Comparative Study of Volatile Compounds and Expression of Related Genes in Fruit from Two Apple Cultivars during Different Developmental Stages
Abstract
1. Introduction
2. Results
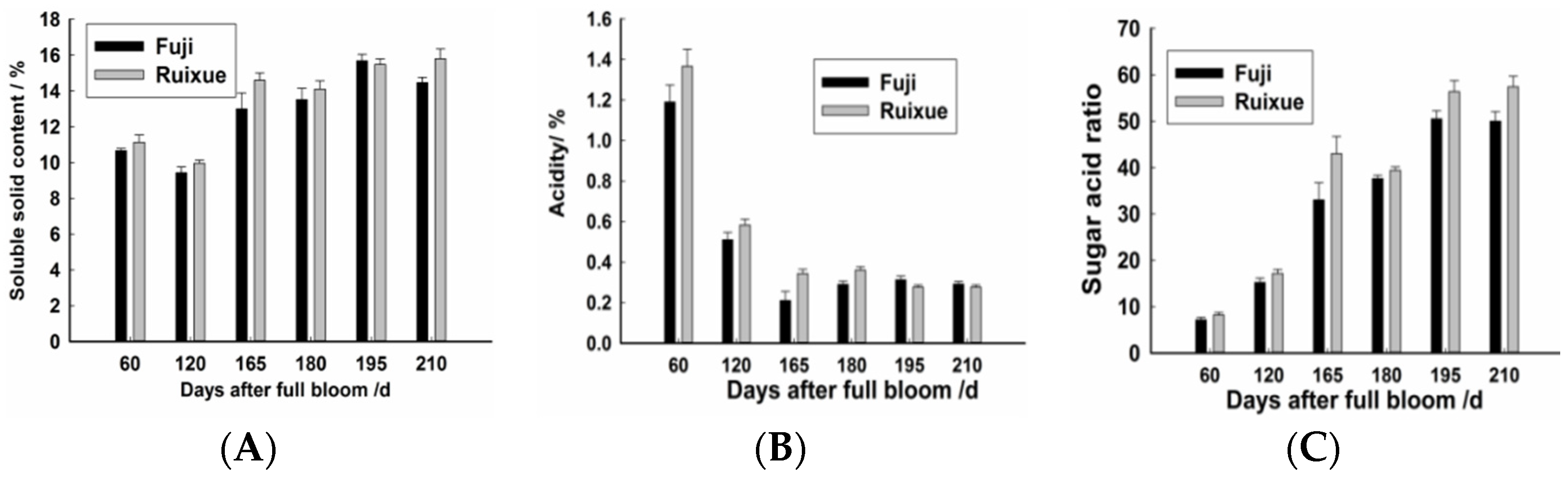
2.1. Soluble Solids and Acidity of Apple Fruit during Different Stages
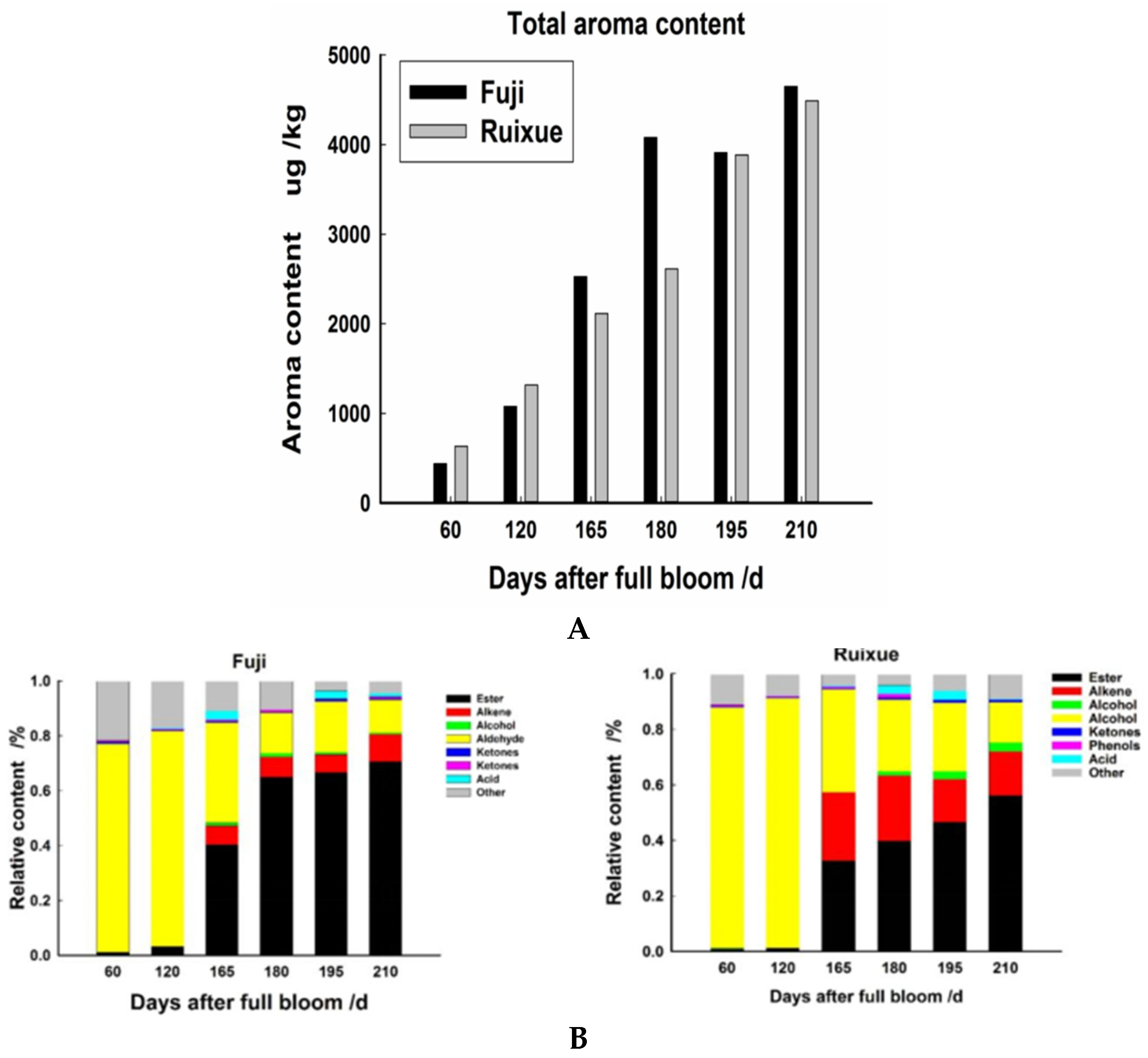
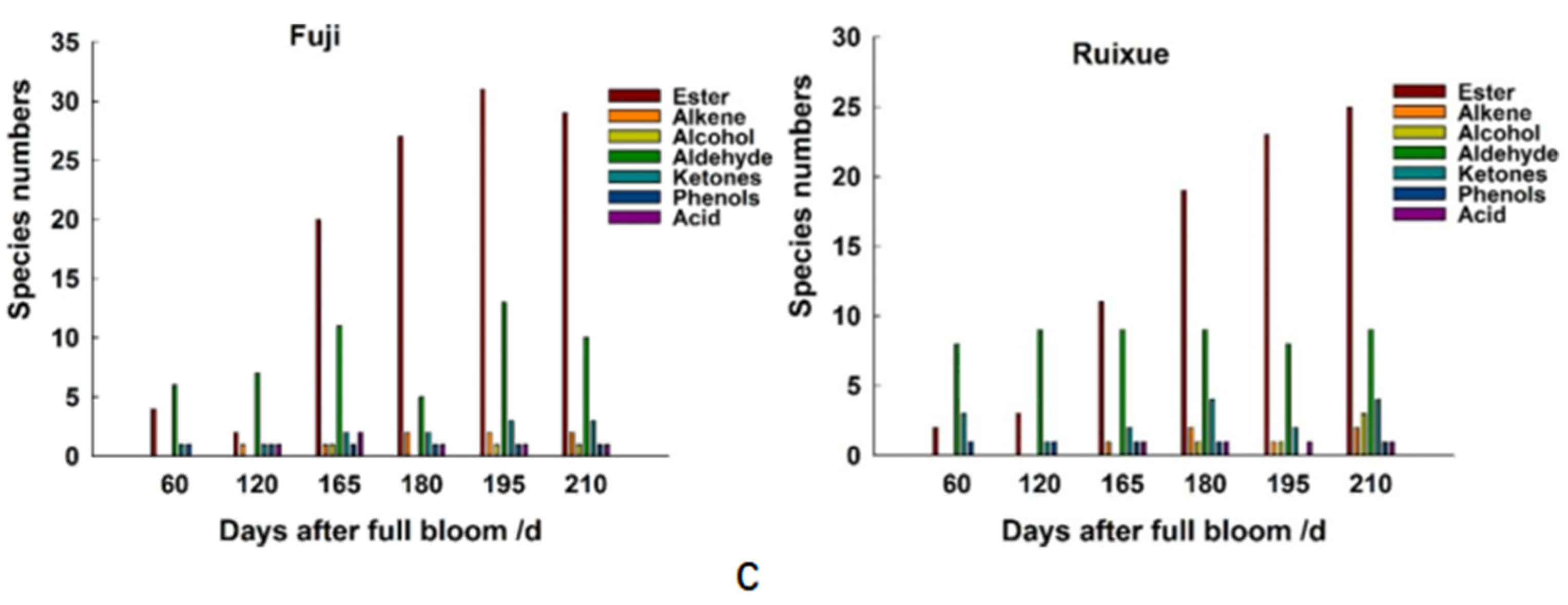
2.2. Volatile Compounds in Apple Fruit during Different Stages
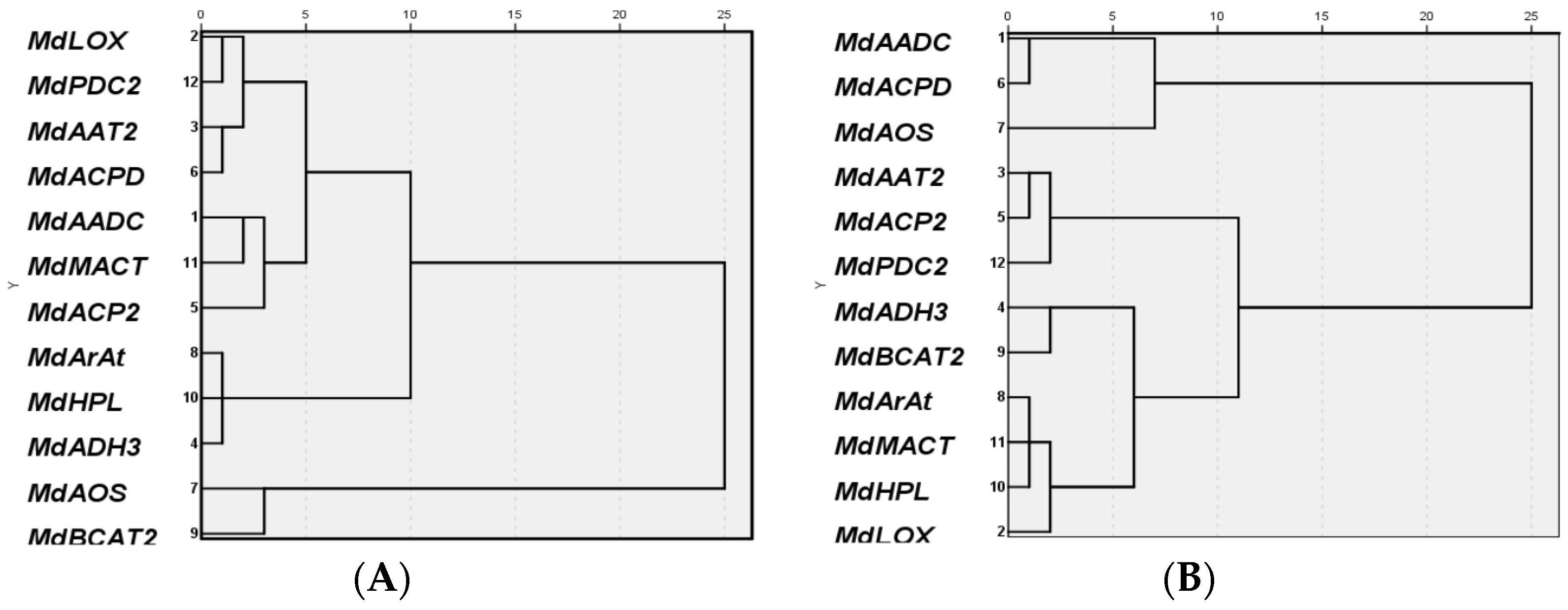
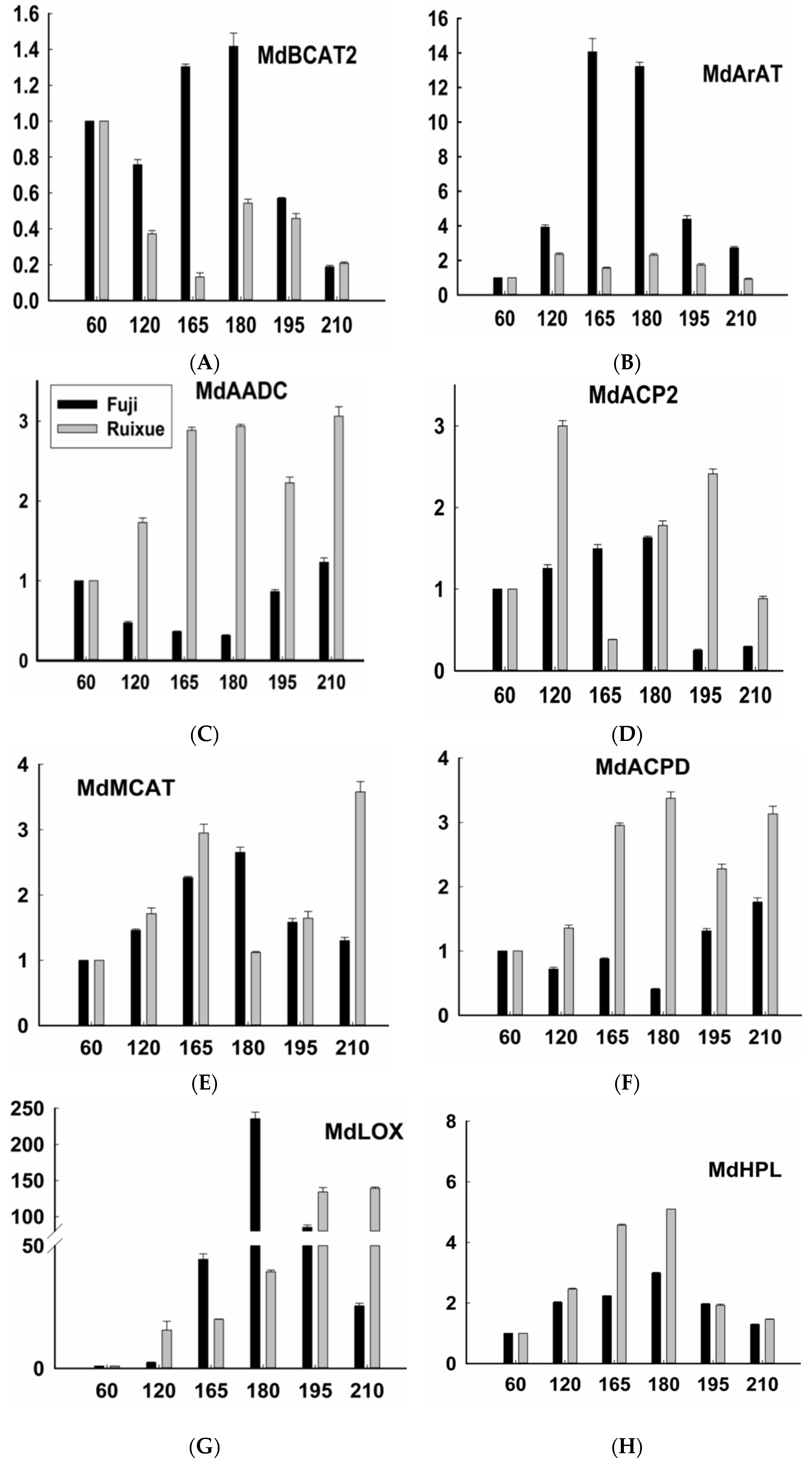
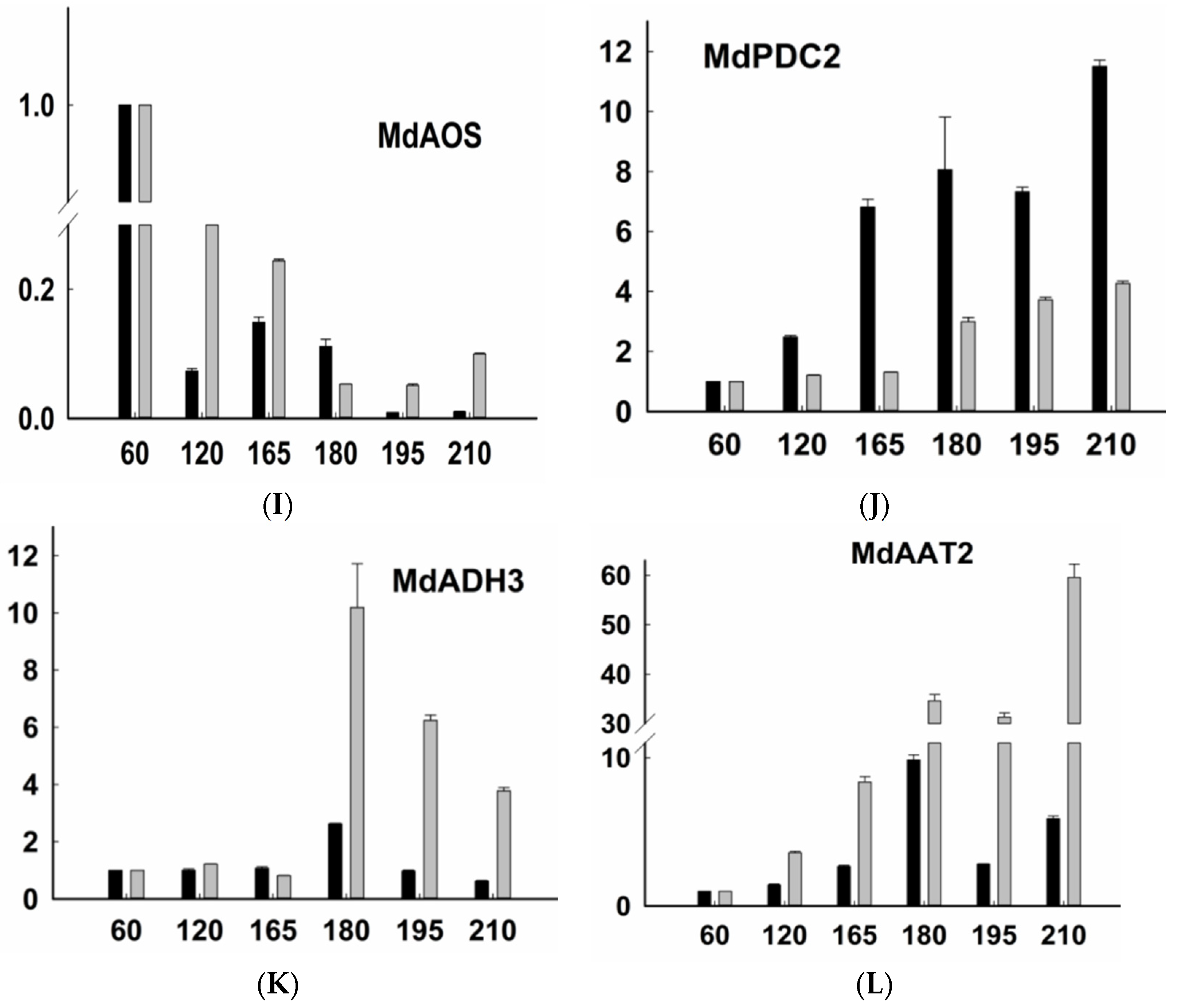
2.3. Expression of Genes Related to Aroma Synthesis during Different Stages
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Determination of Soluble Solids and Acidity
4.3. Extraction and Determination of Volatile Compounds
4.4. Total RNA Extraction, cDNA Synthesis, and Real-time Quantitative PCR
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Park, P.; Sugimoto, N.; Larson, M.D.; Beaudry, R.; van Nocker, S. Identification of genes with potential roles in apple fruit development and biochemistry through large-scale statistical analysis of expressed sequence tags. Plant Physiol. 2006, 141, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Yu, G.; Seo, Y.S.; Han, S.E.; Choi, Y.; Kim, D.; Mok, I.; Kim, W.T.; Sung, S. Microarray analysis of apple gene expression engaged in early fruit development. Plant Cell Rep. 2007, 26, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Shalit, M.; Guterman, I.; Volpin, H.; Bar, E.; Tamari, T.; Menda, N.; Adam, Z.; Zamir, D.; Vainstein, A.; Weiss, D.; et al. Volatile ester formation in roses. identification of an acetyl-Coenzyme A. Geraniol/Citronellol acetyltransferase in developing rose petals. Plant Physiol. 2003, 131, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Paillard, N.M.M. Flavour of apples, pears and quinces. Dev. Food Sci. 1990, 3, 1–41. [Google Scholar]
- Aprea, E.; Charles, M.; Endrizzi, I.; Laura Corollaro, M.; Betta, E.; Biasioli, F.; Gasperi, F. Sweet taste in apple: The role of sorbitol, individual sugars, organic acids and volatile compounds. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Rowan, D.D.; Allen, J.M.; Fielder, S.; Hunt, M.B. Biosynthesis of straight-chain ester volatiles in Red Delicious and Granny Smith apples using deuterium-labeled precursors. J. Agric. Food Chem. 1999, 47, 2553–2562. [Google Scholar] [CrossRef] [PubMed]
- Rowan, D.D.; Lane, H.P.; Allen, J.M.; Fielder, S.; Hunt, M.B. Biosynthesis of 2-Methylbutyl, 2-Methyl-2-butenyl, and 2-Methylbutanoate esters in Red Delicious and Granny Smith apples using deuterium-labeled substrates. J. Agric. Food Chem. 1996, 44, 3276–3285. [Google Scholar] [CrossRef]
- Rowan, D.D.; Hunt, M.B.; Dimouro, A.; Alspach, P.A.; Weskett, R.; Volz, R.K.; Gardiner, S.E.; Chagneé, D. Profiling fruit volatiles in the progeny of a ‘Royal Gala’ × ‘Granny Smith’ apple (Malus × domestica) Cross. J. Agric. Food Chem. 2009, 57, 7953–7961. [Google Scholar] [CrossRef] [PubMed]
- El Hadi, M.; Zhang, F.; Wu, F.; Zhou, C.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, E.; Grosch, W. Character impact odorants of the apple cultivars Elstar and Cox Orange. Food/Nahrung 2002, 46, 187–193. [Google Scholar] [CrossRef]
- Dixon, J.; Hewett, E.W. Factors affecting apple arom/flavour volatile concentration: A review. N. Z. J. Crop Hortic. Sci. 2000, 28, 155–173. [Google Scholar] [CrossRef]
- Young, J.C.; Chu, C.L.G.; Lu, X.; Zhu, H. Ester variability in apple varieties as determined by Solid-Phase Microextraction and Gas Chromatography−Mass Spectrometry. J. Agric. Food Chem. 2004, 52, 8086–8093. [Google Scholar] [CrossRef]
- Song, J.; Bangerth, F. Fatty acids as precursors for aroma volatile biosynthesis in pre-climacteric and climacteric apple fruit. Postharvest Biol. Technol. 2003, 30, 113–121. [Google Scholar] [CrossRef]
- Beekwilder, J.; Alvarez-Huerta, M.; Neef, E.; Verstappen, F.W.A.; Bouwmeester, H.J.; Aharoni, A. Functional characterization of enzymes forming volatile esters from strawberry and banana. Plant Physiol. 2004, 135, 1865–1878. [Google Scholar] [CrossRef] [PubMed]
- Souleyre, E.J.; Greenwood, D.R.; Friel, E.N.; Karunairetnam, S.; Newcomb, R.D. An alcohol acyl transferase from apple (cv. Royal Gala), MpAAT1, produces esters involved in apple fruit flavor. FEBS J. 2005, 272, 3132–3144. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, G.; Graell, J.; López, M.L.; Lara, I. Volatile production, quality and aroma-related enzyme activities during maturation of ‘Fuji’ apples. Postharvest Biol. Technol. 2004, 31, 217–227. [Google Scholar] [CrossRef]
- Tieman, D.; Taylor, M.; Schauer, N.; Fernie, A.R.; Hanson, A.D.; Klee, H.J. Tomato aromatic amino acid decarboxylases participate in synthesis of the flavor volatiles 2-Phenylethanol and 2-Phenylacetaldehyde. Proc. Natl. Acad. Sci. USA 2006, 103, 8287–8292. [Google Scholar] [CrossRef] [PubMed]
- Gonda, I.; Bar, E.; Portnoy, V.; Lev, S.; Burger, J.; Schaffer, A.A.; Tadmor, Y.; Gepstein, S.; Giovannoni, J.J.; Katzir, N.; et al. Branched-chain and aromatic amino acid catabolism into aroma volatiles in Cucumis melo L. fruit. J. Exp. Bot. 2010, 61, 1111–1123. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Dandekar, A.M.; Kader, A.A. Relationship of ethylene biosynthesis to volatile production, related enzymes, and precursor availability in apple peel and flesh tissues. J. Agric. Food Chem. 2005, 53, 3133–3141. [Google Scholar] [CrossRef]
- Larsen, B.; Migicovsky, Z.; Jeppesen, A.A.; Gardner, K.M.; Toldam-Andersen, T.B.; Myles, S.; Orgaard, M.; Petersen, M.A.; Pedersen, C. Genome-wide association studies in apple reveal loci for aroma volatiles, sugar composition, and harvest date. Plant Genome 2019, 12, 80–104. [Google Scholar] [CrossRef] [PubMed]
- Holland, D.; Larkov, O.; Bar-Ya’akov, I.; Bar, E.; Zax, A.; Brandeis, E. Developmental and varietal differences in volatile ester formation and acetyl-CoA: Alcohol acetyl transferase activities in apple (Malus domestica Borkh.) fruit. J. Agric. Food Chem. 2005, 53, 7198–7203. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Shi, J.R.; Ren, X.L.; Tao, Y.S.; Ma, F.W.; Li, R.; Liu, X.R.; Liu, C.H. Insights into the aroma profiles and characteristic aroma of ‘Honeycrisp’ apple (Malus × domestica). Food Chem. 2020, 327, 127074. [Google Scholar] [CrossRef] [PubMed]
- Espino-Díaz, M.; Sepúlveda, D.R.; González-Aguilar, G.; Olivas, G.I. Biochemistry of apple aroma: A Review. Food Technol. Biotechnol. 2016, 54, 375–394. [Google Scholar] [CrossRef]
- EcheverrÕa, G.; Fuentes, T.; Graell, J.; Lara, I.; López, M.L. Aroma volatile compounds of ‘Fuji’ apples in relation to harvest date and cold storage technology. Postharvest Biol. Technol. 2004, 32, 29–40. [Google Scholar] [CrossRef]
- Flath, R.A.; Black, D.R.; Guadagni, D.G.; Mcfadden, W.H.; Schultz, T.H. Identification and organoleptic evaluation of compounds in Delicious Apple Essence. J. Agric. Food Chem. 1967, 15, 29–35. [Google Scholar] [CrossRef]
- Young, H.; Gilbert, J.M.; Murray, S.H.; Ball, R.D. Causal Effects of Aroma Compounds on Royal Gala Apple Flavours. J. Sci. Food Agric. 1996, 71, 329–336. [Google Scholar] [CrossRef]
- Fellman, J.; Mattison, D.; Fan, X.; Mattheis, J. ‘Fuji’ Apple Storage Characteristics in Relation to Growing Conditions and Harvest Maturity in Washington State. In Proceedings of the 7th International Controlled Atmosphere Research Conference, Davis, CA, USA, 13–18 July 1997; University of California: Davis, CA, USA, 1997; Volume 2, pp. 232–234. [Google Scholar]
- Rizzolo, A.; Visai, C. Studies on the quality of ‘Golden Delicious’ apples coming from different localities of Trentino. In Proceedings of the 23rd International Horticultural Congress, Florence, Italy, 30 August 1990. [Google Scholar]
- Yahia, E.M.; Liu, F.W.; Acree, T.E. Changes of some odor-active volatiles in controlled atmosphere-stored apples. J. Food Qual. 1990, 13, 185–202. [Google Scholar] [CrossRef]
- Wang, Y.J.; Yang, C.X.; Liu, C.Y.; Xu, M.; Li, S.H.; Yang, L.; Wang, Y.N. Effects of Bagging on Volatiles and Polyphenols in “Wanmi” Peaches during Endocarp Hardening and Final Fruit Rapid Growth Stages. J. Food Sci. 2010, 75, S455–S460. [Google Scholar] [CrossRef] [PubMed]
- Klieber, A.; Bagnato, N.; Barrett, R.; Sedgleyl, M. Effect of Post-ripeninig Nitrogen Atmosphere Storage on Banana Shelf Life. Visual Appearance Aroma. Postharvest Biol. Technol. 2002, 25, 15–24. [Google Scholar] [CrossRef]
- Argenta, L.C.; Mattheis, J.P.; Fan, X.; Fan, X.; Finger, F.L. Production of volatile compounds by Fuji apples following exposure to high CO2 or low O2. J. Agric. Food Chem. 2004, 52, 5957–5963. [Google Scholar] [CrossRef]
- Streif, J.; Bangerth, F. Production of volatile aroma substances by ‘Golden Delicious’ apple fruits after storage for various times in different CO2 and O2 concentrations. J. Hortic. Sci. 1988, 63, 193–199. [Google Scholar] [CrossRef]
- Brackmann, A.; Streif, J.; Bangerth, F. Relationship between a reduced aroma production and lipid metabolism of apples aft er long-term controlled-atmosphere storage. J. Am. Soc. Hortic. Sci. 1993, 118, 243–247. [Google Scholar] [CrossRef]
- Rizzolo, A.; Grassi, M.; Eccher, Z.P. Influence of harvest date on rip- ening and volatile compounds in the scab-resistant apple cultivar ‘Golden Orange’. Hortic. Sci. Biotechnol. 2016, 8, 681–690. [Google Scholar]
- Lara, I.; Graell, J.; Lopez, M.L.; Echeverrıia, G. Multivariate analysis of modifications in biosynthesis of volatile compounds after CA storage of ‘Fuji’ apples. Postharvest Biol. Technol. 2006, 39, 19–28. [Google Scholar] [CrossRef]
- Kumar, S.; Rowan, D.; Hunt, M.; Chagné, D.; Whitworth, C.; Souleyre, E. Genome-wide scans reveal genetic architecture of apple flavour volatiles. Mol. Breed. 2015, 35, 1–16. [Google Scholar] [CrossRef]
- Schiller, D.; Contreras, C.; Vogt, J.; Dunemann, F.; Defilippi, B.G.; Beaudry, R.; Schwab, W. A dual positional specific lipoxygenase functions in the generation of flavor compounds during climacteric ripening of apple. Hortic. Res. 2015, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, S.; Hackler, L.; Zvara, Á.; Ferreira, S.; Baldé, A.; Dudits, D.; Pais, M.S.; Puskás, L.G. Monitoring gene expression along pear fruit development, ripening and senescence using cDNA microarrays. Plant Sci. 2004, 167, 457–469. [Google Scholar] [CrossRef]
- El-Sharkawy, I.; Manríquez, D.; Flores, F.B.; Regad, F.; Bouzayen, M.; Latché, A.; Pech, J. Functional characterization of a melon alcohol acyl-transferase gene family involved in the biosynthesis of ester volatiles. Identification of the crucial role of a threonine residue for enzyme activity. Plant Mol. Biol. 2005, 59, 345–362. [Google Scholar] [CrossRef]
- Yang, X.; Song, J.; Du, L.; Forney, C.; Campbell-Palmer, L.; Fillmore, S.; Wismer, P.; Zhang, Z. Ethylene and 1-MCP regulate major volatile biosynthetic pathways in apple fruit. Food Chem. 2016, 194, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Dunemann, F.; Ulrich, D.; Malysheva-Otto, L.; Weber, L.; Longhi, S.; Velasco, R.; Costa, F. Functional allelic diversity of the apple alcohol acyl-transferase gene MdAAT1 associated with fruit ester volatile contents in apple cultivars. Mol. Breed. 2012, 29, 609–625. [Google Scholar] [CrossRef]
- Li, D.; Xu, Y.; Xu, G.; Gu, L.; Li, D.; Shu, H. Molecular cloning and expression of a gene encoding alcohol acyltransferase (MdAAT2) from apple (cv. Golden Delicious). Phytochemistry 2006, 67, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.M.; Rudell, D.R.; Matheis, J.P. Characterization of cultivar differences in alcohol acyltransferase and 1-aminocyclopropane-1-carboxylate synthase gene expression and volatile ester emission during apple fruit maturation and ripening. Postharvest Biol. Technol. 2008, 49, 330–339. [Google Scholar] [CrossRef]
- Gambino, G.; Perrone, I.; Gribaudo, I. A rapid and effective method for RNA extraction from different tissues of grapevine and other woody plants. Phytochem. Anal. 2008, 19, 520–525. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using Real-time quantitative PCR and the 2−∆∆Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
| No. | Compound | Relative Content/% | |||||
|---|---|---|---|---|---|---|---|
| 60 DAFB | 120 DAFB | 165 DAFB | 180 DAFB | 195 DAFB | 210 DAFB | ||
| Aldehydes | |||||||
| 1 | Hexanal | 13.3 ± 0.64 | 11.67 ± 0.54 | 3.63 ± 0.31 | 2.51 ± 0.17 | 2.01 ± 0.21 | 2.34 ± 0.05 |
| 2 | 3-Hexenal | 6.08 ± 0.34 | 10.03 ± 0.56 | 2.19 ± 0.25 | 2.57 ± 0.22 | 0.35 ± 0.08 | 0.92 ± 0.04 |
| 3 | 2-Hexenal | 64.89 ± 4.37 | 63.31 ± 5.14 | 25.25 ± 2.35 | 17.47 ± 1.32 | 14.85 ± 0.98 | 14 ± 1.24 |
| 4 | (E)-2-Octenal | 0.08 ± 0.01 | - | 0.35 ± 0.07 | 0.3 ± 0.02 | 0.38 ± 0.04 | 0.31 ± 0.12 |
| 5 | Dodecanal | - | - | 0.2 ± 0.06 | - | 0.26 ± 0.02 | - |
| 6 | (E)-2-Hexenal | - | - | 2.18 ± 0.12 | - | - | 0.97 ± 0.17 |
| 7 | 5-Hydroxymethylfurfural | - | - | - | - | 3.97 ± 0.17 | - |
| 8 | Octanal | - | 0.15 ± 0.04 | - | 0.37 ± 0.07 | 0.34 ± 0.06 | 0.39 ± 0.04 |
| 9 | Nonanal | 0.4 ± 0.08 | 0.57 ± 0.07 | 0.54 ± 0.12 | 0.71 ± 0.06 | - | 0.65 ± 0.07 |
| 10 | (Z)-2-Heptenal | 0.13 ± 0.01 | 0.2 ± 0.08 | 0.42 ± 0.14 | 0.55 ± 0.10 | - | 0.6 ± 0.04 |
| 11 | Decanal | 0.32 ± 0.13 | 0.46 ± 0.11 | - | 0.11 ± 0.02 | - | 0.22 ± 0.02 |
| 12 | (E)-2-Decenal | - | - | - | 0.05 ± 0.01 | - | - |
| 13 | (E,E)-2,4-Hexadienal | 1.32 ± 0.18 | 2.64 ± 0.13 | 0.32 ± 0.04 | - | - | - |
| 14 | Furfural | - | - | - | - | 1.21 ± 0.07 | - |
| 15 | 2-Methyl-4-pentenal | - | 0.27 ± 0.02 | - | - | - | - |
| Esters | |||||||
| 16 | Alkyl isobutyrate | - | - | - | 2.57 ± 0.39 | - | - |
| 17 | Butyl 2-methylbutanoate | - | - | - | - | 1.82 ± 0.23 | - |
| 18 | Isoamyl butyrate | - | - | - | 1.26 ± 0.35 | 1.23 ± 0.29 | 1.64 ± 0.49 |
| 19 | Hexyl acetate | 0.09 ± 0.02 | 0.64 ± 0.17 | 2.14 ± 0.21 | 0.98 ± 0.14 | 0.34 ± 0.01 | 0.23 ± 0.06 |
| 20 | Hexyl propionate | - | - | 0.95 ± 0.19 | 2.58 ± 0.29 | 2.89 ± 0.32 | 3.05 ± 0.28 |
| 21 | Hexyl isovalerate | - | - | 20.89 ± 2.31 | 15.66 ± 2.38 | 11.15 ± 0.55 | 17.57 ± 1.64 |
| 22 | Octaethylene glycol Monododecyl ether | - | 0.42 ± 0.03 | 0.62 ± 0.15 | 0.10 ± 0.01 | 0.22 ± 0.04 | 0.31 ± 0.02 |
| 23 | Butanoic acid, 2-methyl-, pentyl ester | - | - | 0.9 ± 0.19 | 1.32 ± 0.19 | 1.46 ± 0.12 | 1.7 ± 0.16 |
| 24 | Butanoic acid, butyl ester | - | - | 0.39 ± 0.07 | 0.96 ± 0.05 | 2.26 ± 0.25 | 10.91 ± 1.24 |
| 25 | 2-Methylbutyl 2-Methylbutanoate | - | - | 0.61 ± 0.25 | 2.03 ± 0.35 | 1.74 ± 0.24 | 3.05 ± 0.29 |
| 26 | Hexanoic acid, hexyl ester | - | - | 1.39 ± 0.14 | - | 4.2 ± 0.43 | 7.53 ± 0.56 |
| 27 | Octanoic acid, hexyl ester | - | 0.06 ± 0.01 | - | - | - | 0.19 ± 0.02 |
| 28 | Butanoic acid, pentyl ester | - | - | 0.23 ± 0.06 | 0.61 ± 0.19 | 0.68 ± 0.12 | 1.51 ± 0.35 |
| 29 | 2-Methylbutanoic acid-2-methylbutyl ester | - | - | 0.17 ± 0.04 | - | - | - |
| 30 | Propanoic acid, butyl ester | - | - | - | 0.37 ± 0.14 | 0.36 ± 0.03 | 0.41 ± 0.03 |
| 31 | Octylhexanoate | - | - | - | - | - | - |
| 32 | 2-Hexen-1-ol, acetate | 0.43 ± 0.14 | - | - | - | - | - |
| 33 | Hexyl butyrate | - | - | - | 5.06 ± 0.38 | 16.65 ± 1.23 | 10.91 ± 1.05 |
| 34 | Hexanoic acid, 2-methylbutyl ester | - | - | - | 1.84 ± 0.32 | 2.05 ± 0.22 | 3.05 ± 0.21 |
| 35 | Hexanoic acid, pentyl ester | - | - | - | 0.89 ± 0.05 | - | - |
| 36 | Propyl octanoate | - | - | - | - | 0.04 ± 0.01 | 0.05 ± 0.01 |
| 37 | Heptyl 2-methylbutyrate | - | - | - | 0.23 ± 0.01 | 0.28 ± 0.04 | 0.24 ± 0.03 |
| 38 | 2-Methylbutyl octanoate | - | - | - | - | - | 0.37 ± 0.04 |
| 39 | Heptanoic acid, butyl ester | - | - | - | - | - | 0.22 ± 0.02 |
| 40 | Butanoic acid, 2-methyl-, propyl ester | - | - | - | 0.25 ± 0.05 | 0.21 ± 0.19 | 0.18 ± 0.03 |
| 41 | Propyl hexanoate | - | - | - | - | 0.34 ± 0.27 | - |
| 42 | Ethyl butyrate | - | - | - | - | - | 0.17 ± 0.02 |
| 43 | Propanoic acid, pentyl ester | - | - | - | 0.66 ± 0.14 | 0.43 ± 0.06 | 0.63 ± 0.04 |
| 44 | Hexanoic acid, 2-methylpropyl ester | - | - | - | - | 0.08 ± 0.01 | 0.1 ± 0.01 |
| 45 | Pentanoic acid, 2-methylbutyl ester | - | - | - | 0.13 ± 0.02 | 0.22 ± 0.19 | 0.16 ± 0.01 |
| 46 | Propanoic acid, propyl ester | - | - | - | - | 0.12 ± 0.02 | - |
| 47 | Isopentyl hexanoate | - | - | 0.29 ± 0.04 | - | - | - |
| 48 | (Z,Z,Z)-9,12,15-Octadecatrienoic acid, 2,3-dihydroxypropyl ester | - | - | - | 0.09 ± 0.01 | - | - |
| 49 | Butanoic acid, propyl ester | - | - | - | - | - | 0.32 ± 0.05 |
| Acids | |||||||
| 50 | 2-Methyl-mutanoic acid | - | - | 0.35 ± 0.03 | 3.02 ± 0.02 | 1.76 ± 0.12 | 0.16 ± 0.02 |
| 51 | Acetic acid | - | - | - | - | 1.47 ± 0.13 | - |
| Ketones | |||||||
| 52 | 1-Octen-3-one | 0.07 ± 0.01 | - | 0.25 ± 0.03 | 0.32 ± 0.03 | 0.42 ± 0.03 | 0.38 ± 0.04 |
| 53 | 6-Methyl-5-hepten-2-one | 0.35 ± 0.03 | 0.45 ± 0.06 | 0.24 ± 0.02 | 0.36 ± 0.02 | 0.45 ± 0.02 | 0.27 ± 0.03 |
| 54 | (E)-6,10-Dimethyl-,5,9-undecadien-2-one | - | - | - | 0.07 ± 0.01 | - | 0.11 ± 0.01 |
| 55 | 4,4-Dimethyl-3-phenyl-2,5-cyclohexadien-1-one | - | - | - | - | - | 0.23 ± 0.03 |
| 56 | Acetone | 0.1 ± 0.01 | - | - | 0.05 ± 0.01 | - | - |
| Alcohols | |||||||
| 57 | 1-Hexanol | - | - | - | 1.39 ± 0.15 | - | 1.45 ± 0.20 |
| 58 | 1-Butanol, 2-methyl-, (S)- | - | - | - | - | 1.21 ± 0.14 | 1.04 ± 0.17 |
| 59 | 1-Undecanol | - | - | - | - | - | 0.14 ± 0.02 |
| Alkene | |||||||
| 60 | (E)-á-Famesene | - | - | - | 0.19 ± 0.02 | - | - |
| 61 | à-Farnesene | - | - | 27.99 ± 1.96 | 22.99 ± 1.65 | 15.22 ± 1.23 | 15.47 ± 1.35 |
| Others | |||||||
| 62 | Estragole | - | - | 0.11 ± 0.01 | - | - | 0.08 ± 0.01 |
| 63 | 1-Nonene | - | - | - | - | - | 0.18 ± 0.02 |
| 64 | Melezitose | - | - | - | - | 0.48 ± 0.03 | - |
| 65 | 2,4-Di-tert-butylphenol | 0.63 ± 0.05 | 0.34 ± 0.02 | 0.51 ± 0.04 | 1.44 ± 0.12 | - | 0.09 ± 0.01 |
| No. | Compound | Relative Content/% | |||||
|---|---|---|---|---|---|---|---|
| 60 DAFB | 120 DAFB | 165 DAFB | 180 DAFB | 195 DAFB | 210 DAFB | ||
| Aldehydes | |||||||
| 1 | Hexanal | 16.51 ± 0.7 | 16.4 ± 0.31 | 3.27 ± 0.18 | 1.84 ± 0.27 | 1.26 ± 0.23 | 1.3 ± 0.07 |
| 2 | 3-Hexenal | 13.94 ± 0.46 | 10.67 ± 0.48 | 5.49 ± 0.98 | 0.86 ± 0.16 | 0.12 ± 0.02 | 0.47 ± 0.03 |
| 3 | 2-Hexenal | 40.76 ± 0.22 | 45.86 ± 0.49 | 19.9 ± 1.13 | 11.02 ± 0.82 | 11.5 ± 0.85 | 8.33 ± 0.68 |
| 4 | (E)-2-Octenal | - | - | 0.37 ± | 0.2 ± 0.05 | 0.6 ± 0.14 | 0.21 ± 0.05 |
| 5 | Dodecanal | - | - | - | - | 0.09 ± 0.19 | - |
| 6 | (E)-2-Hexenal | - | - | 4.06 ± 0.77 | - | 2.58 ± 0.34 | - |
| 7 | Octanal | - | - | 0.28 ± 0.03 | - | 0.38 ± 0.24 | 0.25 ± 0.04 |
| 8 | Nonanal | - | - | - | - | 0.87 ± 0.05 | 0.56 ± 0.13 |
| 9 | (Z)-2-Heptenal | - | 0.31 ± 0.04 | 0.69 ± 0.07 | - | 0.58 ± 0.17 | 0.33 ± 0.05 |
| 10 | (Z)-2-Nonenal | - | - | - | 0.09 ± 0.01 | 0.14 ± 0.04 | 0.14 ± 0.01 |
| 11 | Decanal | 0.4 ± 0.02 | 0.51 ± 0.03 | - | - | - | - |
| 12 | (E)-2-Decenal | - | - | 0.07 ± 0.01 | - | 0.12 ± 0.07 | 0.1 ± 0.01 |
| 13 | (E,E)-2,4-Hexadienal | 4.83 ± 0.51 | 6.11 ± 0.15 | 0.24 ± 0.13 | - | - | - |
| 14 | Furfural | - | - | 2.07 ± 0.23 | - | 0.77 ± 0.22 | - |
| 15 | 2-Methyl-4-Pentenal | 0.52 ± 0.23 | 0.19 ± 0.05 | 0.45 ± 0.17 | - | 0.08 ± 0.02 | 0.03 ± 0.01 |
| Esters | |||||||
| 16 | Butyl 2-methylbutanoate | - | - | - | 3.39 ± 0.35 | 2.7 ± 0.04 | 1.97 ± 0.24 |
| 17 | Isoamyl butyrate | - | - | 0.62 ± 0.04 | 0.75 ± 0.19 | 0.57 ± 0.12 | 0.74 ± 0.04 |
| 18 | Hexyl acetate | 0.34 ± 0.02 | 0.93 ± 0.08 | 4.79 ± 0.58 | 4.85 ± 0.46 | 6.13 ± 0.23 | 4.02 ± 0.34 |
| 19 | Hexyl propionate | - | - | 0.79 ± 0.15 | 2.8 ± 0.35 | 2.98 ± 0.40 | 2.45 ± 0.25 |
| 20 | Hexyl isovalerate | - | - | 10.78 ± 0.77 | 15.73 ± 0.87 | 14.16 ± 0.55 | 12.48 ± 0.64 |
| 21 | Octaethylene glycol monododecyl ether | 0.86 ± 0.21 | 2.06 ± 0.70 | 0.68 ± 0.04 | - | 0.34 ± 0.31 | - |
| 22 | Acetic acid, butyl ester | - | - | 0.94 ± 009 | 1.33 ± 0.24 | 1.67 ± 0.17 | 1.65 ± 0.33 |
| 23 | Butanoic acid, 2-methyl-, pentyl ester | - | - | 0.93 ± 0.12 | 1.29 ± 0.04 | 0.94 ± 0.16 | 1.39 ± 0.14 |
| 24 | Butanoic acid, butyl ester | - | - | 1.19 ± 0.22 | 1.68 ± 0.34 | 2.46 ± 0.23 | 2.05 ± 0.30 |
| 25 | Butyl 2-methylbutanoate | - | - | 1.74 ± 0.07 | - | 1.56 ± 0.18 | 1.72 ± 0.18 |
| 26 | Hexyl Hexanoate | - | - | 3.83 ± 0.18 | - | 5.55 ± 0.37 | 12.42 ± 0.47 |
| 27 | Hexyl octanoate | - | - | - | 0.42 ± 0.26 | 0.23 ± 0.04 | 0.37 ± 0.02 |
| 28 | 1-Butanol, 2-methyl-, acetate | - | - | 4.59 ± 0.97 | 4.2 ± 0.62 | 2.09 ± 0.17 | 5.58 ± 0.34 |
| 29 | Propanoic acid, butyl ester | - | - | - | 1.22 ± 0.24 | 0.27 ± 0.09 | 1.64 ± 0.04 |
| 30 | Acetic acid, pentyl ester | - | - | - | 0.45 ± 0.07 | 0.09 ± 0.01 | 0.91 ± 0.09 |
| 31 | Octylhexanoate | - | - | 0.17 ± 0.03 | - | 0.19 ± 0.03 | - |
| 32 | Hexanoic acid, 2-methylbutyl ester | - | - | 1.96 ± 0.04 | 2.54 ± 0.33 | 1.79 ± 0.23 | 2.95 ± 0.24 |
| 33 | Hexanoic acid, pentyl ester | - | - | 0.92 ± 0.01 | - | - | - |
| 34 | Propyl octanoate | - | - | 0.08 ± 0.01 | 0.37 ± 0.18 | 0.71 ± 0.21 | 0.55 ± 0.04 |
| 35 | 2-Methylbutyl 2-methylbutanoate | - | - | - | 0.3 ± 0.10 | 0.22 ± 0.04 | 0.27 ± 0.03 |
| 36 | Butyl caprylate | - | - | - | 1.96 ± 0.09 | 2.03 ± 0.31 | - |
| 37 | 2-Methylbutyl octanoate | - | - | - | 1.15 ± 0.24 | 0.72 ± 0.14 | 0.92 ± 0.04 |
| 38 | Heptanoic acid, butyl ester | - | - | - | 0.28 ± 0.08 | 2.18 ± 0.22 | 0.58 ± 0.16 |
| 39 | Butyl caprate | - | - | - | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.04 ± 0.1 |
| 40 | n-Propyl acetate | - | - | - | - | - | 0.28 ± 0.18 |
| 41 | Heptyl 2-methylbutyrate | - | - | - | 0.65 ± 0.21 | 0.14 ± 0.01 | 1.28 ± 0.007 |
| 42 | Propyl acetate | - | - | 0.22 ± 0.07 | 0.96 ± 0.17 | 0.84 ± 0.03 | 1.93 ± 0.16 |
| 43 | Butanoic acid3-methyl-, pentyl ester | - | - | 5.03 ± 0.53 | - | 10.66 ± 0.56 | 8.14 ± 0.29 |
| 44 | Acetic acid, heptyl ester | - | - | - | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 |
| 45 | Acetic acid, 2-phenylethyl ester | 0.12 ± 0.01 | - | - | - | - | - |
| 46 | Propanoic acid, pentyl ester | - | - | - | 0.71 ± 0.13 | 1.16 ± 0.07 | 1.57 ± 0.26 |
| 47 | Hexanoic acid, 2-methylpropyl ester | - | - | 0.13 ± 0.01 | 0.08 ± 0.01 | 0.1 ± 0.01 | 0.1 ± 0.04 |
| 48 | Pentanoic acid, 2-methylbutyl ester | - | - | - | 0.08 ± 0.01 | 0.05 ± 0.01 | 0.13 ± 0.06 |
| 49 | Propanoic acid, propyl ester | - | - | - | 0.22 ± 0.05 | 0.21 ± 0.03 | 0.3 ± 0.18 |
| 50 | Octanoic acid, 2-ethylhexyl ester | - | - | 0.36 ± 0.07 | - | - | - |
| 51 | Cis-3-Hexenyl isovalerate | - | - | - | 0.06 ± 0.01 | - | - |
| 52 | Octanoic acid, 2-ethylhexyl ester | - | - | 0.36 ± 0.07 | - | - | - |
| 53 | Heptyl 2-methylbutyrate | - | - | 0.88 ± 0.02 | - | - | - |
| Acids | |||||||
| 54 | 2-Methyl-butanoic acid | - | - | 1.35 ± 0.18 | 2.81 ± 0.42 | 1.85 ± 0.22 | 1.28 ± 0.21 |
| Ketones | |||||||
| 55 | 1-Octen-3-one | - | - | 0.23 ± 0.04 | 0.13 ± 0.20 | 0.3 ± 0.02 | 0.21 ± 0.02 |
| 56 | 6-Methyl-5-hepten-2-one | 0.37 ± 0.01 | 0.61 ± 0.10 | 0.17 ± 0.02 | - | 0.26 ± 0.02 | 0.2 ± 0.04 |
| 57 | (E)-6,10-Dimethyl-,5,9-undecadien-2-one | - | - | - | 0.11 ± 0.19 | 0.08 ± 0.01 | 0.06 ± 0.01 |
| Alcohols | |||||||
| 58 | 1-Hexanol | - | - | - | - | 0.61 ± 0.34 | 0.6 ± 0.01 |
| 59 | (S)-2-Methyl-1-butanol | - | - | 0.41 ± 0.05 | - | - | - |
| Alkene | |||||||
| 60 | (E)-á-Famesene | - | - | - | 0.04 ± 0.01 | - | 0.13 ± 0.02 |
| 61 | à-Farnesene | - | 0.12 ± 0.01 | 16.44 ± 1.49 | 11.37 ± 1.12 | 6.65 ± 0.71 | 9.88 ± 0.54 |
| 62 | 1-Nonene | - | - | - | - | 0.11 ± 0.04 | - |
| Others | |||||||
| 63 | Estragole | - | - | - | 0.04 ± 0.01 | - | 0.1 ± 0.01 |
| 64 | 2,4-Di-tert-butylphenol | 0.56 ± 0.02 | 0.41 ± 0.03 | 0.63 ± 0.17 | 0.8 ± 0.12 | 0.39 ± 0.06 | 0.75 ± 0.03 |
| 65 | Carbamic acid, monoammonium salt | - | 1.26 ± 0.13 | - | - | - | - |
| Gene Name | Protein Product | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) | GenBank Accession No. |
|---|---|---|---|---|
| MdBCAT2 | Branched-chain amino acid aminotransferase 2 | ACTCGTTCCTCAACCTGGTA | AAGCAAGGGATTTTCGGATGTT | CV082955 |
| MdArAT | Aromatic amino acid aminotransferase | CAGTCGTCACAAACCAACC | GCTGATGAAGTTTATGGGCA | GO511145 |
| MdAADC | Amino acid decarboxylase | GTTGATGATGGCGGGTTTATC | AGGCAATCTTCACGGAGTCTT | CN888142 |
| MdACP2 | Acyl carrier protein 2 | CCGAGTACATAACCAGTCTACCA | CGCAGATGTTATTGAGAAGCTC | CV630926 |
| MdMCAT | Malonyl-CoA:ACP transacylase | AGGCCGCACGTGACATCAAC | GACGCCTTGTTCGCCGATTAC | MD177270 |
| MdACPD | Acyl-ACP desaturase | CTCAGCCCAATCCTCTAATGACTT | CTCCACTCTTCACTCCTCCACC | ES790074 |
| MdLOX | Lipoxygenase | GTCACGCTGTCCGAGATAGA | ACTCCAGAGTATGAGGAGCTCAG | DY742295 |
| MdHPL | Hydroperoxide lyase | GTGTGAACTGAGTTGGAAGTCCT | TTGCAACTGGTTCAGTCAGTAGT | GO537614 |
| MdAOS | Alene oxide synthase | CGACTCGACTTGAGGAGGTAG | AAGAAGGATATCTTCACCGGAAC | DR991430 |
| MdPDC2 | Pyruvate decarboxylase 2 | TGGTCAGTTGGAGCAACTCTT | GACACATCTTGAGCAGTCACCT | DR992977 |
| MdADH3 | Alcohol dehydrogenase 3 | CCGTCGATCATGAGATTATCC | AACTTGCAAGGAGTGGAACTG | DY256060 |
| MdAAT2 | Alcohol acyltransferase 2 | AGGACAACCAATAATTCCATCAG | GATGTCACACTTGAGCAACTAGG | AY517491 |
| MdActin (reference gene) | GCCAGATCTTCTCCATGTCATCC | TGTGTTTCCTAGTATTGTTGGTCGC | XM008393049 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, S.; Yan, C.; Zhang, T.; Ji, M.; Tao, R.; Gao, H. Comparative Study of Volatile Compounds and Expression of Related Genes in Fruit from Two Apple Cultivars during Different Developmental Stages. Molecules 2021, 26, 1553. https://doi.org/10.3390/molecules26061553
Feng S, Yan C, Zhang T, Ji M, Tao R, Gao H. Comparative Study of Volatile Compounds and Expression of Related Genes in Fruit from Two Apple Cultivars during Different Developmental Stages. Molecules. 2021; 26(6):1553. https://doi.org/10.3390/molecules26061553
Chicago/Turabian StyleFeng, Shuaishuai, Chengtai Yan, Tianhao Zhang, Miaomiao Ji, Ru Tao, and Hua Gao. 2021. "Comparative Study of Volatile Compounds and Expression of Related Genes in Fruit from Two Apple Cultivars during Different Developmental Stages" Molecules 26, no. 6: 1553. https://doi.org/10.3390/molecules26061553
APA StyleFeng, S., Yan, C., Zhang, T., Ji, M., Tao, R., & Gao, H. (2021). Comparative Study of Volatile Compounds and Expression of Related Genes in Fruit from Two Apple Cultivars during Different Developmental Stages. Molecules, 26(6), 1553. https://doi.org/10.3390/molecules26061553






