 7409–7413. [Google Scholar] [CrossRef]
- Gierschner, J.; Ehni, M.; Egelhaaf, H.-J.; Medina, B.M.; Beljonne, D.; Benmansur, H.; Bazan, G.C. Solid-state optical prpperties of linear polyconjugated molecules: π-stack contra herringbone. J. Chem. Phys. 2005, 123, 144914. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, C.; Qin, J. An aggregation-induced blue shift of emission and the self-assembly of nanoparticles from a novel amphiphilic oligofluorene. Chem. Commun. 2008, 47, 6303–6305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, L.; Qin, J.; Yang, C. New water-soluble cationic poly(p-phenylenevinylene) derivative: The interaction with DNA and selective fluorescence enhancement induced by ssDNA. Macromol. Rapid Commun. 2013, 34, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.K.; Scherf, U. Cationic main-chain polyelectrolytes with pyridinium-based p-phenylenevinylene units and their aggregation-induced gelation. Macromol. Chem. Phys. 2016, 218, 1600374. [Google Scholar] [CrossRef]
- Martelo, L.M.; Fonseca, S.M.; Marques, A.T.; Burrows, H.D.; Valente, A.J.M.; Justino, L.L.G.; Scherf, U.; Pradhan, S.; Song, Q. Effects of charge density on photophysics and aggregation behavior of anionic fluorene-arylene conjugated polyelectrolytes. Polymers 2018, 10, 258. [Google Scholar] [CrossRef] [PubMed]
- Burrows, H.D.; Fonseca, S.M.; Silva, C.L.; Pais, A.A.C.C.; Tapia, M.J.; Pradhan, S.; Scherf, U. Aggregation of the hairy rod conjugated polyelectrolytes poly{1,4-phenylene-[9,9-bis(4-phenoxybutylsulfonate)]fluorene-2,7-diyl} in aqueous solution: An experimental and molecular modelling study. Phys. Chem. Chem. Phys. 2008, 10, 4420–4428. [Google Scholar] [CrossRef]
- Wacha, A.; Varga, Z.; Bota, A. Credo: A new general-purpose laboratory instrument for small-angle x-ray scattering. J. Appl. Cryst. 2014, 47, 1749–1754. [Google Scholar] [CrossRef]
- Wacha, A. Optimized pinhole geometry for small-angle scattering. J. Appl. Cryst. 2015, 48, 1843–1848. [Google Scholar] [CrossRef]
- Knaapila, M.; Bright, D.W.; Stepanyan, R.; Torkkeli, M.; Almásy, L.; Schweins, R.; Vainio, U.; Preis, E.; Galbrecht, F.; Scherf, U.; et al. Network structure of polyfluorene membranes as a function of side chain length. Phys. Rev. E 2011, 83, 051803. [Google Scholar] [CrossRef]
- Rahman, M.H.; Chen, C.-Y.; Liao, S.-C.; Chen, H.-L.; Tsao, C.-S.; Chen, J.-H.; Liao, J.-L.; Ivanov, V.A.; Chen, S.-A. Segmental alignment in the aggregate domains of poly(9,9-dioctylfluorene) in semidilute solution. Macromolecules 2007, 40, 6572–6578. [Google Scholar] [CrossRef]




| Polymer | Mn a | Mw b | PDI c | dn/dc (mL/g) | Rh (nm) d | Rg (nm) e |
|---|---|---|---|---|---|---|
| 1 | 96.5 | 181.8 | 1.88 | 0.1417 | 13.3 | 50.0 |
| 2 | 103.6 | 155.8 | 1.50 | 0.0878 | 12.7 | 24.4 |
| 3 | 107.8 | 130.0 | 1.21 | 0.0885 | 12.4 | 24.2 |
| 4 | 96.6 | 108.1 | 1.12 | 0.1089 | 11.8 | 33.0 |
| 5 | 103.9 | 124.1 | 1.19 | 0.0945 | 12.2 | 28.8 |
| Polymer | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| DMSO | 1–10% I a 20% B a 30% L a | 20% B 30% L | 1–30% I 40% L | 1–40% I 50% L | 1–30% I 35% L |
| CH3CN | 10% B 20% L | 20% L | 1–20% I 30% L | − | − |
| CH3OH | 10% B 20% L | − | 20% B 30% L | − | 30% B 40% L |
| Polymer:Solvent Ratio | 50:50 | 60:40 | 80:20 | |
|---|---|---|---|---|
| Analyzed q-range (nm−1) | ||||
| P1:DMSO | ||||
| α | 0.21–0.48 | 3.36 ± 0.06 | 2.67 ± 0.05 | - |
| 0.48–1.49 | 1.03 ± 0.04 | 0.98 ± 0.03 | - | |
| d (nm) | 2.0 ± 0.1 | 2.0 ± 0.1 | - | |
| P1:CH3CN | ||||
| model | aggregate | sheet | sheet | |
| α | 0.21–0.71 | 3.59 ± 0.03 | - | - |
| 1.27–2.50 | - | - | 1.60 ± 0.03 | |
| ξsheet (nm) | 0.21–(1.37)–2.42 | - | 10.15 ± 0.25 | 5.90 ± 0.08 |
| L (nm) | - | 3.30 ± 0.07 | 4.71 ± 0.01 | |
| d (nm) | 2.53–4.00 | 1.95 ± 0.1 | 2.0 ± 0.1 | 2.0 ± 0.1 |
| Polymer | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| UV λabs (nm) a | 347 | 347 | 347 | 351 (429) | 347 |
| Band gap (eV) | 3.04 | 3.04 | 3.04 | 2.99 | 3.04 |
| UV λabs (nm) | 343 b | 342 c | − | − | 343 b |
| Molar Absorptivity (M−1cm−1) | ε343 = 83,630 | ε342 = 79,331 | − | − | ε343 = 82,086 |
| PL λem DMSO (nm) | 534 | 534 | 533 | 538 | 533 |
| PL λem CH3CN (nm) | 535 | 533 | 534 | − | 531 |
| PL λem CH3OH(nm) | 528 | 533 | 527 | 527 | 528 |
| AQY(ΦF) d | 0.040 | 0.051 | − | − | 0.038 |
| PL λem THF (nm) | − | 517 | 493 | − | 497 |
| PL λem CHCl3 (nm) | − | 524 | 524 | − | 518 |
| PL λem film (nm) | 503 e | 495 f | 480 e | − | 493 e |
| PL λem (powdered) g | 542 | 529 | 514 | − | 517 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhowmik, P.K.; Jo, T.S.; Koh, J.J.; Park, J.; Biswas, B.; Principe, R.C.G.; Han, H.; Wacha, A.F.; Knaapila, M. Poly(Pyridinium Salt)s Containing 2,7-Diamino-9,9′-Dioctylfluorene Moieties with Various Organic Counterions Exhibiting Both Lyotropic Liquid-Crystalline and Light-Emitting Properties. Molecules 2021, 26, 1560. https://doi.org/10.3390/molecules26061560
Bhowmik PK, Jo TS, Koh JJ, Park J, Biswas B, Principe RCG, Han H, Wacha AF, Knaapila M. Poly(Pyridinium Salt)s Containing 2,7-Diamino-9,9′-Dioctylfluorene Moieties with Various Organic Counterions Exhibiting Both Lyotropic Liquid-Crystalline and Light-Emitting Properties. Molecules. 2021; 26(6):1560. https://doi.org/10.3390/molecules26061560
Chicago/Turabian StyleBhowmik, Pradip K., Tae S. Jo, Jung J. Koh, Jongwon Park, Bidyut Biswas, Ronald Carlo G. Principe, Haesook Han, András F. Wacha, and Matti Knaapila. 2021. "Poly(Pyridinium Salt)s Containing 2,7-Diamino-9,9′-Dioctylfluorene Moieties with Various Organic Counterions Exhibiting Both Lyotropic Liquid-Crystalline and Light-Emitting Properties" Molecules 26, no. 6: 1560. https://doi.org/10.3390/molecules26061560
APA StyleBhowmik, P. K., Jo, T. S., Koh, J. J., Park, J., Biswas, B., Principe, R. C. G., Han, H., Wacha, A. F., & Knaapila, M. (2021). Poly(Pyridinium Salt)s Containing 2,7-Diamino-9,9′-Dioctylfluorene Moieties with Various Organic Counterions Exhibiting Both Lyotropic Liquid-Crystalline and Light-Emitting Properties. Molecules, 26(6), 1560. https://doi.org/10.3390/molecules26061560






