A Microwave-Assisted Boudouard Reaction: A Highly Effective Reduction of the Greenhouse Gas CO2 to Useful CO Feedstock with Semi-Coke
Abstract
1. Introduction
2. Results and Discussions
2.1. Temperature Correction of the Microwave Fixed Bed Reactor
2.2. The Boudouard Reaction in Both Microwave and Conventional Thermal Fields
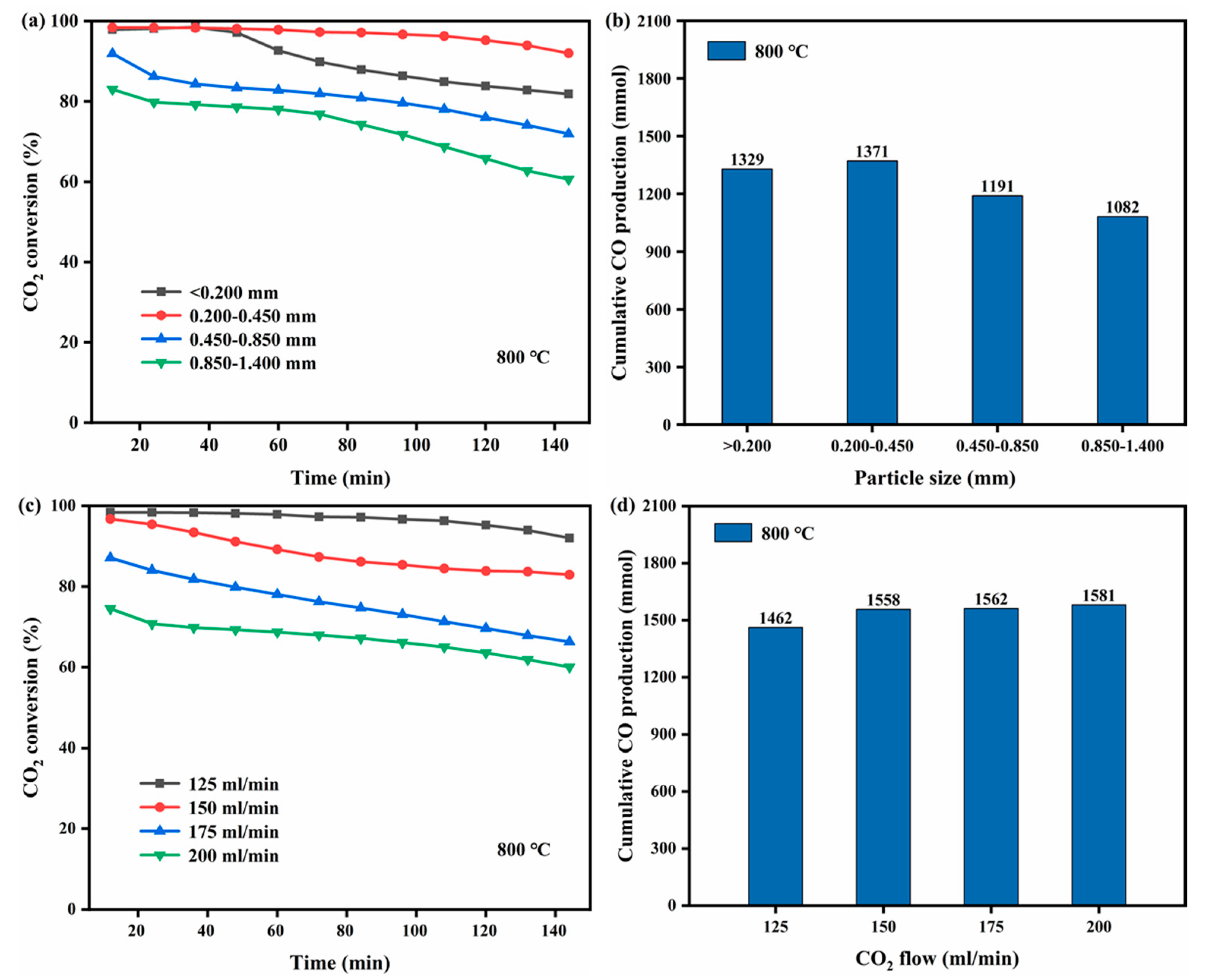
2.3. Influence of the SC Particle Size
2.4. Influence of the CO2 Gas Flow Rate
2.5. Influence of the Catalyst
2.6. Analysis of Gasification Kinetics
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. Carbon Characterization and Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Das Neves Gomes, C.; Blondiaux, E.; Thuery, P.; Cantat, T. Metal-free reduction of CO2 with hydroboranes: Two effi-cient pathways at play for the reduction of CO2 to methanol. Chemistry 2014, 20, 7098–7106. [Google Scholar] [CrossRef] [PubMed]
- Lahijani, P.; Zainal, Z.A.; Mohammadi, M.; Mohamed, A.R. Conversion of the greenhouse gas CO2 to the fuel gas CO via the Boudouard reaction: A review. Renew. Sustain. Energy Rev. 2015, 41, 615–632. [Google Scholar] [CrossRef]
- Beller, M. Catalytic Carbonylation Reaction. Organomet. Chem. 2006, 18, 1–283. [Google Scholar]
- Ratnasamy, C.; Wagner, J.P. Water Gas Shift Catalysis. Catal. Rev. 2009, 51, 325–440. [Google Scholar] [CrossRef]
- Leadbeater, N.E. Microwave Heating as a Tool for Sustainable Chemistry; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Bykov, Y.V.; Rybakov, K.I.; Semenov, V.E. High temperature microwave processing. Appl. Phys. 2001, 34, 55–75. [Google Scholar]
- Gabriel, C.; Gabriel, S.; Grant, E.H.; Halstead, B.S.J.; Mingos, D.M.P. Dielectric parameters relevant to microwave dielectric heating. Chem. Soc. Rev. 1998, 27, 213–224. [Google Scholar] [CrossRef]
- Jia, Z.; Lin, K.; Wu, G.; Xing, H.; Wu, H. Recent Progresses of High-Temperature Micro-wave-Absorbing Materials. NANO 2018, 13, 1830005. [Google Scholar] [CrossRef]
- Bermúdez, J.M.; Ruisánchez, E.; Arenillas, A.; Moreno, A.H.; Menéndez, J.A. New concept for energy storage: Mi-crowave-induced carbon gasification with CO2. Energy Convers. Manag. 2014, 78, 559–564. [Google Scholar] [CrossRef]
- Lahijani, P.; Mohammadi, M.; Zainal, Z.A.; Mohamed, A.R. Improvement of biomass char-CO2 gasification reactivity using microwave irradiation and natural catalyst. Thermochim. Acta 2015, 604, 61–66. [Google Scholar] [CrossRef]
- Lahijani, P.; Zainal, Z.A.; Mohamed, A.R.; Mohammadi, M. Microwave-enhanced CO2 gasification of oil palm shell char. Bioresour. Technol. 2014, 158, 193–200. [Google Scholar] [CrossRef]
- Chun, Y.N.; Song, H.G. Microwave-induced carbon-CO2 gasification for energy conversion. Energy 2020, 190, 116386. [Google Scholar] [CrossRef]
- Chun, Y.N.; Song, H.G. Microwave-enhanced gasification of sewage sludge waste. Environ. Eng. Res. 2018, 24, 591–599. [Google Scholar] [CrossRef]
- Lartey-Young, G.; Ma, L. Remediation with Semicoke-Preparation, Characterization, and Adsorption Application. Materials 2020, 13, 4334. [Google Scholar] [CrossRef]
- Ramirez, A.; Hueso, J.L.; Abian, M.; Alzueta, M.U.; Mallada, R.; Santamaria, J. Escaping undesired gas-phase chemistry: Microwave-driven selectivity enhancement in heterogeneous catalytic reactors. Sci. Adv. 2019, 5, eaau9000. [Google Scholar] [CrossRef]
- Sugawara, H.; Kashimura, K.; Hayashi, M.; Ishihara, S.; Mitani, T.; Shinohara, N. Behavior of microwave-heated silicon carbide particles at frequencies of 2.0–13.5 GHz. Appl. Phys. Lett. 2014, 105, 034103. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Chou, T.W. Microwave processing fundamentals and applications. Compos. Part A Appl. Sci. 1999, 30, 1055–1071. [Google Scholar] [CrossRef]
- Liu, Y.; Guan, Y.; Zhang, K. CO2 gasification performance and alkali/alkaline earth metals catalytic mechanism of Zhundong coal char. Korean J. Chem. Eng. 2018, 35, 859–866. [Google Scholar] [CrossRef]
- Uwaoma, R.C.; Strydom, C.A.; Bunt, J.R.; Okolo, G.N.; Matjie, R.H. The catalytic effect of Benfield waste salt on CO2 gasification of a typical South African Highveld coal. J. Therm. Anal. Calorim. 2018, 135, 2723–2732. [Google Scholar] [CrossRef]
- Lahijani, P.; Mohammadi, M.; Mohamed, A.R. Catalytic CO2 gasification of rubber seed shell-derived hydrochar: Reactivity and kinetic studies. Environ. Sci. Pollut. Res. 2019, 26, 11767–11780. [Google Scholar] [CrossRef]
- Irfan, M.F.; Usman, M.R.; Kusakabe, K. Coal gasification in CO2 atmosphere and its kinetics since 1948: A brief review. Energy 2011, 36, 12–40. [Google Scholar] [CrossRef]
- Wen, X.D.; Man, S.F.; Long, Y.J.; Xin, J.; Yan, Z.h.; Jian, G.Q.; Xin, L.J. Combustion characteristics of unburned pulverized coal and its reaction kinetics with CO2. Int. J. Miner. Metall. Mater. 2019, 26, 811–821. [Google Scholar]
- Zhang, Y.; Geng, P.; Zheng, Y. Exploration and practice to improve the kinetic analysis of char-CO2 gasification via thermogravimetric analysis. Chem. Eng. J. 2019, 359, 298–304. [Google Scholar] [CrossRef]
- Bhui, B.; Vairakannu, P. Experimental and kinetic studies on in-situ CO2 gasification based chemical looping combus-tion of low ash coal using Fe2O3 as the oxygen carrier. J. CO2 Util. 2019, 29, 103–116. [Google Scholar] [CrossRef]
- Hunt, J.; Ferrari, A.; Lita, A.; Crosswhite, M.; Ashley, B.; Stiegman, A.E. Microwave-Specific Enhancement of the Carbon–Carbon Dioxide (Boudouard) Reaction. J. Phys. Chem. C 2013, 117, 26871–26880. [Google Scholar] [CrossRef]






| Sample | Raw SC | CH-SC | MW-SC | MW-SC/BaCO3 |
|---|---|---|---|---|
| Sbet (m2/g) | 2.2 | 3.6 | 18.2 | 14.3 |
| Vtotal (cm3/g) | 1.3 × 10−3 | 1.7 × 10−3 | 2.8 × 10−3 | 2.5 × 10−3 |
| Parameters | Raw SC | CH-SC |
|---|---|---|
| Ea/kJ·mol−1 | 47.1 | 148.9 |
| A/min−1 | 0.3 | 1088.6 |
| Ultimate Analysis (%) | Proximate Analysis (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| C | H | N | O | S | FC | M | V | Ash |
| 82.3 | 0.7 | 1.15 | 14.8 | 0.9 | 81.7 | 1.5 | 2.9 | 14.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, H.; Zhao, H.; Chen, S.; Jiang, B. A Microwave-Assisted Boudouard Reaction: A Highly Effective Reduction of the Greenhouse Gas CO2 to Useful CO Feedstock with Semi-Coke. Molecules 2021, 26, 1507. https://doi.org/10.3390/molecules26061507
Dai H, Zhao H, Chen S, Jiang B. A Microwave-Assisted Boudouard Reaction: A Highly Effective Reduction of the Greenhouse Gas CO2 to Useful CO Feedstock with Semi-Coke. Molecules. 2021; 26(6):1507. https://doi.org/10.3390/molecules26061507
Chicago/Turabian StyleDai, Huan, Hong Zhao, Siyuan Chen, and Biao Jiang. 2021. "A Microwave-Assisted Boudouard Reaction: A Highly Effective Reduction of the Greenhouse Gas CO2 to Useful CO Feedstock with Semi-Coke" Molecules 26, no. 6: 1507. https://doi.org/10.3390/molecules26061507
APA StyleDai, H., Zhao, H., Chen, S., & Jiang, B. (2021). A Microwave-Assisted Boudouard Reaction: A Highly Effective Reduction of the Greenhouse Gas CO2 to Useful CO Feedstock with Semi-Coke. Molecules, 26(6), 1507. https://doi.org/10.3390/molecules26061507








