Collagen Peptides Derived from Sipunculus nudus Accelerate Wound Healing
Abstract
1. Introduction
2. Results
2.1. The Extracted Collagen from Sipunculus nudus (SNC) is Type I
2.2. SNCP Consists of Peptides with Small Molecular Weight Less Than 5 kDa
2.3. SNCP is Rich in Gly and Arg
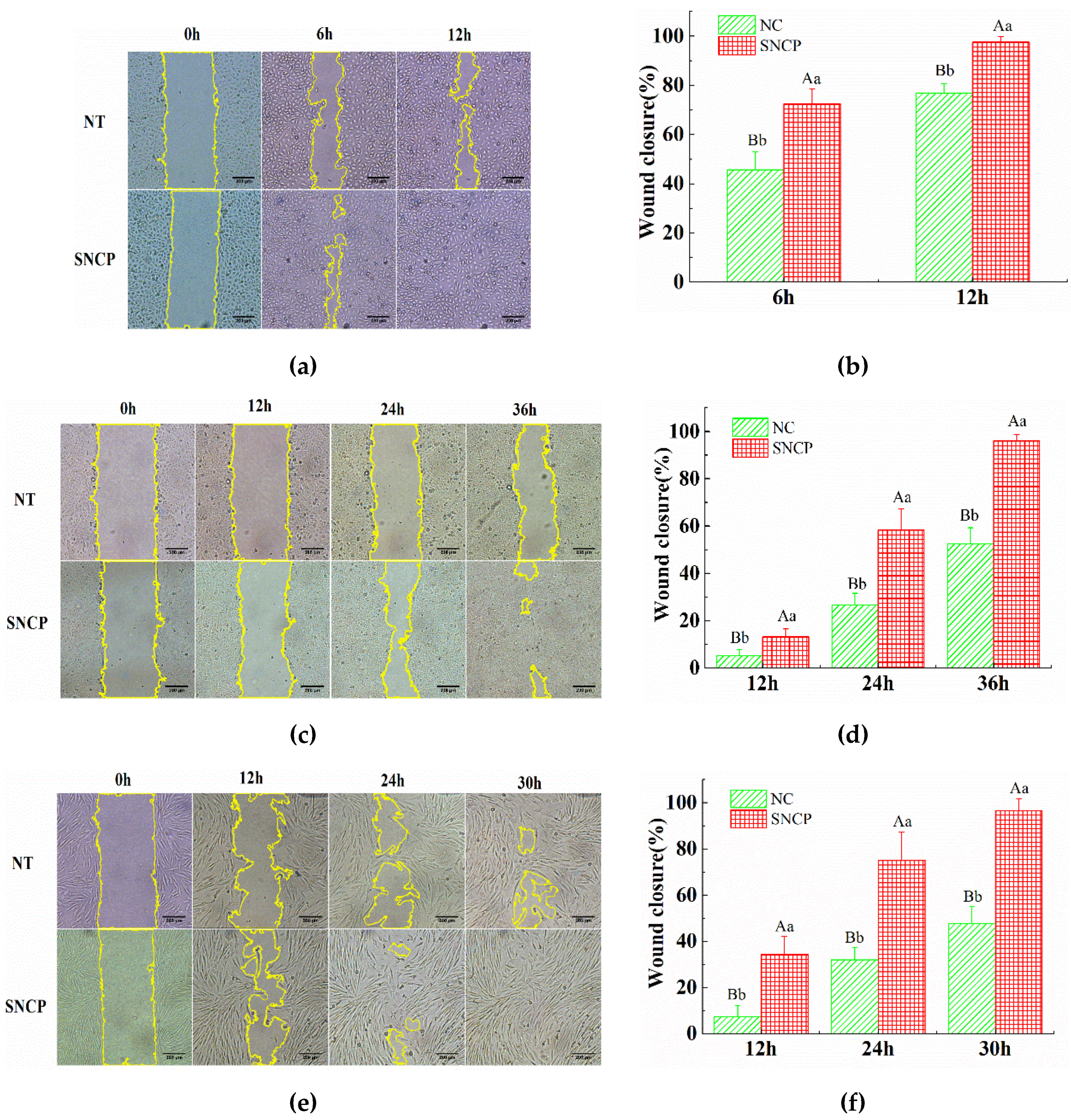
2.4. SNCP Possesses an Outstanding Capacity to Induce HUVEC, HaCaT and HSF Cells Proliferation and Migration
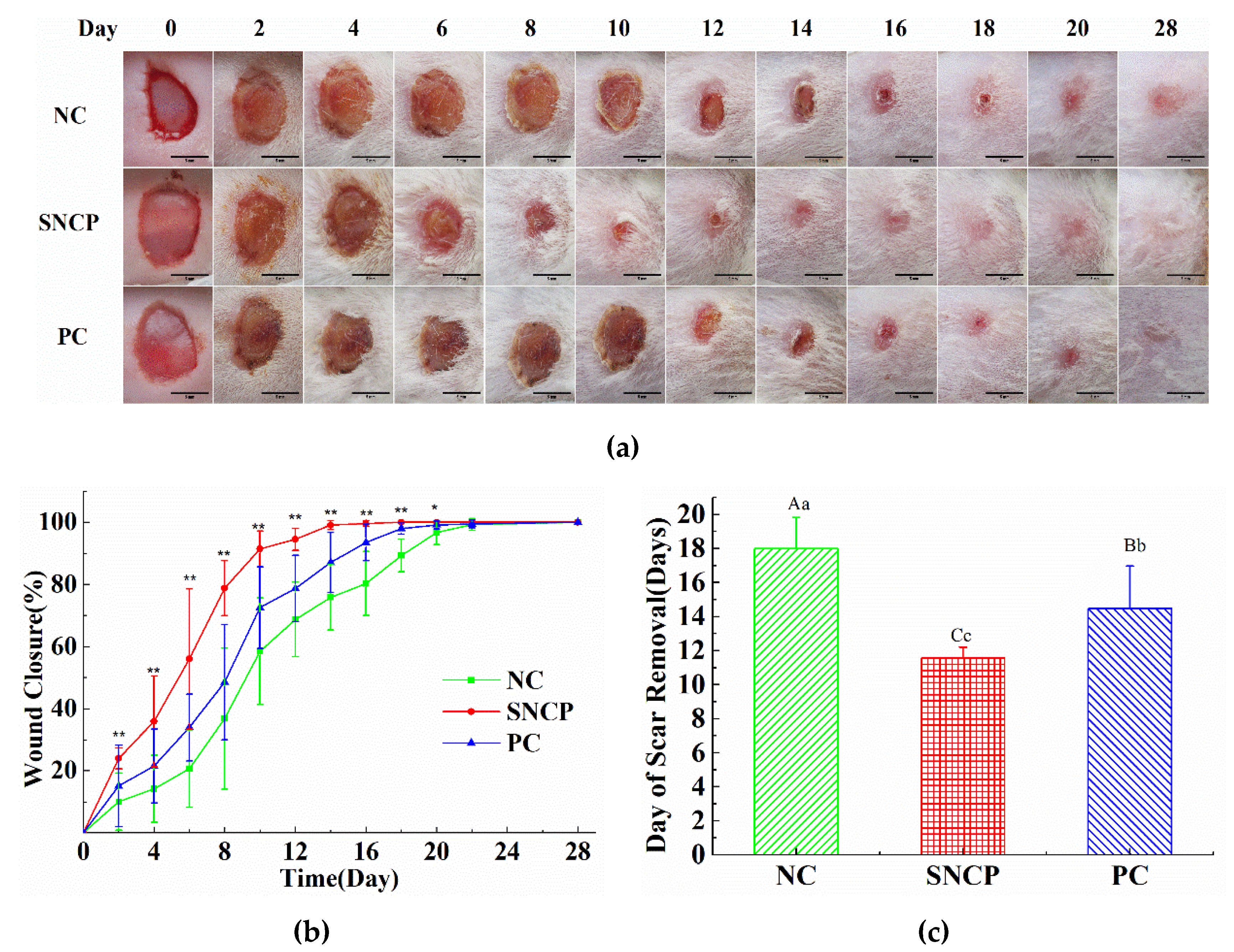
2.5. SNCP Accelerates the Wound Healing In Vivo
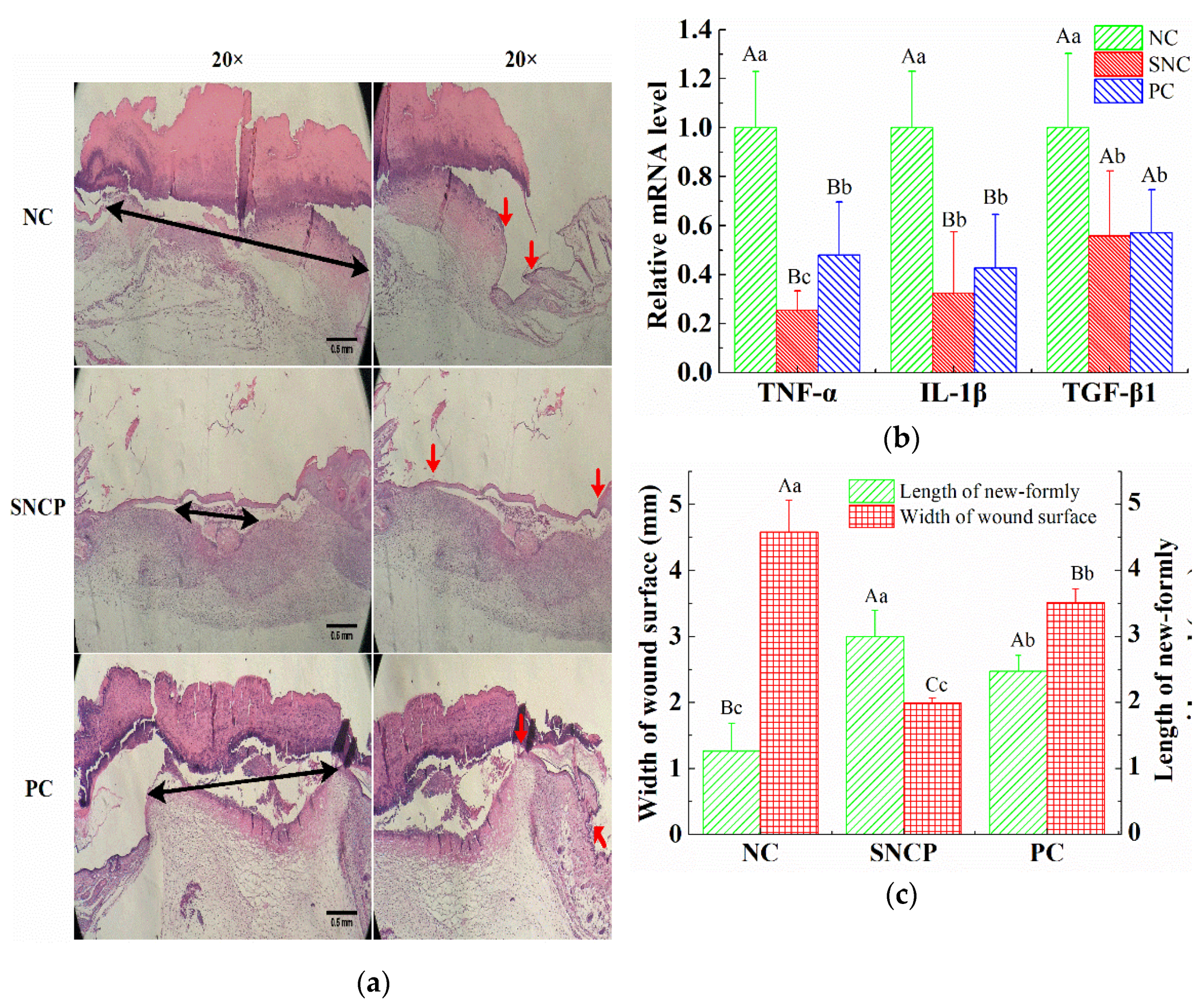
2.6. SNCP Reduces Excessive Inflammation Response
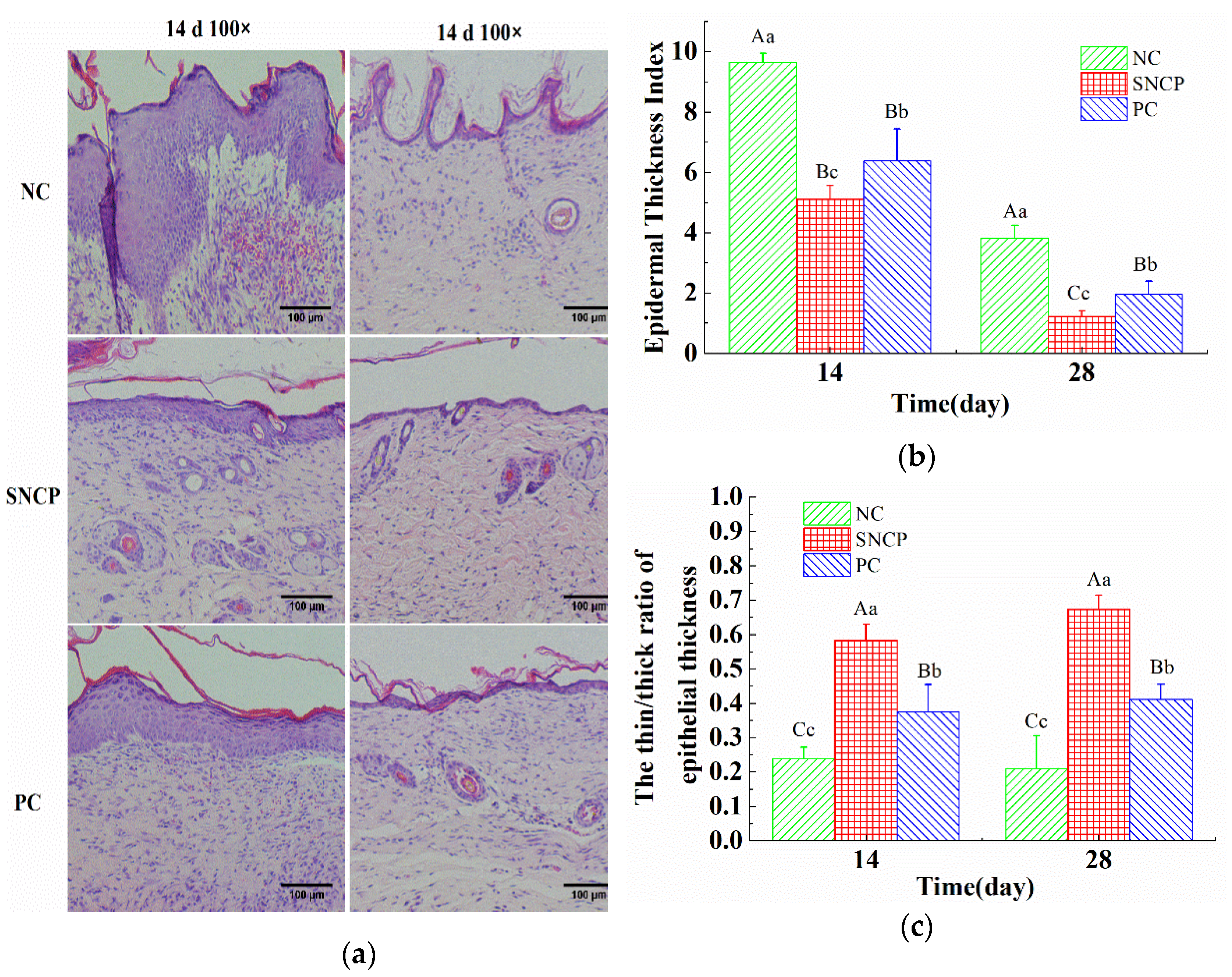
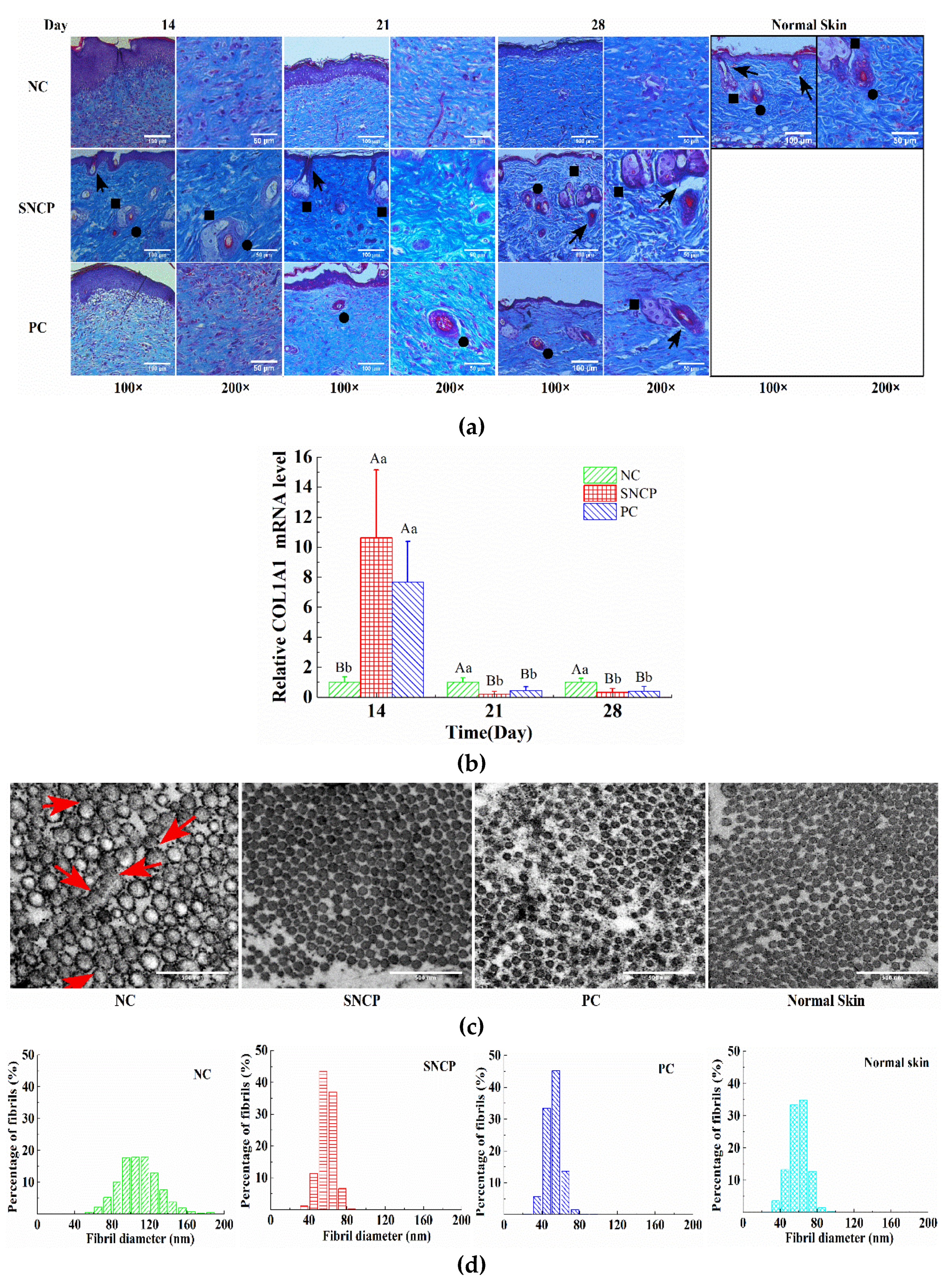
2.7. SNCP Improves the Healing Quality of Wound Epidermis
2.8. SNCP Improves Collagen Deposition and Recombination at the Wound Dermis
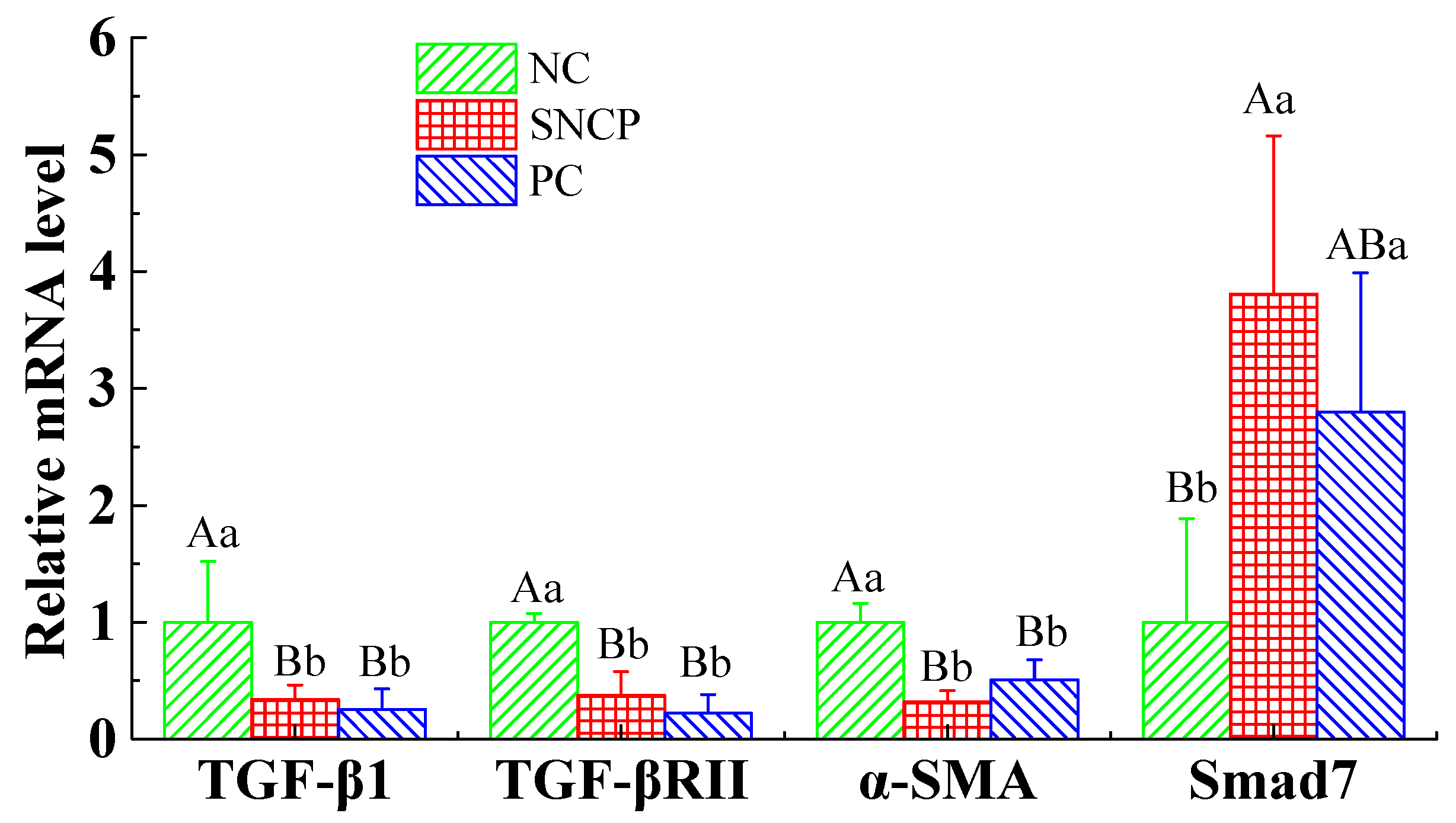
2.9. SNCP Blockades the TGF-β/Smad Signaling Pathway to Achieve Scarless Healing
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction of the Collagen and Preparation of Collagen Peptides
4.3. Sodium Dodecyl Sulphate Polyacrylamide-Gel Electrophoresis (SDS-PAGE)
4.4. Fourier Transform Infrared Spectroscopy (FTIR)
4.5. Differential Scanning Calorimetry (DSC)
4.6. Determination of Molecular Weight Distribution of SNCP
4.7. Amino Acid Composition Measurement of SNCP
4.8. In Vitro Scratch Assay
4.9. In Vivo Wound Healing Assay
4.10. Macroscopic Evaluation of Wounded Tissues
4.11. Microscopic Evaluation of HE-Stained Sections
4.12. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
4.13. TEM Analysis
4.14. Statistics Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vinklarkova, L.; Masteikova, R.; Foltynova, G.; Muselik, J.; Pavlokova, S.; Bernatoniene, J.; Vetchy, D. Film wound dressing with local anesthetic based on insoluble carboxymethycellulose matrix. J. Appl. Biomed. 2017, 15, 313–320. [Google Scholar] [CrossRef]
- Olsson, M.; Jarbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.M.; Qin, X.M.; Zhang, T.; Lin, H.S.; Zhang, C.H. Evaluation of Small Molecular Polypeptides from the Mantle of Pinctada Martensii on Promoting Skin Wound Healing in Mice. Molecules 2019, 24, 4231. [Google Scholar] [CrossRef]
- Greaves, N.S.; Ashcroft, K.J.; Baguneid, M.; Bayat, A. Current understanding of molecular and cellular mechanisms in fibroplasia and angiogenesis during acute wound healing. J. Dermatol. Sci. 2013, 72, 206–217. [Google Scholar] [CrossRef]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, C.; Lin, H.; Zeng, S.; Chen, H. Wound-healing acceleration of mice skin by Sipunculus nudus extract and its mechanism. J. Biomed. Eng. (Chengdu, China) 2020, 37, 460–468. [Google Scholar]
- Mei, F.; Liu, J.; Wu, J.; Duan, Z.; Chen, M.; Meng, K.; Chen, S.; Shen, X.; Xia, G.; Zhao, M. Collagen Peptides Isolated from Salmo salar and Tilapia nilotica Skin Accelerate Wound Healing by Altering Cutaneous Microbiome Colonization via Upregulated NOD2 and BD14. J. Agric. Food Chem. 2020, 68, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Jin, S.J.; Tang, Y.P. Marine Collagen Peptides Promote Cell Proliferation of NIH-3T3 Fibroblasts via NF-kappa B Signaling Pathway. Molecules 2019, 24, 4201. [Google Scholar] [CrossRef] [PubMed]
- Carmo, J.; Cavalcante-Araujo, P.; Silva, J.; Ferro, J.; Correia, A.C.; Lagente, V.; Barreto, E. Uvaol Improves the Functioning of Fibroblasts and Endothelial Cells and Accelerates the Healing of Cutaneous Wounds in Mice. Molecules 2020, 25, 4982. [Google Scholar] [CrossRef]
- Kumar, N.S.S.; Nazeer, R.A.; Jaiganesh, R. Wound Healing Properties of Collagen from the Bone of Two Marine Fishes. Int. J. Pept. Res. Ther. 2012, 18, 185–192. [Google Scholar] [CrossRef]
- Su, X.R.; Sun, B.; Li, Y.Y.; Hu, Q.H. Biological characterization of collagen of Sipunculid. Nat. Prod. Res. Dev. 2009, 21, 48–52. [Google Scholar]
- Chen, J.J.; Gao, K.L.; Liu, S.; Wang, S.J.; Elango, J.; Bao, B.; Dong, J.; Liu, N.; Wu, W.H. Fish Collagen Surgical Compress Repairing Characteristics on Wound Healing Process In Vivo. Mar. Drugs 2019, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Jridi, M.; Bardaa, S.; Moalla, D.; Rebaii, T.; Souissi, N.; Sahnoun, Z.; Nasri, M. Microstructure, rheological and wound healing properties of collagen-based gel from cuttlefish skin. Int. J. Biol. Macromol. 2015, 77, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.X.; Chen, X.L.; Shen, X.R.; He, Y.; Chen, W.; Luo, Q.; Ge, W.H.; Yuan, W.H.; Tang, X.; Hou, D.Y.; et al. Preparation of chitosan-collagen-alginate composite dressing and its promoting effects on wound healing. Int. J. Biol. Macromol. 2018, 107, 93–104. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, P.; Zhou, C.; Li, S.; Hong, P. Marine collagen peptides from the skin of Nile Tilapia (Oreochromis niloticus): Characterization and wound healing evaluation. Mar. Drugs 2017, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Leon-Lopez, A.; Morales-Penaloza, A.; Martinez-Juarez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Alvarez, G. Hydrolyzed Collagen-Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Ding, Y.; Dai, X.; Li, Y. Oral administration of marine collagen peptides from Chum Salmon skin enhances cutaneous wound healing and angiogenesis in rats. J. Sci. Food Agric. 2011, 91, 2173–2179. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Teixeira, C.; Ferraz, R.; Prudencio, C.; Gomes, P. Wound-Healing Peptides for Treatment of Chronic Diabetic Foot Ulcers and Other Infected Skin Injuries. Molecules 2017, 22, 1743. [Google Scholar] [CrossRef] [PubMed]
- Witte, M.B.; Thornton, F.J.; Tantry, U.; Barbul, A. L-Arginine supplementation enhances diabetic wound healing: Involvement of the nitric oxide synthase and arginase pathways. Metabolism 2002, 51, 1269–1273. [Google Scholar] [CrossRef]
- Zhang, C.X.; Dai, Z.R. Anti-hypoxia activity of a polysaccharide extracted from the Sipunculus nudus L. Int. J. Biol. Macromol. 2011, 49, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.M.; Li, M.; Chen, Y.J.; Liu, Y.M.; He, Y.; Jiang, D.W.; Tong, J.; Li, J.X.; Shen, X.R. Protective effects of polysaccharides from Sipunculus nudus on beagle dogs exposed to gamma-radiation. PLoS ONE 2014, 9, e104299. [Google Scholar] [CrossRef]
- Su, J.; Jiang, L.L.; Wu, J.N.; Liu, Z.Y.; Wu, Y.P. Anti-tumor and anti-virus activity of polysaccharides extracted from Sipunculus nudus(SNP) on Hepg2.2.15. Int. J. Biol. Macromol. 2016, 87, 597–602. [Google Scholar] [CrossRef]
- Zhang, C.X.; Dai, Z.R. Immunomodulatory activities on macrophage of a polysaccharide from Sipunculus nudus L. Food Chem. Toxicol. 2011, 49, 2961–2967. [Google Scholar] [CrossRef]
- Zhang, C.X.; Dai, Z.R.; Cai, Q.X. Anti-inflammatory and anti-nociceptive activities of Sipunculus nudus L. extract. J. Ethnopharmacol. 2011, 137, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Karnchanatat, A. Anti-inflammatory action of two novel peptides derived from peanut worms (Sipunculus nudus) in lipopolysaccharide-induced RAW264.7 macrophages. Food Funct. 2020, 11, 552–560. [Google Scholar] [CrossRef]
- Su, X.R.; Sun, B.; Li, Y.Y.; Hu, Q.H. Characterization of acid-soluble collagen from the coelomic wall of Sipunculida. Food Hydrocolloids 2009, 23, 2190–2194. [Google Scholar] [CrossRef]
- Chinh, N.T.; Manh, V.Q.; Trung, V.Q.; Lam, T.D.; Huynh, M.D.; Tung, N.Q.; Trinh, N.D.; Hoang, T. Characterization of collagen derived from tropical freshwater carp fish scale wastes and its amino acid sequence. Nat. Prod. Commun. 2019, 14, 1–12. [Google Scholar] [CrossRef]
- Wei, P.; Zheng, H.; Shi, Z.Y.; Li, D.; Xiang, Y.L. Isolation and characterization of acid-soluble collagen and pepsin-soluble collagen from the skin of hybrid sturgeon. J. Wuhan Univ. Technol., Mater. Sci. Ed. 2019, 34, 950–959. [Google Scholar] [CrossRef]
- Zhou, C.S.; Li, Y.H.; Yu, X.J.; Yang, H.; Ma, H.L.; Yagoub, A.E.A.; Cheng, Y.; Hu, J.L.; Otu, P.N.Y. Extraction and characterization of chicken feet soluble collagen. LWT-Food Sci. Technol. 2016, 74, 145–153. [Google Scholar] [CrossRef]
- Jeong, H.S.; Venkatesan, J.; Kim, S.K. Isolation and characterization of collagen from marine fish (Thunnus obesus). Biotechnol. Bioprocess Eng. 2013, 18, 1185–1191. [Google Scholar] [CrossRef]
- Woo, J.W.; Yu, S.J.; Cho, S.M.; Lee, Y.B.; Kim, S.B. Extraction optimization and properties of collagen from yellowfin tuna (Thunnus albacares) dorsal skin. Food Hydrocolloids 2008, 22, 879–887. [Google Scholar] [CrossRef]
- Ahmed, R.; Haq, M.; Chun, B.S. Characterization of marine derived collagen extracted from the by-products of bigeye tuna (Thunnus obesus). Int. J. Biol. Macromol. 2019, 135, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Shiau, C.Y.; Su, Y.C.; Liu, Y.H.; Huang, Y.R. Isolation and characterization of collagens from the skin of giant grouper (Epinephelus lanceolatus). J. Aquat. Food Prod. Technol. 2016, 25, 93–104. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Characterisation of acid-soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus). Food Chem. 2005, 89, 363–372. [Google Scholar] [CrossRef]
- Pati, F.; Adhikari, B.; Dhara, S. Isolation and characterization of fish scale collagen of higher thermal stability. Bioresour. Technol. 2010, 101, 3737–3742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.T.; Tu, X.; Wang, W.X.; Nan, J.; Wei, B.M.; Xu, C.Z.; He, L.; Xu, Y.L.; Li, S.; Wang, H.B. Insight into the role of grafting density in the self-assembly of acrylic acid-grafted-collagen. Int. J. Biol. Macromol. 2019, 128, 885–892. [Google Scholar] [CrossRef]
- Yamamoto, S.; Deguchi, K.; Onuma, M.; Numata, N.; Sakai, Y. Absorption and urinary excretion of peptides after collagen tripeptide ingestion in humans. Biol. Pharm. Bull. 2016, 39, 428–434. [Google Scholar] [CrossRef]
- Sun, X.X.; Chai, Y.L.; Wang, Q.Q.; Liu, H.X.; Wang, S.R.; Xiao, J.X. A Natural Interruption Displays Higher Global Stability and Local Conformational Flexibility than a Similar Gly Mutation Sequence in Collagen Mimic Peptides. Biochemistry-Us 2015, 54, 6106–6113. [Google Scholar] [CrossRef]
- Raynaud-Simon, A.; Belabed, L.; Le Naour, G.; Marc, J.; Capron, F.; Cynober, L.; Darquy, S. Arginine plus proline supplementation elicits metabolic adaptation that favors wound healing in diabetic rats. Am. J. Physiol.: Regul., Integr. Comp. Physiol 2012, 303, R1053–R1061. [Google Scholar] [CrossRef]
- Martin, P.; Leibovich, S.J. Inflammatory cells during wound, repair: The good, the bad and the ugly. Trends Cell Biol. 2005, 15, 599–607. [Google Scholar] [CrossRef]
- Redd, M.J.; Cooper, L.; Wood, W.; Stramer, B.; Martin, P. Wound healing and inflammation: Embryos reveal the way to perfect repair. Philos. Trans. R. Soc., B 2004, 359, 777–784. [Google Scholar] [CrossRef]
- Roh, J.L.; Lee, J.; Kim, E.H.; Shin, D. Plasticity of oral mucosal cell sheets for accelerated and scarless skin wound healing. Oral Oncol. 2017, 75, 81–88. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Invest. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Mauviel, A.; Heino, J.; Kahari, V.M.; Hartmann, D.J.; Loyau, G.; Pujol, J.P.; Vuorio, E. Comparative effects of interleukin-1 and tumor necrosis factor-alpha on collagen production and corresponding procollagen mRNA levels in human dermal fibroblasts. J. Invest. Dermatol. 1991, 96, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Arno, A.I.; Gauglitz, G.G.; Barret, J.P.; Jeschke, M.G. New molecular medicine-based scar management strategies. Burns 2014, 40, 539–551. [Google Scholar] [CrossRef]
- Enoch, S.; Leaper, D.J. Basic science of wound healing. Surgery 2008, 26, 31–37. [Google Scholar]
- Beanes, S.R.; Dang, C.; Soo, C.; Ting, K. Skin repair and scar formation: The central role of TGF-beta. Expert. Rev. Mol. Med. 2003, 5, 1–22. [Google Scholar] [CrossRef]
- Cheng, L.; Sun, X.; Hu, C.; Jin, R.; Sun, B.; Shi, Y.; Cui, W.; Zhang, Y. In vivo early intervention and the therapeutic effects of 20(s)-ginsenoside rg3 on hypertrophic scar formation. PLoS ONE 2014, 9, e113640. [Google Scholar] [CrossRef]
- Jeon, E.Y.; Choi, B.H.; Jung, D.; Hwang, B.H.; Cha, H.J. Natural healing-inspired collagen-targeting surgical protein glue for accelerated scarless skin regeneration. Biomaterials 2017, 134, 154–165. [Google Scholar] [CrossRef]
- Puig, A.; Anton, J.M.; Mangues, M. A new decorin-like tetrapeptide for optimal organization of collagen fibres. Int. J. Cosmet. Sci. 2008, 30, 97–104. [Google Scholar] [CrossRef]
- Tang, B.; Zhu, B.; Liang, Y.; Bi, L.; Hu, Z.; Chen, B.; Zhang, K.; Zhu, J. Asiaticoside suppresses collagen expression and TGF-β/Smad signaling through inducing Smad7 and inhibiting TGF-βRI and TGF-βRII in keloid fibroblasts. Arch. Dermatol. Res. 2011, 303, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.T.; Han, Y.P.; Yan, C.; Shaw, M.C.; Garner, W.L. TNF-alpha suppresses alpha-smooth muscle actin expression in human dermal fibroblasts: An implication for abnormal wound healing. J. Invest. Dermatol. 2007, 127, 2645–2655. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wang, C.; Wen, C.; Wang, D. miR-21 promotes collagen production in keloid via Smad7. Burns 2017, 43, 555–561. [Google Scholar] [CrossRef]
- Kamiya, Y.; Miyazono, K.; Miyazawa, K. Smad7 inhibits transforming growth factor-beta family type i receptors through two distinct modes of interaction. J. Biol. Chem. 2010, 285, 30804–30813. [Google Scholar] [CrossRef]
- Zhang, Z.; Finnerty, C.; He, J.; Herndon, D.N. Smad ubiquitination regulatory factor 2 expression is enhanced in hypertrophic scar fibroblasts from burned children. Burns 2012, 38, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Chen, B.; Zheng, Q.; Zhu, G.; Cao, W.; Qin, X.; Zhang, C. Ameliorative Effects of Peptides from the Oyster (Crassostrea hongkongensis) Protein Hydrolysates against UVB-Induced Skin Photodamage in Mice. Mar. Drugs 2020, 18, 288. [Google Scholar] [CrossRef] [PubMed]

| Amino Acids. | Free Amino Acid (g/100 g) | Free Amino Acid Residues per 1000 Residues | Hydrolyzed Amino Acids (g/100 g) | Hydrolyzed Amino Acid Residues per 1000 Residues |
|---|---|---|---|---|
| Aspartic acid (Asp) | 0.04 | 10 | 4.09 * | 40 * |
| Threonine (Thr) | 0.19 | 52 | 4.75 | 51 |
| Serine (Ser) | 0.09 | 28 | 4.01 | 49 |
| Proline (Pro) | 0.34 | 96 | 4.00 | 45 |
| Glutamic acid (Glu) | 0.19 | 42 | 16.64 # | 145 # |
| Glycine (Gly) | 0.12 | 52 | 20.86 | 357 |
| Alanine (Ala) | 0.24 | 88 | 8.50 | 123 |
| Cysteine (Cys) | 0.03 | 8 | 0.58 | 6 |
| Valine (Val) | 0.18 | 50 | 1.58 | 17 |
| Methionine (Met) | 0.06 | 13 | 0.49 | 4 |
| Isoleucine (Ile) | 0.26 | 65 | 1.40 | 14 |
| Leucine (Leu) | 0.51 | 127 | 2.32 | 23 |
| Tyrosine (Tyr) | 0.42 | 76 | 0.08 | 1 |
| Phenylalanine (Phe) | 0.38 | 75 | 1.03 | 8 |
| Lysine (Lys) | 0.22 | 49 | 1.74 | 15 |
| Histidine (His) | 0.08 | 17 | 0.62 | 5 |
| Arginine (Arg) | 0.81 | 152 | 13.11 | 97 |
| Total | 4.16 | 1000 | 85.81 | 1000 |
| Gene | Forward | Reverse |
|---|---|---|
| GADPH | CAGGAGGCATTGCTGATGAT | GAAGGCTGGGGCTCATTT |
| TNF-α | GGTCAATCTGCCCAAGTA | CACCCATTCCCTTCACAG |
| IL-1β | TATGGGCTGGACTGTTTCTAATGC | TTCTTGTGACCCTGAGCGACCT |
| TGF-β1 | CCGCAACAACGCCATCTAT | CCAAGGTAACGCCAGGAAT |
| COL1A1 | ACGCCATCAAGGTCTACTGC | GAATCCATCGGTCATGCTCT |
| α-SMA | AGACATCAGGGAGTAATGGTTG | GAAGCTCGTTATAGAAAGAGTGG |
| TGF-βRII | TGAGAAGCCGCATGAAGT | AGAGTGAAGCCGTGGTAGGT |
| Smad7 | TTTACAACCGCAGCAGTTAC | GGCTGTAGGCTTTCTCATAGT |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Zheng, Z.; Yuan, J.; Zhang, C.; Cao, W.; Qin, X. Collagen Peptides Derived from Sipunculus nudus Accelerate Wound Healing. Molecules 2021, 26, 1385. https://doi.org/10.3390/molecules26051385
Lin H, Zheng Z, Yuan J, Zhang C, Cao W, Qin X. Collagen Peptides Derived from Sipunculus nudus Accelerate Wound Healing. Molecules. 2021; 26(5):1385. https://doi.org/10.3390/molecules26051385
Chicago/Turabian StyleLin, Haisheng, Zhihong Zheng, Jianjun Yuan, Chaohua Zhang, Wenhong Cao, and Xiaoming Qin. 2021. "Collagen Peptides Derived from Sipunculus nudus Accelerate Wound Healing" Molecules 26, no. 5: 1385. https://doi.org/10.3390/molecules26051385
APA StyleLin, H., Zheng, Z., Yuan, J., Zhang, C., Cao, W., & Qin, X. (2021). Collagen Peptides Derived from Sipunculus nudus Accelerate Wound Healing. Molecules, 26(5), 1385. https://doi.org/10.3390/molecules26051385







