Removing Iron and Organic Substances from Water over the Course of Its Treatment with the Application of Average and Highly Alkaline Polyaluminium Chlorides
Abstract
1. Introduction
2. Results and Discussion
2.1. Water Samples
2.2. Removing Iron and Organic Substances from Water over the Course of Its Treatment
2.2.1. Aeration
2.2.2. Microfiltration
2.2.3. Coagulation
2.2.4. Filtration Through a Catalytic-Oxidative Bed
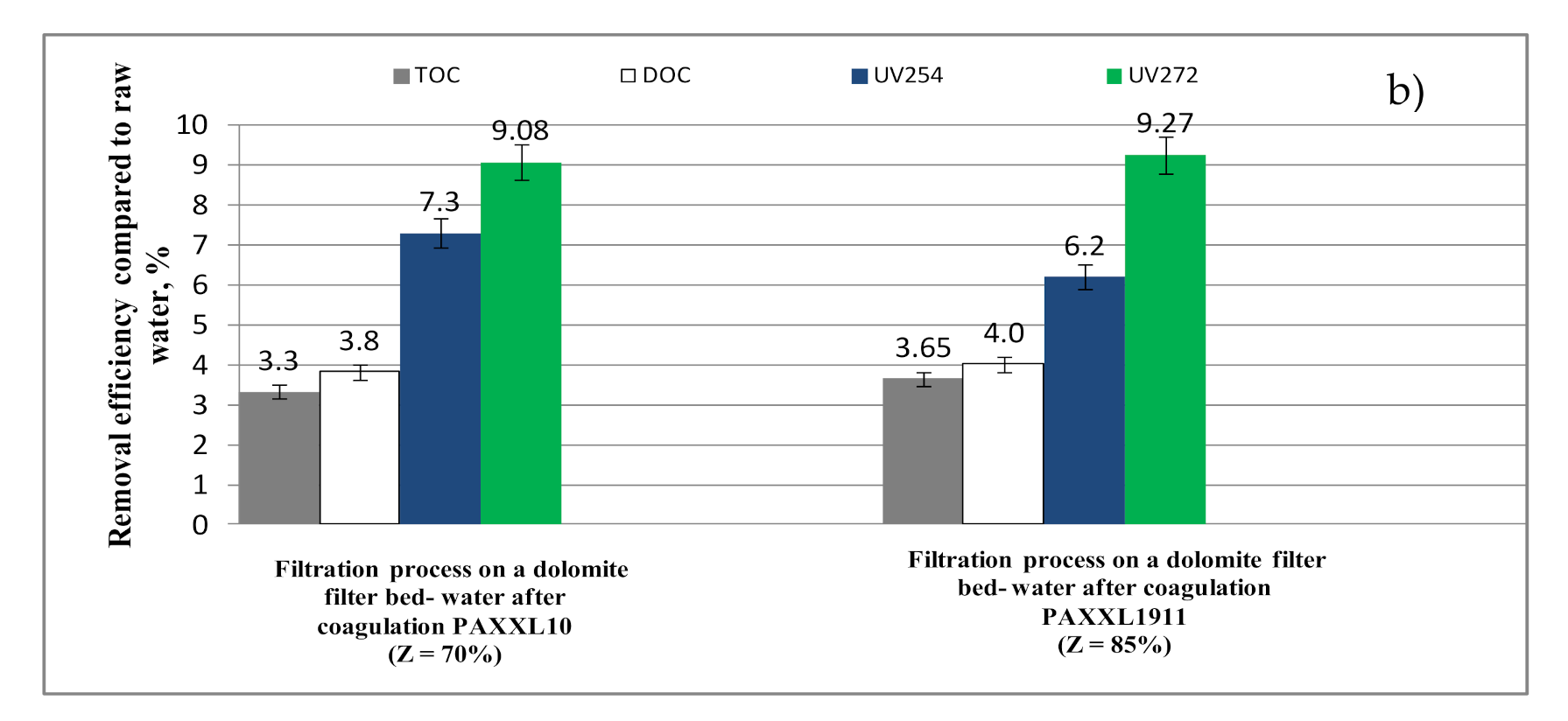
2.2.5. Filtration Through a Dolomite Bed
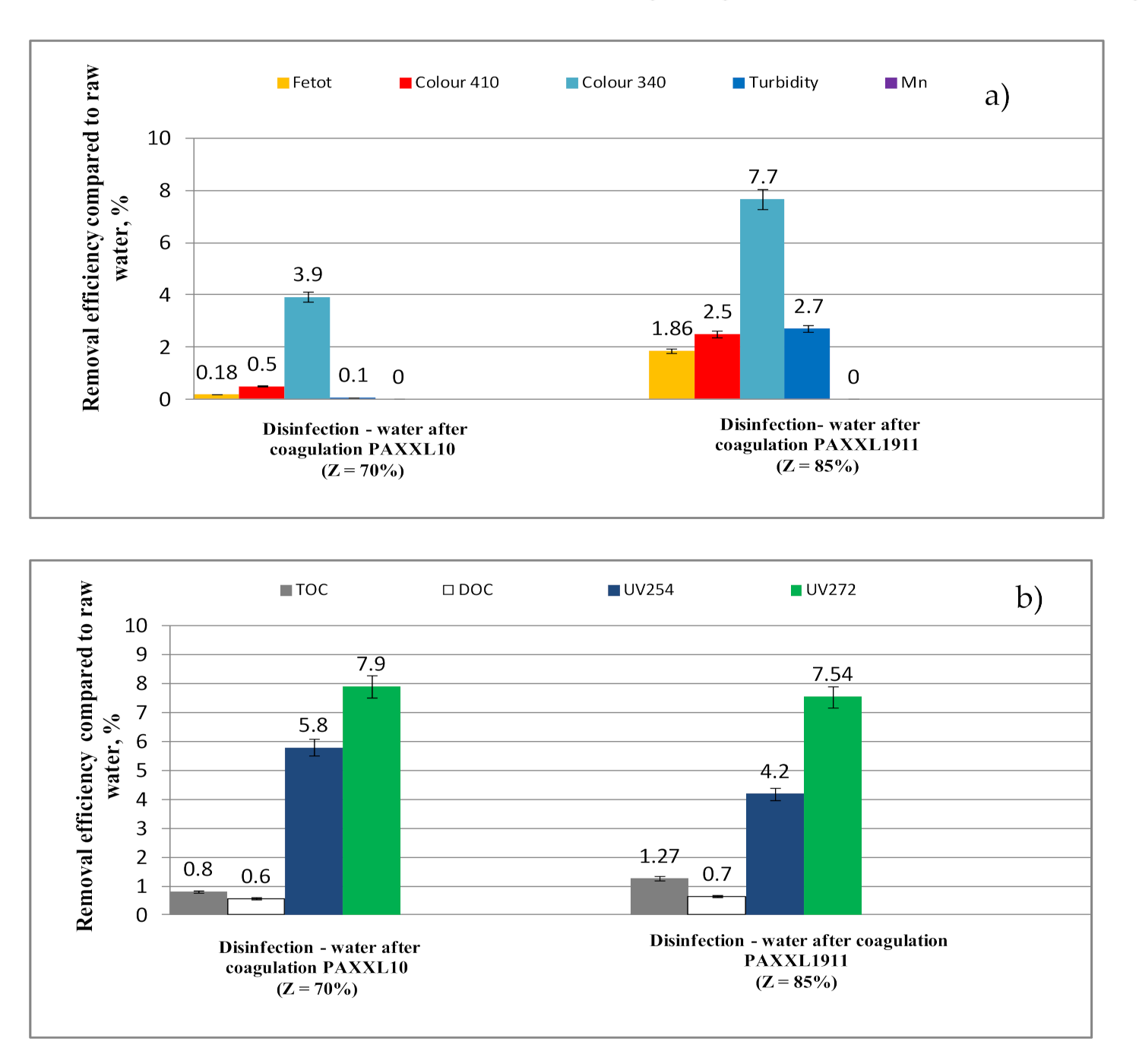
2.2.6. Disinfection
2.3. Jar Test
Removing Iron and Organic Substances from Water
3. Materials and Methods
3.1. Water Samples
3.2. Jar Test
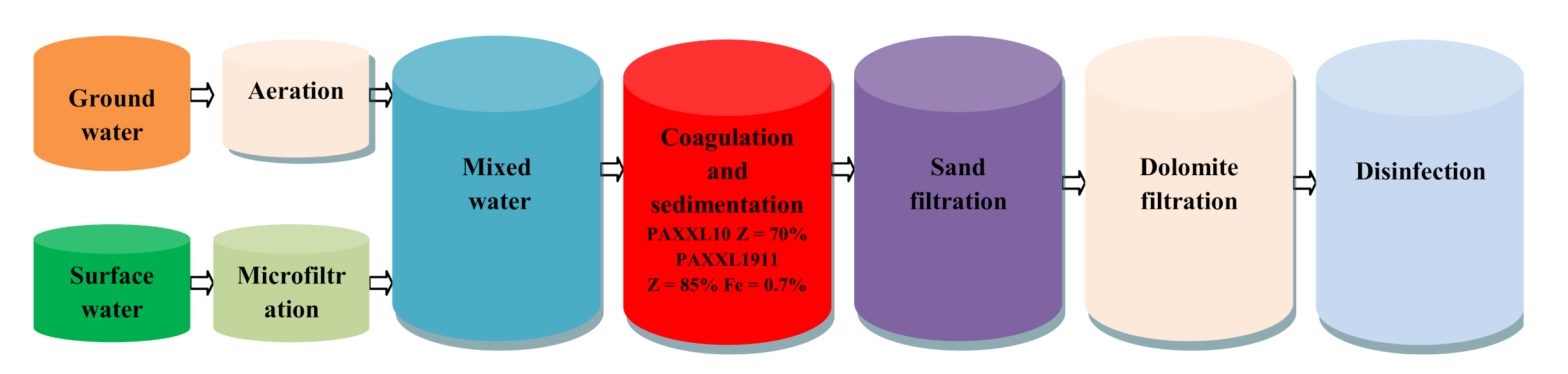
3.3. Characteristics of Water Treatment Technology
3.4. Analytical Methods
4. Conclusions
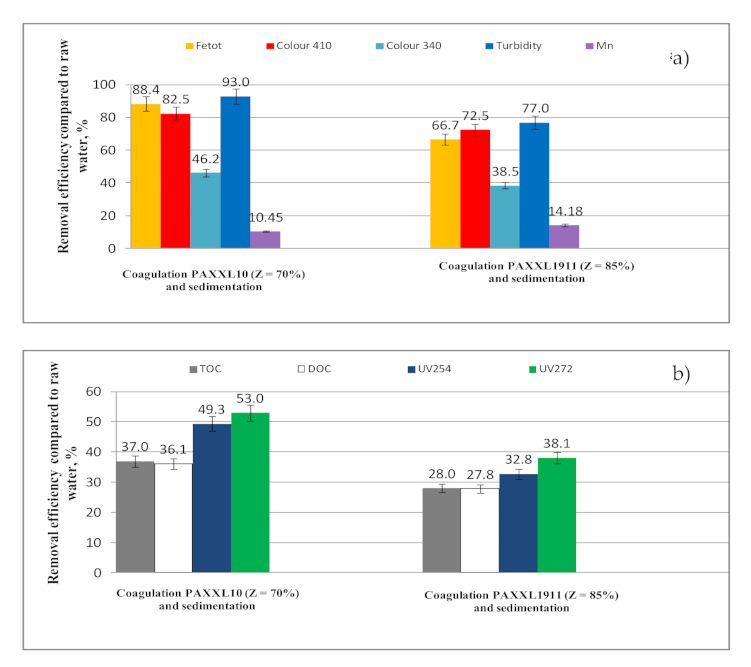
- Iron compounds, as well as organic substances, but also color and turbidity, were removed at the highest levels in the coagulation process, which also had a significant effect on the final effectiveness of water purification. The efficiency of iron removal during the coagulation process was 66.7 and 88.4%, and of organic substances: 28.0 and 37.0% (TOC), 27.8 and 36.1% (DOC).
- The effectiveness of purifying water in the coagulation process was determined by the type of tested polyaluminium chloride. Polyaluminium chloride PAXXL1911, which is characterized by a higher alkalinity and additionally contains iron ions in its composition, caused a significantly lower effectiveness of removing iron compounds (66.7%) and organic substances (28.0%-TOC, 27.8%-DOC), and also decreasing the color (72.5%-color at wavelength 410 nm; 38.5%-color at wavelength 340 nm), and turbidity (77.0%) as well as the zeta potential (23.0%), despite the fact that it contained higher amounts of polymer forms of aluminum (Alb = 40%) than PAXXL10 (Alb = 29%). The reason behind the lower effectiveness of water purification in the coagulation process upon applying PAXXL1911 was the formation of iron-organic connections, most likely in the form of protective colloids.
- The reason behind the formation of colored iron–organic connections during coagulation with the highly alkaline PAXXL1911 coagulant may have been the high pH of approx. 8, at which the functional groups of organic substances are more reactive in relation to the metal ions, especially to iron, and the introduction of additional iron ions along with the coagulant which may have reacted with the organic substances present in the purified water, contributing to the formation of additional iron–organic connections.
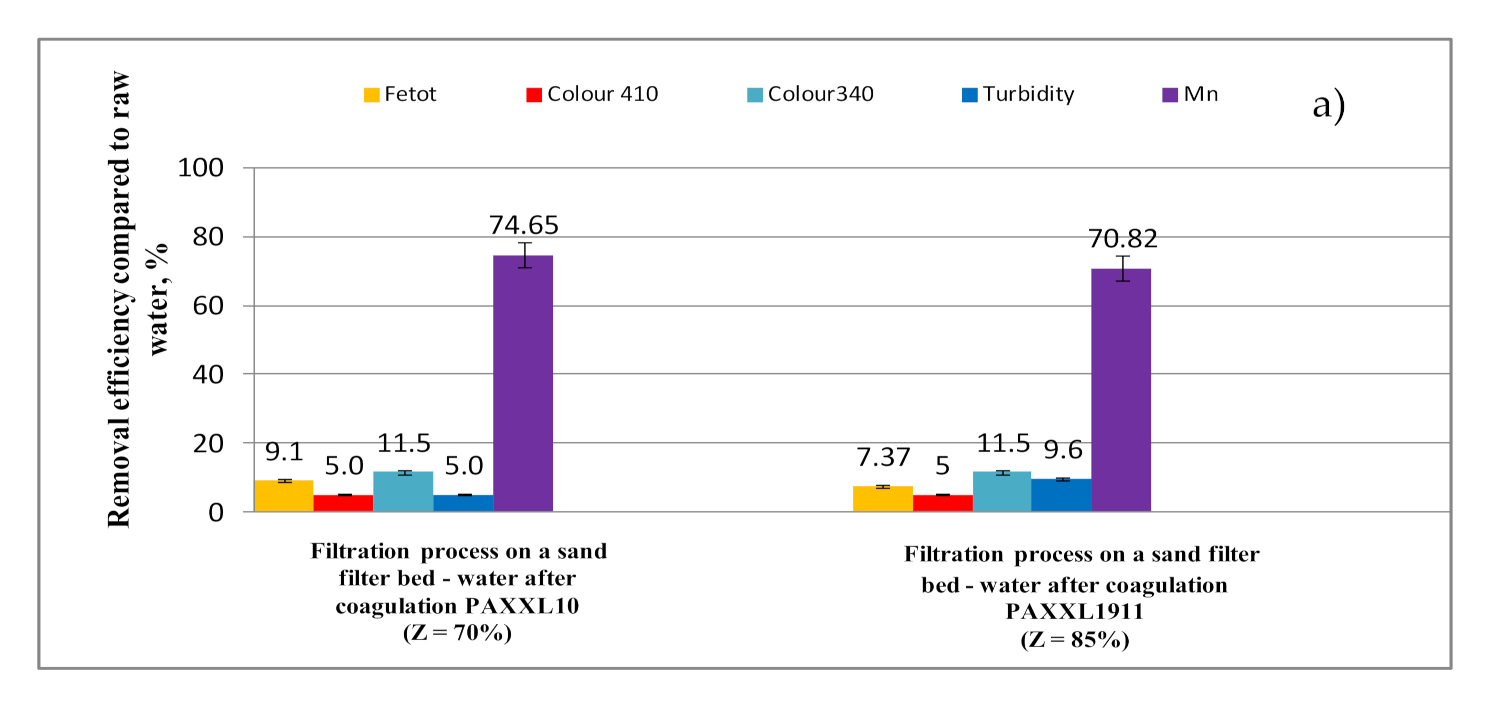
- Over the course of filtration on a catalytic-oxidative filter bed, the effectiveness of removing iron compounds (7.37 and 9.10%), as well as decreasing color (5.0%-color at wavelength 410 nm; 11.5%-color at wavelength 340 nm) turbidity (5.0 and 9.6%) and organic substances (4.57 and 5.00%-TOC; 3.00 and 3.80%-DOC) was also increased, though manganese compounds were removed to the largest extent (70.82 and 74.65%).
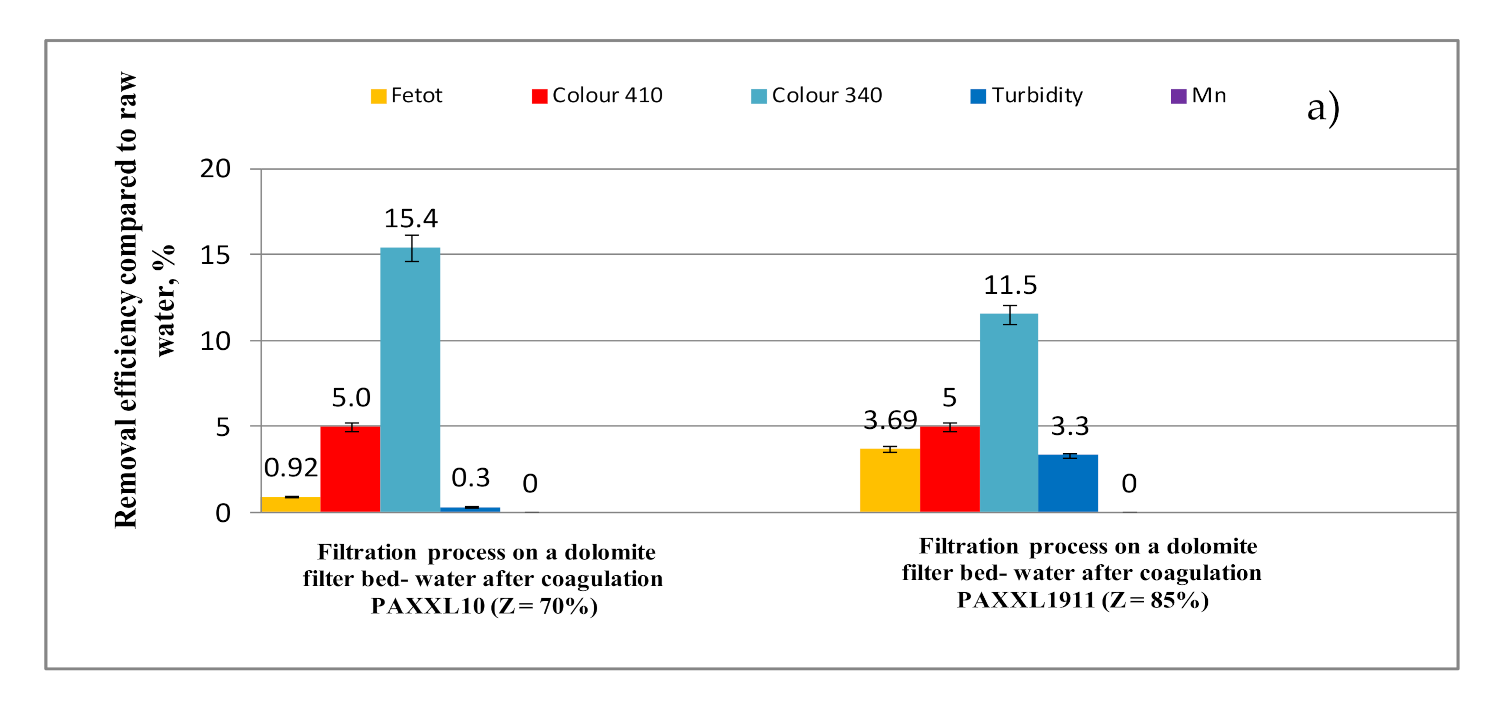
- The second level of filtration on a dolomite bed mainly increased the effectiveness of removing organic substances containing aromatic rings (6.20 and 7.30%-UV254; 9.08 and 9.27%-UV272), as well as lowering the color measured at a wavelength of 340 nm (11.5 and 15.4%), at which they absorb mainly organic substances containing chromophoric groups. The processes of removing organic substances from water as a result of filtration through a dolomite bed, and especially removing organic substances containing aromatic rings, is connected with the ability of these substances to create complexes with calcium and magnesium ions.
- The disinfection process using chlorine dioxide caused the partial oxidation of organic substances containing aromatic rings (4.20 and 5.80%-UV254; 7.54 and 7.90%-UV272). The analyses of the obtained results also showed that only after the disinfection process in water purified with highly-alkaline polyaluminium chloride (Z = 85%) containing iron compounds in its composition, water quality parameters of water intended for human consumption were not met due to iron (0.22 mgFe/L), turbidity (1.10 NTU) and total organic carbon (5.145 mgC/L), while the calculated value of the SUVA254 indicator was over 2, thus not fulfilling the production criterion for safe water, as a result of the presence of precursors of oxidation and disinfection by-products. Therefore, the use of highly alkaline polyaluminum chlorides, which additionally contain iron ions in their composition, should not be recommended for the treatment of water containing excessively high concentrations of organic substances, including humic substances, due to the possibility of creating colored iron-organic connections difficult to remove in the entire technological system.
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Wolska, M. Removal of precursors of chlorinated organic compounds in selected water treatment processes. Desalin. Water Treat. 2013, 52, 3938–3946. [Google Scholar] [CrossRef]
- Surbhi, T.; Bhanau Prakash, V. Natural organic matter as precursor to disinfection byproducts and its removal using con-ventional and advanced processes: State of the art review. J. Water Health 2018, 16, 681–703. [Google Scholar]
- Phuoc Dan, N.; Thanh Do Quang, L.; Nhat Huy, N.; Kim Thach, T.; Minh TriNguyen, K.; An, H. Reducing disinfection by-product precursors and chlorine consuming substances by a special integration of biofiltration and ozonation: A pilot study. J. Water Process Eng. 2020, 37, 101419. [Google Scholar]
- Krupińska, I.; Kowalczyk, W.; Szczepaniak, G. Effect of coexistence ratio of organic substances and total iron in groundwater on its treatment efficacy. Ochr. Sr. 2013, 35, 27–34. [Google Scholar]
- Krupińska, I. Impact of the Oxidant Type on the Efficienty of the Oxidation and Removal of Iron Compounds from Groundwater Containing Humic Substances. Molecules 2020, 25, 3380. [Google Scholar] [CrossRef]
- Krupińska, I. Effect of Organic Substances on the Efficiency of Fe(Ii) to Fe(Iii) Oxidation and Removal of Iron Compounds from Groundwater in the Sedimentation Process. Civ. Environ. Eng. Rep. 2017, 26, 15–29. [Google Scholar] [CrossRef]
- Krupińska, I.; Świderska-Bróż, M. Effect of the presence of organic substances on the extent of iron compound removal from water via oxidation and sedimentation processes. Ochr. Sr. 2008, 30, 3–7. [Google Scholar]
- Krupińska, I. Importance of Humic Substances for Methods of Groundwater Treatment. Pol. J. Soil Sci. 2016, 48, 161. [Google Scholar] [CrossRef]
- Albrektiene, R.; Rimeika, M.; Grazeniene, R. Organic fractions and metal-organic complexes in the groundwater [CD]. In Proceedings of the 9th International Conference, Environmental Engineering, Vilnius, Lithuania, 22–23 May 2014; pp. 1–7. [Google Scholar]
- Thurman, E.M. Organic Geochemistry of Natural Waters; Springer International Publishing: Dordrecht, The Netherlands, 1985. [Google Scholar]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry, 3rd ed.; John Wiley Sons Inc.: New York, NY, USA, 1996. [Google Scholar]
- Bond, T.; Goslan, E.; Parsons, S.; Jefferson, B. Treatment of disinfection by-product precursors. Environ. Technol. 2011, 32, 1–25. [Google Scholar] [CrossRef]
- Miao, H.; Tao, W. Ozonation of humic acid in water. J. Chem. Technol. Biotechnol. 2008, 83, 336–344. [Google Scholar] [CrossRef]
- Liu, H.J.; Liu, R.P.; Tian, C.; Jiang, H.; Liu, X.L.; Zhang, R.; Qu, J.H. Removal of natural organic matter for controlling disin-fection by-products formation by enhanced coagulation: A case study. Sep. Purif. Technol. 2012, 84, 41–45. [Google Scholar] [CrossRef]
- Chowdhury, S.; Champagne, P.; McLellan, P.J. Models for predicting disinfection byproduct (DBP) formation in drinking waters: A chronological review. Sci. Total. Environ. 2009, 407, 4189–4206. [Google Scholar] [CrossRef] [PubMed]
- Krupinska, I. Removal of natural organic matter from groundwater by coagulation using prehydrolysed and non-prehydrolysed coagulants. Desalin. Water Treat. 2018, 132, 244–252. [Google Scholar] [CrossRef]
- Nowacka, A.; Włodarczyk-Makuła, M.; Macherzyński, B. Comparison of effectiveness of coagulation with aluminum sulfate and pre-hydrolyzed aluminum coagulants. Desalin. Water Treat. 2013, 52, 3843–3851. [Google Scholar] [CrossRef]
- Duan, J.; Gregory, J. Coagulation by hydrolysing metal salts. Adv. Colloid Interface Sci. 2003, 100–102, 475–502. [Google Scholar] [CrossRef]
- Dafne, C.; Marcio, P.; Ana, R.; Wilson, C. Charge Neutralization Mechanism Efficiency in Water with High Color Turbidity Ratio Using Aluminium Sulfate and Flocculation Index. Water 2020, 12, 572. [Google Scholar]
- Park, M.; Kang, Y.-J.; Jang, J.-H.; Seo, J.-D.; Kim, J.; Paek, S.-M.; Lim, W.T.; Komarneni, S. Formation mechanism of an Al13 Keggin cluster in hydrated layered polysilicates. Dalton Trans. 2020, 49, 4920–4926. [Google Scholar] [CrossRef]
- Gumińska, J.; Kłos, M. Effect of polyaluminium chlorides overdosage on effectiveness of coagulation and filtration. Environ. Prot. Eng. 2015, 41, 5–14. [Google Scholar] [CrossRef]
- Du, P.; Li, X.; Yang, Y.; Fan, X.; Fang, X.; Zhou, Z. Enhanced coagulation by two-stage alum addition: The role of solution pH, floc breakage and assistant of non-ionic polyacrylamide. Environ. Technol. 2020, 41, 1–10. [Google Scholar] [CrossRef]
- Wang, D.; Luan, Z.; Tang, H. Differences in coagulation efficiencies between PACl and PICl. J. Am. Water Work. Assoc. 2003, 95, 79–86. [Google Scholar] [CrossRef]
- Wang, D.; Tang, H.; Gregory, J. Relative importance of charge neutralization and precipitation on coagulaation of Kaolin with PACl: Effect of sulfate ion. Environ. Sci. Technol. 2002, 36, 1815–1820. [Google Scholar] [CrossRef]
- Chen, Y.; Nakazawa, Y.; Matsui, Y.; Shirasaki, N.; Matsushita, T. Sulfate ion in raw water affects performance of high-basicity PACl coagulants produced by Al(OH)3 dissolution and base-titration: Removal of SPAC particles by coagula-tion-flocculation, sedimentation, and sand filtration. Water Res. 2020, 183, 116093. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Abdullah, S.R.S.; Imron, M.F.; Said, N.S.M.; Ismail, N.I.; Abu Hasan, H.; Othman, A.R.; Purwanti, I.F. Challenges and Opportunities of Biocoagulant/Bioflocculant Application for Drinking Water and Wastewater Treatment and Its Potential for Sludge Recovery. Int. J. Environ. Res. Public Health 2020, 17, 9312. [Google Scholar] [CrossRef] [PubMed]
- Matilainen, A.; Vepsäläinen, M.; Sillanpää, M. Natural organic matter removal by coagulation during drinking water treatment: A review. Adv. Colloid Interface 2010, 159, 189–197. [Google Scholar] [CrossRef]
- Kang, M.; Kamei, T.; Magara, Y. Comparing polyaluminum chloride and ferric chloride for antimony removal. Water Res. 2003, 37, 4171–4179. [Google Scholar] [CrossRef]
- Krupińska, I. The impact of the oxidising agent type and coagulant type on the effectiveness of coagulation in the removal of pollutants from underground water with an increased content of organic substances. J. Environ. Eng. Landsc. Manag. 2016, 24, 70–78. [Google Scholar] [CrossRef]
- Krupińska, I. Removal of iron and organic substances from groundwater in an alkaline medium. J. Environ. Eng. Landsc. Manag. 2019, 27, 12–21. [Google Scholar] [CrossRef]
- Machi, J.; Mołczan, J. Methods for natural organic matter characterization in water taken and treated for human consuption. Ochr. Sr. 2016, 38, 25–32. [Google Scholar]
- Szlachta, M.; Adamski, W. Assessing the efficiency of Natural Organic Matter (NOM) removal from water by coagulation. Ochr. Sr. 2008, 30, 9–11. [Google Scholar]
- International Standard, Water Quality—Examination and Determination of Colour. Technical Committee ISO/TC 147/SC 2 Physical, Chemical and Biochemical Methods: 2011; ISO 7887. Available online: https://www.iso.org/obp/ui/#iso:std:iso:7887:en (accessed on 9 February 2021).
- Aiken, G.; McKnight, D.; Thorn, K.; Thurman, E. Isolation of hydrophilic organic acids from water using nonionic macroporous resins. Org. Geochem. 1992, 18, 567–573. [Google Scholar] [CrossRef]
- Aiken, G.R.; Mcknight, D.M.; Wershaw, R.L.; Maccarthy, P. Humic Substances in Soil, Sediment and Water: Geochemistry, Isolation and Characterization; John Wiley Sons: New York, NY, USA, 1985. [Google Scholar]
- Rho, H.; Chon, K.; Park, J.; Cho, J. Rapid and Effective Isolation of Dissolved Organic Matter Using Solid-Phase Extraction Cartridges Packed with Amberlite XAD 8/4 Resins. Water 2019, 11, 67. [Google Scholar] [CrossRef]
- Kwakye-Awuah, B.; Sefa-Ntiri, B.; Von-Kiti, E.; Nkrumah, I.; Williams, C. Adsorptive Removal of Iron and Manganese from Groundwater Samples in Ghana by Zeolite Y Synthesized from Bauxite and Kaolin. Water 2019, 11, 1912. [Google Scholar] [CrossRef]
- Michel, M.; Reczek, L.; Papciak, D.; Włodarczyk-Makuła, M.; Siwiec, T.; Yuliia Trach, Y. Mineral Materials Coated with and Consisting of MnOx—Characteristics and Application of Filter Media for Groundwater Treatment: A Review. Materials 2020, 13, 2232. [Google Scholar] [CrossRef] [PubMed]
- Regulation of the Minister of Health dated December 7, 2017 amending the regulation on the quality of drinking water mean for human consumption. Available online: http://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20170002294 (accessed on 1 March 2021).
- Kutus, B.; Gaona, X.; Pallagi, A.; Pálinkó, I.; Altmaier, M.; PálSipos, P. Recent advances in the aqueous chemistry of the cal-cium(II)-gluconate system—Equilibria, structure and composition of the complexes forming in neutral and in alkaline solu-tions. Coord. Chem. Rev. 2020, 417, 213337. [Google Scholar] [CrossRef]
- Boguta, P.; Sokołowska, Z. Zinc Binding to Fulvic acids: Assessing the Impact of pH, Metal Concentrations and Chemical Properties of Fulvic Acids on the Mechanism and Stability of Formed Soluble Complexes. Molecules 2020, 25, 1297. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Wu, F.; Feng, W.; Tang, Z.; Giesy, J.P.; Guo, F.; Shi, D.; Liu, X.; Qin, N.; Xing, B.; et al. Fluorescence regional integra-tion and differential fluorescence spectroscopy for analysis of structural characteristics and proton binding properties of fulvic acid sub-fractions. J. Environ. Sci. 2018, 74, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Deborde, M.; von Gunten, U. Reactions of chlorine with inorganic and organic compounds during water treatment—Kinetics and mechanisms: A critical review. Water Res. 2008, 42, 13–51. [Google Scholar] [CrossRef] [PubMed]
- Świetlik, J.; Raczyk-Stanisławiak, U.; Nawrocki, J. The influence of disinfection on aquatic biodegradable organic carbon formation. Water Res. 2009, 43, 463–473. [Google Scholar] [CrossRef]
- Albrektienė, R.; Karaliūnas, K.; Bazienė, K. Sustainable Reuse of Groundwater Treatment Iron Sludge for Organic Matter Removal from River Neris Water. Sustainability 2019, 11, 639. [Google Scholar] [CrossRef]
- Albrektiene, R.; Rimeika, M.; Lubyte, E. The removal of iron-organic complexes from drinking water using coagulation process [CD]. In Proceedings of the 8th International Conference “Environmental Engineering”, Vilnius, Lithuania, 19–20 May 2011; pp. 509–512. [Google Scholar]
- Helal, A.A.; Murad, G. Characterization of different humic materials by various analytical techniques. Arab. J. Chem. 2011, 4, 51–54. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part A: Theory and Applications in Inorganic Chemistry, 6th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2009. [Google Scholar]
- Silverstein, R.; Webster, F.; Kiemle, D. Spektroskopowe Metody Identyfikacji Związków Organicznych. (Spectroscopic Methods for Identification of Organic Compounds); PWN: Warszawa, Poland, 2012. [Google Scholar]
- Jerzykiewicz, M. Formation of new radicals in humic acids upon interaction Pb(II) ions. Geoderma 2004, 122, 305–309. [Google Scholar] [CrossRef]
- Zhou, W.; Gao, B.; Yue, Q.; Liu, L.; Wang, Y. Al-Ferron kinetics and quantitative calculation of Al(III) species in polyalumi-num chloride coagulants. Colloid Surf. A 2006, 278, 235–240. [Google Scholar] [CrossRef]
- Manufacturer’s Specification (Coagulants: PAX XL10, PAXXL1911 were Produced by Kemipol in Police Poland). Available online: https://www.kemipol.com.pl/nowoczesne-technologie// (accessed on 1 March 2021).
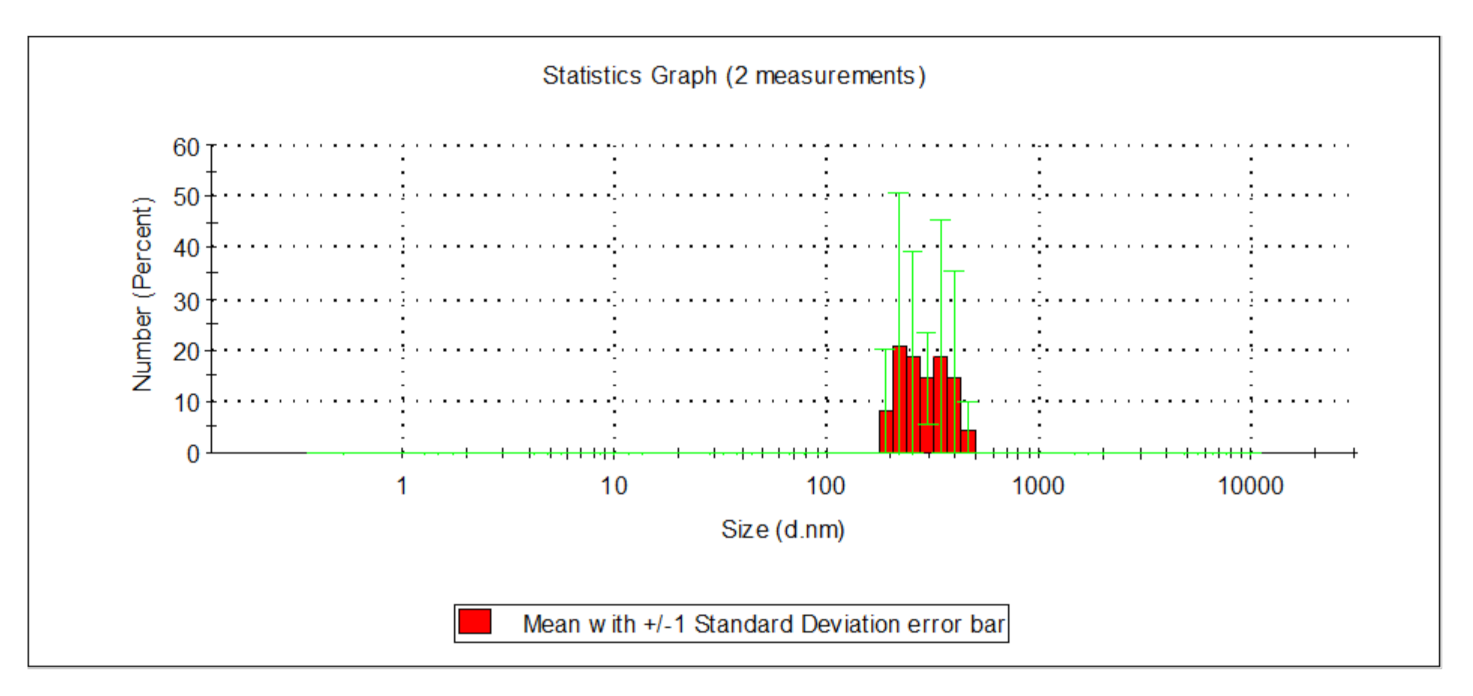
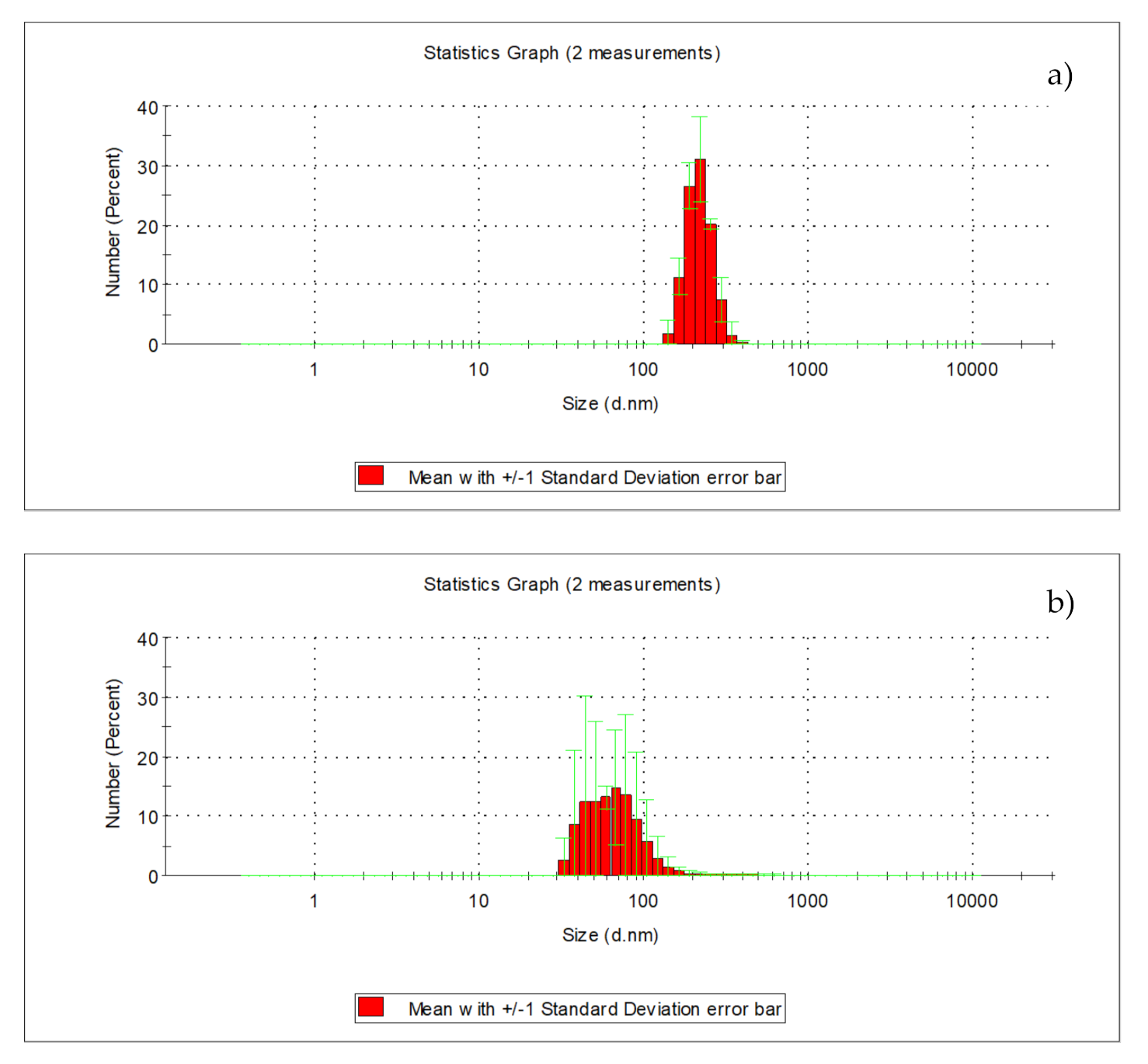
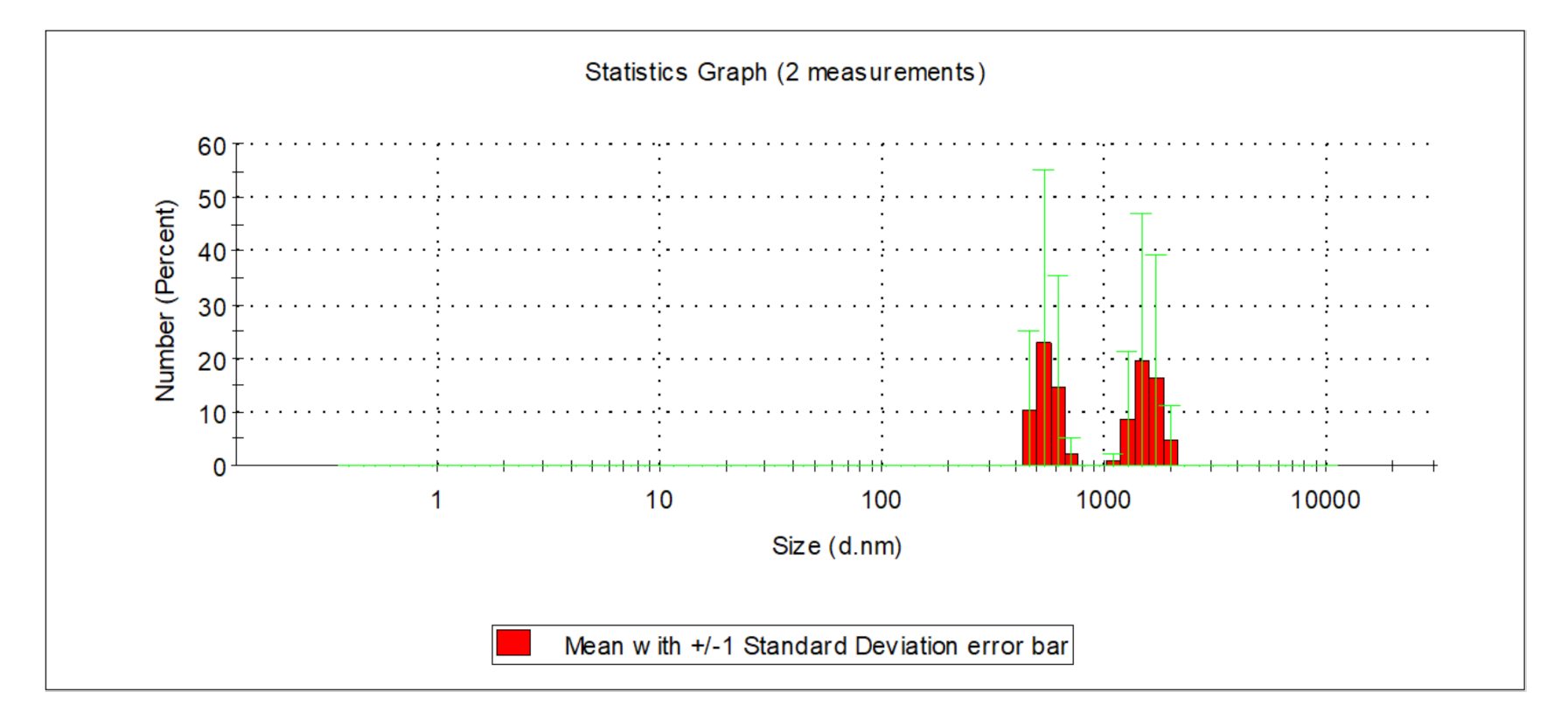
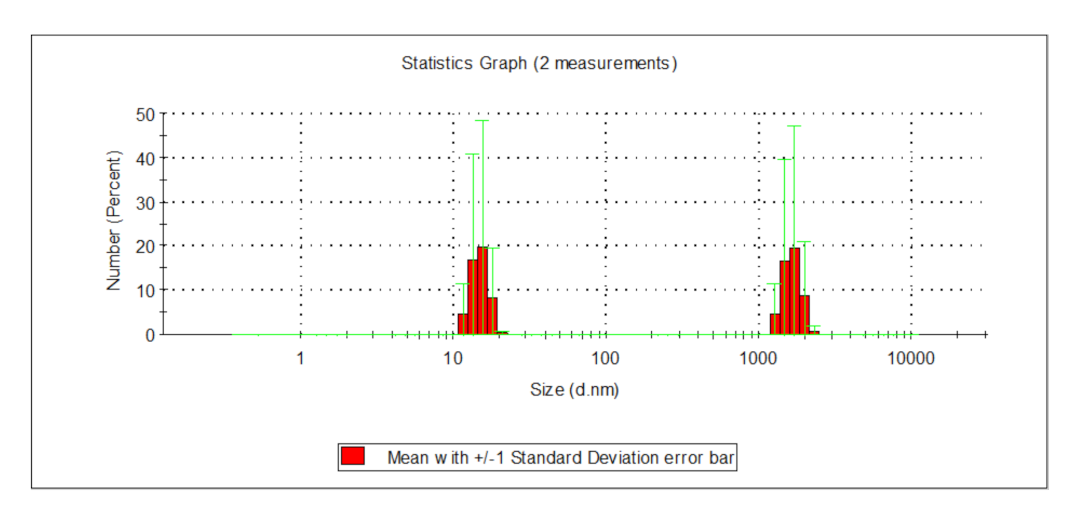

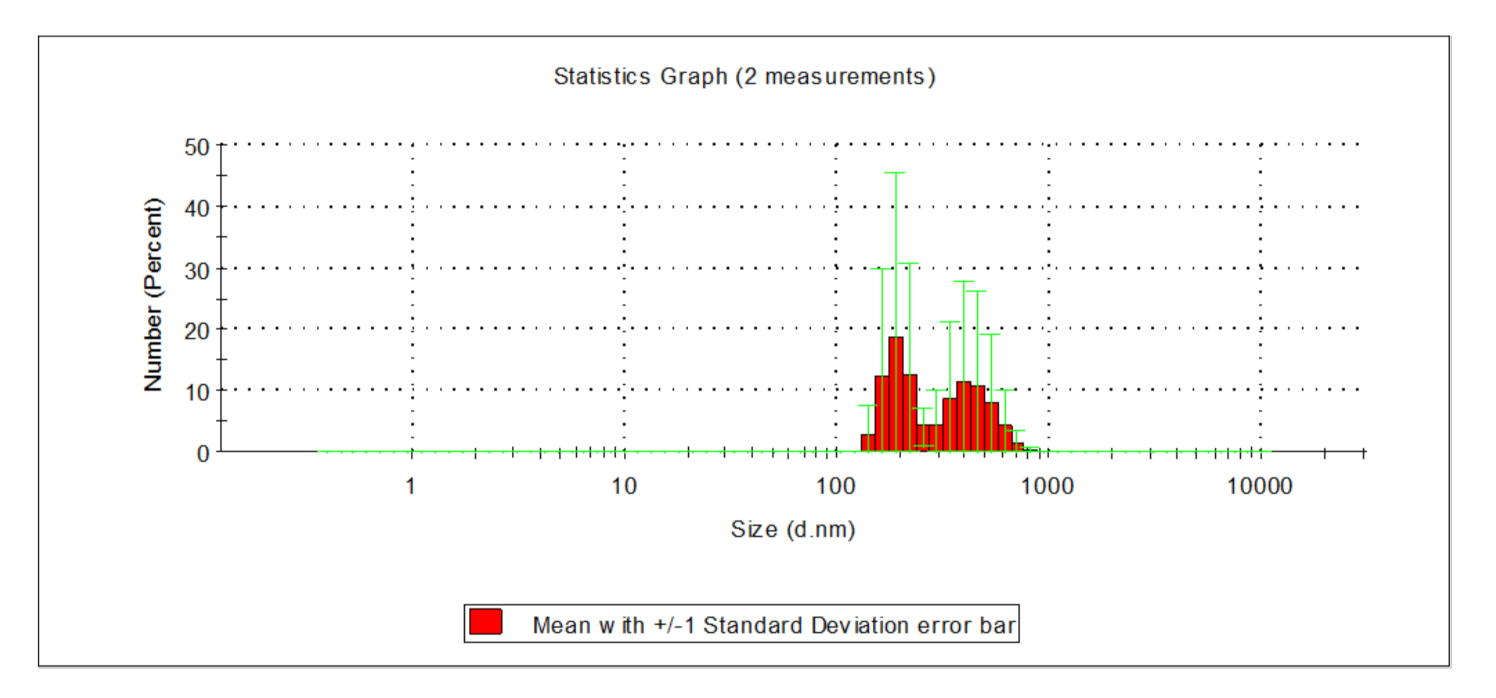
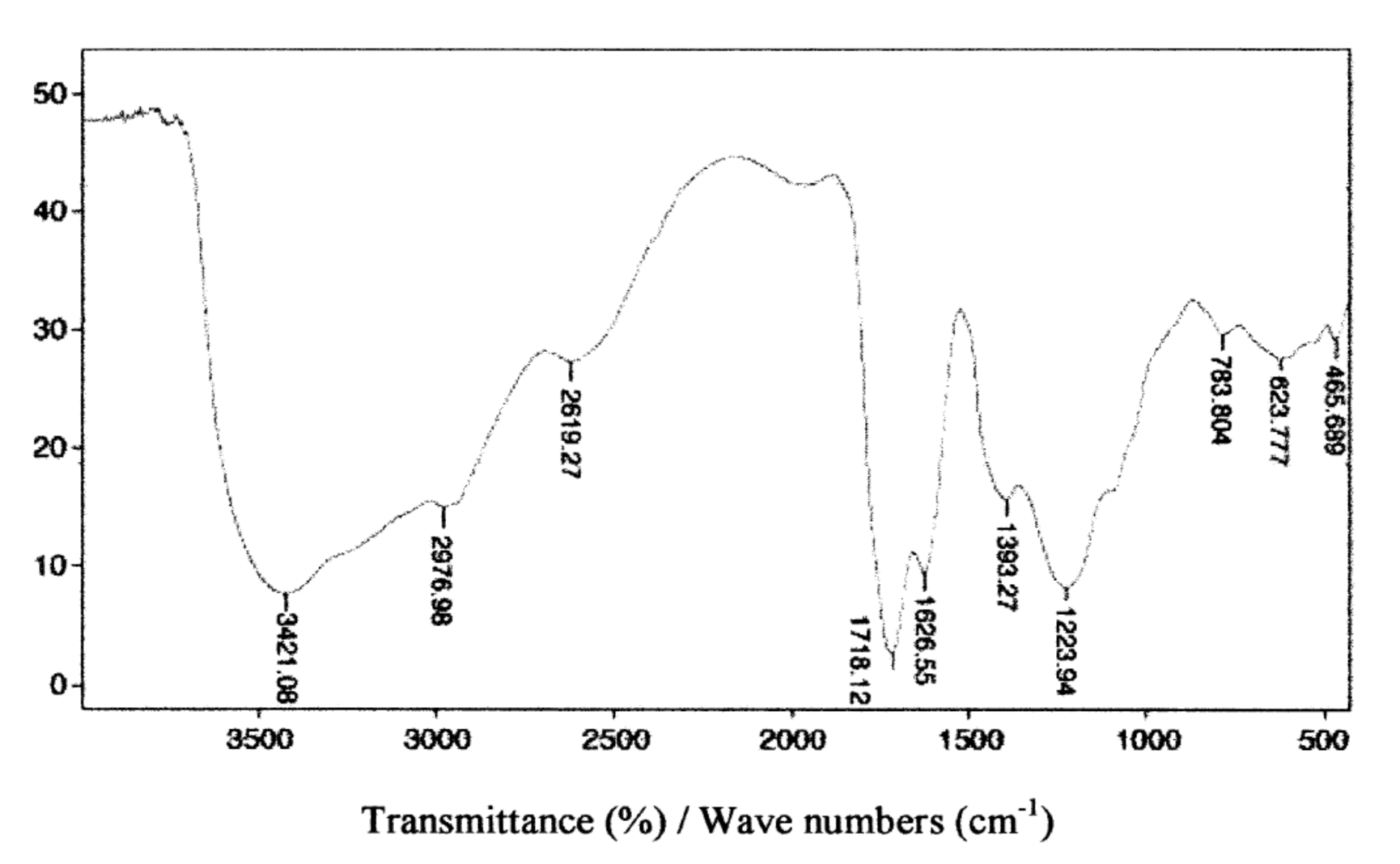
| Indicator | Unit | Value | ||||
|---|---|---|---|---|---|---|
| Groundwater | Groundwater Following Aeration | Surface Water | Surface Water Following Microfilters | Mixed Water | ||
| pH | - | 7.31 ± 0.1 | 7.70 ± 0.1 | 8.26 ± 0.1 | 8.30 ± 0.1 | 8.10 ± 0.1 |
| Alkalinity | mmol/L | 3.40 ± 0.05 | 3.40 ± 0.05 | 3.60 ± 0.05 | 3.60 ± 0.05 | 3.50 ± 0.05 |
| Turbidity | NTU | 15.00 ± 0.1 | 18.00 ± 0.1 | 2.10 ± 0.1 | 1.40 ± 0.1 | 15 ± 0.1 |
| Colour 340 nm | mgPt/L | 20 ± 1 | 22 ± 1 | 36 ± 1 | 35 ± 1 | 26 ± 1 |
| Colour 410 nm | mgPt/L | 23 ± 1 | 34 ± 1 | 48 ± 1 | 48 ± 1 | 40 ± 1 |
| TOC | mgC/L | 6.000 ± 0.05 | 6.000 ± 0.05 | 10.110 ± 0.05 | 9.468 ± 0.05 | 8.231 ± 0.05 |
| DOC | mgC/L | 5.800 ± 0.05 | 5.800 ± 0.05 | 9.431 ± 0.05 | 9.400 ± 0.05 | 7.827 ± 0.05 |
| UV254 | m−1 | 14.49 ± 0.200 | 14.49 ± 0.200 | 23.110 ± 0.200 | 22.570 ± 0.200 | 19.338 ± 0.200 |
| UV272 | m−1 | 12.94 ± 0.200 | 12.94 ± 0.200 | 19.060 ± 0.200 | 18.310 ± 0.200 | 16.165 ± 0.200 |
| Iron total | mgFe/L | 2.353 ± 0.100 | 2.353 ± 0.100 | 0.128 ± 0.100 | 0.090 ± 0.100 | 1.080 ± 0.100 |
| Iron (II) | mgFe/L | 1.900 ± 0.100 | 0.091 ± 0.100 | 0.078 ± 0.100 | 0.078 ± 0.100 | 0.085 ± 0.100 |
| Iron (III) | mgFe/L | 0.453 ± 0.100 | 2.262 ± 0.100 | 0.05 ± 0.010 | 0.012 ± 0.100 | 0.995 ± 0.010 |
| Iron (II)/Iron total | % | 81 | 4 | 61 | 87 | 8 |
| Elektrokinetic Potential | mV | −14.3 | −12.2 | −10.47 | −10.47 | −11.4 |
| Manganese | mgMn/L | 0.240 ± 0.020 | 0.240 ± 0.020 | 0.086 ± 0.020 | 0.063 ± 0.020 | 0.134 ± 0.020 |
| D = TOC/Iron total | mgC/mgFe | 2.549 | 2.549 | 78.984 | 105.2 | 7.621 |
| D’ = DOC/Iron total | mgC/mgFe | 2.465 | 2.465 | 73.679 | 104.4 | 7.247 |
| SUVA254 | m2/gC | 3.144 | 3.144 | 2.450 | 2.401 | 2.471 |
| Sampling Point | pH | Alkalinity mval/L | Turbidity NTU | Irontotal mg/L | Iron (II) mg/L | Mn mg/L | Al mg/L |
|---|---|---|---|---|---|---|---|
| Mixed water | 8.10 | 3.50 | 15.00 | 1.080 | 0.085 | 0.134 | 0 |
| After PAXXL10 coagulation and sedimentation | 7.56 | 3.40 | 1.05 | 0.125 | 0.008 | 0.120 | 0.362 |
| After PAXXL1911 coagulation and sedimentation | 7.80 | 3.50 | 3.43 | 0.360 | 0.017 | 0.115 | 0.172 |
| After the filtration process on a catalytic-oxidative sand filter bed (PAXXL10 coagulation) | 7.57 | 3.35 | 0.30 | 0.027 | 0.006 | 0.02 | 0.036 |
| After the filtration process on a catalytic-oxidative sand filter bed (PAXXL1911 coagulation) | 7.85 | 3.40 | 2.00 | 0.280 | 0.015 | 0.02 | 0.029 |
| After the filtration process on a dolomite filter bed (PAXXL10 coagulation) | 7.75 | 3.45 | 0.25 | 0.017 | 0.005 | 0.02 | 0.020 |
| After the filtration process on a dolomite filter bed (PAXXL1911 coagulation) | 7.95 | 3.50 | 1.50 | 0.240 | 0.015 | 0.02 | 0.020 |
| After disinfection (PAXXL10 coagulation) | 7.65 | 3.45 | 0.24 | 0.015 | 0.005 | 0.02 | 0.020 |
| After disinfection (PAXXL1911 coagulation) | 7.75 | 3.50 | 1.10 | 0.220 | 0.013 | 0.02 | 0.020 |
| Sampling Point | TOC mg/L | DOC mg/L | UV254 m−1 | UV272 m−1 | Colour 410 nm | Colour 340 nm | Zeta Potential mV | SUVA254 m2/gC |
|---|---|---|---|---|---|---|---|---|
| Mixed water | 8.231 | 7.827 | 19.338 | 16.165 | 40 | 26 | −11.4 | 2.471 |
| After PAXXL10 coagulation and sedimentation | 5.185 | 5.000 | 9.800 | 7.600 | 7 | 14 | −5.30 | 1.960 |
| After PAXXL1911 coagulation and sedimentation | 5.926 | 5.650 | 13.00 | 10.00 | 11 | 16 | −8.77 | 2.300 |
| After the filtration process on a catalytic-oxidative sand filter bed (PAXXL10 coagulation) | 4.774 | 4.700 | 8.900 | 6.900 | 5 | 11 | −5.00 | 1.840 |
| After the filtration process on a catalytic-oxidative sand filter bed (PAXXL1911 coagulation) | 5.550 | 5.415 | 12.40 | 9.500 | 9 | 13 | −8.77 | 2.290 |
| After the filtration process on a dolomite filter bed (PAXXL10 coagulation) | 4.500 | 4.400 | 7.50 | 5.430 | 3 | 7 | −4.50 | 1.700 |
| After the filtration process on a dolomite filter bed (PAXXL1911 coagulation) | 5.250 | 5.100 | 11.20 | 8.000 | 7 | 10 | −7.00 | 2.190 |
| After disinfection (PAXXL10 coagulation) | 4.432 | 4.356 | 6.900 | 4.154 | 2 | 6 | −3.50 | 1.580 |
| After disinfection (PAXXL1911 coagulation) | 5.145 | 5.049 | 10.39 | 7.380 | 6 | 8 | −6.00 | 2.060 |
| Linear Correlation Equation | Coefficient of the Pearson Correlation (R) |
|---|---|
| Fe(III) = 0.0206 UV254 − 0.1334 Fe(III) = 0.0523 DOC − 0.1554 Fe(III) = 0.0474TOC − 0.1500 Turbidity = 9.9331Fe(III) + 1.2595 Turbidity = 0.2431UV254 − 0.8480 | 0.9389 0.9225 0.8936 0.9489 0.8526 |
| Turbidity = 0.613DOC − 1.0858 Turbidity = 0.5509TOC − 0.9885 Colour410 = 74.105Fe(III) +34.041 Colour340 = 4.156TOC − 5.8122 Colour340 = 4.4747DOC − 5.6988 Colour340 = 1.7273UV254 − 3.3383 | 0.8322 0.7982 0.8834 0.9743 0.9829 0.9802 |
| Indicator | Type of Coagulant | |
|---|---|---|
| PAXXL10 | PAXXL1911 | |
| Alkalinity ratio, [OH−]/[Al3+] | 2.10 | 2.55 |
| Alkalinity, % | 70 | 85 |
| Al3+, % | 5.0 | 11.5 |
| Fetot, % | - | 0.7 |
| Cl−, % | 11.5 | 7.0 |
| Monomeric Al species (Ala), % | 22.4 | 14.3 |
| Polymerized Al species (Alb), % | 29.0 | 40.0 |
| Colloidal Al species (Alc), % | 48.6 | 45.7 |
| Sample Number No. | Water Sample Collection Points |
|---|---|
| 1 | Groundwater |
| 2 | Groundwater, following aeration |
| 3 | Surface water from the Obrzyca River |
| 4 | Surface water, following microfilters |
| 5 | Mixed water—groundwater after aeration and surface water from the Obrzyca River after the microstraining process, mixed in a volume ratio of 1:2 |
| 6 | After PAX XL10 coagulation and sedimentation |
| 7 | After PAX XL1911 coagulation and sedimentation |
| 8 | After the filtration process on a catalytic-oxidative sand filter bed |
| 9 | After the filtration process on a dolomite filter bed |
| 10 | After disinfection with chlorine dioxide-water directed to the network |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krupińska, I. Removing Iron and Organic Substances from Water over the Course of Its Treatment with the Application of Average and Highly Alkaline Polyaluminium Chlorides. Molecules 2021, 26, 1367. https://doi.org/10.3390/molecules26051367
Krupińska I. Removing Iron and Organic Substances from Water over the Course of Its Treatment with the Application of Average and Highly Alkaline Polyaluminium Chlorides. Molecules. 2021; 26(5):1367. https://doi.org/10.3390/molecules26051367
Chicago/Turabian StyleKrupińska, Izabela. 2021. "Removing Iron and Organic Substances from Water over the Course of Its Treatment with the Application of Average and Highly Alkaline Polyaluminium Chlorides" Molecules 26, no. 5: 1367. https://doi.org/10.3390/molecules26051367
APA StyleKrupińska, I. (2021). Removing Iron and Organic Substances from Water over the Course of Its Treatment with the Application of Average and Highly Alkaline Polyaluminium Chlorides. Molecules, 26(5), 1367. https://doi.org/10.3390/molecules26051367







