On the Proton-Bound Noble Gas Dimers (Ng-H-Ng)+ and (Ng-H-Ng’)+ (Ng, Ng’ = He-Xe): Relationships between Structure, Stability, and Bonding Character
Abstract
1. Introduction
2. Method of Bonding Analysis
3. Computational Details
4. Results and Discussion
4.1. The NgH+ and the Homonuclear (Ng-H-Ng)+ (Ng = He-Xe)
4.2. The Heteronuclear (Ng-H-Ng’)+ (Ng = He-Xe)
4.3. An Overview of the (Ng-H-Ng’)+ (Ng = Ng’ or Ng ≠ Ng’)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Barlow, M.J.; Swinyard, B.M.; Owen, P.J.; Cernicharo, J.; Gomez, H.L.; Ivison, R.J.; Krause, O.; Lim, T.L.; Matsuura, M.; Miller, S.; et al. Detection of a Noble Gas Molecular Ion, 36ArH+, in the Crab Nebula. Science 2013, 342, 1343–1345. [Google Scholar] [CrossRef] [PubMed]
- Schilke, P.; Neufeld, D.A.; Müller, H.S.P.; Comito, C.; Bergin, E.A.; Lis, D.C.; Gerin, M.; Black, J.H.; Wolfire, M.; Indriolo, N.; et al. Ubiquitous Argonium (ArH+) in the Diffuse Interstellar Medium: A Molecular Tracer of almost Purely Atomic Gas. Astron. Astrophys. 2014, 566, A29. [Google Scholar] [CrossRef]
- Müller, H.S.P.; Muller, S.; Schilke, P.; Bergin, E.A.; Black, J.H.; Gerin, M.; Lis, D.C.; Neufeld, D.A.; Suri, S. Detection of Extragalactic Argonium, ArH+, toward PKS 1830-211. Astron. Astrophys. 2015, 582, L4. [Google Scholar] [CrossRef]
- Güsten, R.; Wiesemeyer, H.; Neufeld, D.; Menten, K.M.; Graf, U.U.; Jacobs, K.; Klein, B.; Ricken, O.; Risacher, C.; Stutzki, J. Astrophysical Detection of the Helium Hydride Ion HeH+. Nature 2019, 568, 357–362. [Google Scholar]
- Grandinetti, F. Noble Gas Chemistry, Structure, Bonding, and Gas-Phase Chemistry; Wiley-VCH: Weinheim, Germany, 2018; pp. 203–258. [Google Scholar]
- Fortenberry, R.C. The Case for Gas-phase Astrochemistry without Carbon. Mol. Astrophys. 2020, 18, 100062. [Google Scholar] [CrossRef]
- Das, A.; Sil, M.; Bhat, B.; Gorai, P.; Chakrabarti, S.K.; Caselli, P. Exploring the Possibility of Identifying Hydride and Hydroxyl Cations of Noble Gas Species in the Crab Nebula Filament. Astrophys. J. 2020, 902, 131. [Google Scholar] [CrossRef]
- Fehsenfeld, F.C.; Schmeltekopf, A.L.; Ferguson, E.E. Thermal—Energy Ion-Neutral Reaction Rates. VII. Some Hydrogen—Atom Abstraction Reactions. J. Chem. Phys. 1967, 46, 2802–2808. [Google Scholar] [CrossRef]
- Adams, N.G.; Bohme, D.K.; Ferguson, E.E. Reactions of He2+, Ne2+, Ar2+, and Rare—Gas Hydride Ions with Hydrogen at 200 °K. J. Chem. Phys. 1970, 52, 5101–5105. [Google Scholar] [CrossRef]
- Collins, C.B.; Lee, F.W. Measurement of the Rate Coefficients for the Bimolecular and Termolecular Ion-Molecule Reactions of He2+ with Selected Atomic and Molecular Species. J. Chem. Phys. 1978, 68, 1391–1401. [Google Scholar] [CrossRef]
- Collins, C.B.; Lee, F.W. Measurement of the Rate Coefficients for the Bimolecular and Termolecular Ion-Molecule Reactions of Ar2+ with Selected Atomic and Molecular Species. J. Chem. Phys. 1979, 71, 184–191. [Google Scholar] [CrossRef]
- Rakshit, A.B.; Warneck, P. A Drift Chamber Study of the Reaction ArH+ + H2→ H3+ + Ar and Related Reactions. J. Chem. Phys. 1981, 74, 2853–2859. [Google Scholar] [CrossRef]
- Raksit, A.B. Reactions of Ar2+ Ions with Neutral Molecules. Int. J. Mass Spectrom. Ion Processes 1985, 66, 109–119. [Google Scholar] [CrossRef]
- Shul, R.J.; Passarella, R.; Upschulte, B.L.; Keesee, R.G.; Castleman, A.W., Jr. Thermal Energy Reactions Involving Ar+ Monomer and Dimer with N2, H2, Xe, and Kr. J. Chem. Phys. 1987, 86, 4446–4451. [Google Scholar] [CrossRef]
- Bedford, D.K.; Smith, D. Variable-Temperature Selected Ion Flow Tube Studies of the Reactions of Ar+, Ar2+ and ArHn+ (n = 1-3) ions with H2, HD and D2 at 300 K and 80 K. Int. J. Mass Spectrom. Ion Processes 1990, 98, 179–190. [Google Scholar] [CrossRef]
- Hvistendahl, G.; Saastad, O.W.; Uggerud, E. Ion/Molecule Reactions in a Mixture of Ar and H2: High Pressure Mass Spectrometry and Quantum Chemical Calculations. Int. J. Mass Spectrom. Ion Processes 1990, 98, 167–177. [Google Scholar] [CrossRef]
- Kojima, T.M.; Kobayashi, N.; Kaneko, Y. Formation of Helium Cluster Ions HHex+ (x ≤ 14) and H3Hex+ (x ≤ 13) in a Very Low Temperature Drift Tube. Z. Phys. D Atoms Mol. Clust. 1992, 23, 181–185. [Google Scholar] [CrossRef]
- Fárník, M.; Toennies, J.P. Ion-Molecule Reactions in 4He Droplets: Flying Nano-Cryo-Reactors. J. Chem. Phys. 2005, 122, 014307. [Google Scholar] [CrossRef]
- Bartl, P.; Leidlmair, C.; Denifl, S.; Scheier, P.; Echt, O. Cationic Complexes of Hydrogen with Helium. ChemPhysChem 2013, 14, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Gatchell, M.; Martini, P.; Kranabetter, L.; Rasul, B.; Scheier, P. Magic Sizes of Cationic and Protonated Argon Clusters. Phys. Rev. A 2018, 98, 022519. [Google Scholar] [CrossRef]
- Gatchell, M.; Martini, P.; Schiller, A.; Scheier, P. Protonated Clusters of Neon and Krypton. J. Am. Soc. Mass Spectrom. 2019, 30, 2632–2636. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, L.; Bartl, P.; Leidlmar, C.; Scheier, P.; Gatchell, M. Protonated and Cationic Helium Clusters. Molecules 2020, 25, 1066. [Google Scholar] [CrossRef]
- Császár, A.G.; Szidarovszky, T.; Asvany, O.; Schlemmer, S. Fingerprints of Microscopic Superfluidity in HHen+ Clusters. Mol. Phys. 2019, 117, 1559–1583. [Google Scholar] [CrossRef]
- McDonald, D.C., II; Mauney, D.T.; Leicht, D.; Marks, J.H.; Tan, J.A.; Kuo, J.-L.; Duncan, M.A. Communication: Trapping a Proton in Argon: Spectroscopy and Theory of the Proton-Bound Argon Dimer and its Solvation. J. Chem. Phys. 2016, 145, 231101. [Google Scholar] [CrossRef]
- Asvany, O.; Schlemmer, S.; Szidarovszky, T.; Császár, A.G. Infrared Signatures of the HHen+ and DHen+ (n = 3–6) Complexes. J. Phys. Chem. Lett. 2019, 10, 5325–5330. [Google Scholar] [CrossRef] [PubMed]
- Töpfer, M.; Jensen, A.; Nagamori, K.; Kohguchi, H.; Szidarovsky, T.; Császár, A.G.; Schlemmer, S.; Asvany, O. Spectroscopic Signatures of HHe2+ and HHe3+. Phys. Chem. Chem. Phys. 2020, 22, 22885–22888. [Google Scholar] [CrossRef] [PubMed]
- Bondybey, V.E.; Pimentel, G.C. Infrared Absorptions of Interstitial Hydrogen Atoms in Solid Argon and Krypton. J. Chem. Phys. 1972, 56, 3832–3836. [Google Scholar] [CrossRef]
- Milligan, D.E.; Jacox, M.E. Infrared Spectroscopic Evidence for the Stabilization of HArn+ in Solid Argon at 14 K. J. Mol. Spec. 1973, 46, 460–469. [Google Scholar] [CrossRef]
- Wight, C.A.; Ault, B.S.; Andrews, L. On Microwave Discharge Sources of New Chemical Species for Matrix—Isolation Spectroscopy and the Identification of Charged Species. J. Chem. Phys. 1976, 65, 1244. [Google Scholar] [CrossRef]
- Kunttu, H.; Seetula, J.; Räsänen, M.; Apkarian, V.A. Photogeneration of Ions via Delocalized Charge Transfer States. I. Xe2H+ and Xe2D+ in Solid Xe. J. Chem. Phys. 1992, 96, 5630–5635. [Google Scholar] [CrossRef]
- Kunttu, H.M.; Seetula, J.A. Photogeneration of Ionic Species in Ar, Kr and Xe Matrices Doped with HCl, HBr and HI. Chem. Phys. 1994, 189, 273–292. [Google Scholar] [CrossRef]
- Fridgen, T.D.; Parnis, J.M. Electron Bombardment Matrix Isolation of Rg/Rg’/Methanol Mixtures (Rg = Ar, Kr, Xe): Fourier-transform Infrared Characterization of the Proton-Bound Dimers Kr2H+, Xe2H+, (ArHKr)+ and (ArHXe)+ in Ar Matrices and (KrHXe)+ and Xe2H+ in Kr Matrices. J. Chem. Phys. 1998, 109, 2155–2161. [Google Scholar] [CrossRef]
- Lundell, J.; Pettersson, M.; Räsänen, M. The Proton-Bound Rare Gas Compounds (RgHRg’)+ (Rg = Ar, Kr, Xe)—A Computational Approach. Phys. Chem. Chem. Phys. 1999, 1, 4151–4155. [Google Scholar] [CrossRef]
- Tsuge, M.; Kalinowski, J.; Gerber, R.B.; Lee, Y.-P. Infrared Identification of Proton-Bound Rare-Gas Dimers (XeHXe)+, (KrHKr)+, and (KrHXe)+ and their Deuterated Species in Solid Hydrogen. J. Phys. Chem. A 2015, 119, 2651–2660. [Google Scholar] [CrossRef]
- Poshusta, R.D.; Haugen, J.A.; Zetik, D.F. Ab Initio Predictions for Very Small Ions. J. Chem. Phys. 1969, 51, 3343–3351. [Google Scholar] [CrossRef]
- Poshusta, R.D.; Siems, W.F. Ab Initio Calculations on He2H+. J. Chem. Phys. 1971, 55, 1995–1996. [Google Scholar] [CrossRef]
- Milleur, M.B.; Matcha, R.L.; Hayes, E.F. Theoretical Studies of Hydrogen—Rare Gas Complexes: HenH and HenH+ Clusters. J. Chem. Phys. 1974, 60, 674–679. [Google Scholar] [CrossRef]
- Dykstra, C.E. The strong Hydrogen Bond in HeHHe+ and its Weak Counterpart in HeH3+. J. Mol. Struct. 1983, 103, 131–138. [Google Scholar] [CrossRef]
- Lee, J.S.; Secrest, D.A. Calculation of the Rotation-Vibration States of He2H+. J. Chem. Phys. 1986, 85, 6565–6575. [Google Scholar] [CrossRef]
- Kim, S.T.; Lee, J.S. Ab Initio Study of He2H+ and Ne2H+: Accurate Structure and Energetics. J. Chem. Phys. 1999, 110, 4413–4418. [Google Scholar] [CrossRef]
- Panda, A.N.; Sathyamurthy, N. Bound and Quasibound States of He2H+ and He2D+. J. Phys. Chem. A 2003, 107, 7125–7131. [Google Scholar] [CrossRef]
- Liang, J.-J.; Yang, C.-L.; Wang, L.-Z.; Zhang, Q.-G. A New Analytical Potential Energy Surface for the Singlet State of He2H+. J. Chem. Phys. 2012, 136, 094307. [Google Scholar] [CrossRef] [PubMed]
- Stephan, C.J.; Fortenberry, R.C. The Interstellar Formation and Spectra of the Noble Gas, Proton-Bound HeHHe+, HeHNe+ and HeHAr+ Complexes. Mon. Not. R. Astron. Soc. 2017, 469, 339–346. [Google Scholar] [CrossRef]
- Fortenberry, R.C.; Wiesenfeld, L. A Molecular Candle Where Few Molecules Shine: HeHHe+. Molecules 2020, 25, 2183. [Google Scholar] [CrossRef] [PubMed]
- Matcha, R.L.; Milleur, M.B.; Meier, P.F. Theoretical Studies of Hydrogen Rare Gas Complexes. II. NenH and NenH+ Clusters. J. Chem. Phys. 1978, 68, 4748–4751. [Google Scholar] [CrossRef]
- Matcha, R.L.; Milleur, M.B. Theoretical Studies of Hydroegn Rare Gas Complexes. III. ArnH and ArnH+ Clusters. J. Chem. Phys. 1978, 69, 3016–3024. [Google Scholar] [CrossRef]
- Rosenkrantz, M.E. Ab Initio Study of ArH, ArH+, Ar2H, Ar2H+, and Ar4H+. Chem. Phys. Lett. 1990, 173, 378–383. [Google Scholar] [CrossRef]
- Lundell, J.; Kunttu, H. Structure, Spectra, and Stability of Ar2H+, Kr2H+, and Xe2H+: An Effective Core Potential Approach. J. Phys. Chem. 1992, 96, 9774–9781. [Google Scholar] [CrossRef]
- Nieminen, J.; Kauppi, E. Potential Energy Surface and Vibrational Analysis along the Stretching Vibrations of the ArHAr+ Ion. Chem. Phys. Lett. 1994, 217, 31–35. [Google Scholar] [CrossRef]
- Giju, K.T.; Roszak, S.; Leszczynski, J. A Theoretical Study of Protonated Argon Clusters: ArnH+ (n = 1-7). J. Chem. Phys. 2002, 117, 4803–4809. [Google Scholar] [CrossRef]
- Qu, J.Y.; Li, W.; Guo, R.; Zhao, X.S. A Global Potential Energy Surface of Ar2H+ Based on Ab Initio Calculations. J. Chem. Phys. 2002, 117, 2592–2598. [Google Scholar] [CrossRef]
- Ritschel, T.; Zülicke, L.; Kuntz, P.J. Cationic Van-der-Waals Complexes: Theoretical Study of Ar2H+ Structure and Stability. Z. Phys. Chem. 2004, 218, 377–390. [Google Scholar] [CrossRef]
- Ritschel, T.; Kuntz, P.J.; Zülicke, L. Structure and Dynamics of Cationic Van-der-Waals Clusters. I. Binding and Structure of Protonated Argon Clusters. Eur. Phys. J. D 2005, 33, 421–432. [Google Scholar] [CrossRef]
- Ritschel, T.; Kuntz, P.J.; Zülicke, L. Structure and Dynamics of Cationic van-der-Waals Clusters. II. Dynamics of Protonated Argon Clusters. Eur. Phys. J. D 2007, 41, 127–141. [Google Scholar] [CrossRef]
- Fortenberry, R.C. Rovibrational Characterization and Interstellar Implications of the Proton-Bound, Noble Gas Complexes: ArHAr+, NeHNe+, and ArHNe+. ACS Earth Space Chem. 2017, 1, 60–69. [Google Scholar] [CrossRef]
- Tan, J.A.; Kuo, J.-L. A Theoretical Study on the Infrared Signatures of Proton-Bound Rare Gas Dimers (Rg–H+–Rg), Rg = {Ne, Ar, Kr, and Xe}. J. Chem. Phys. 2019, 150, 124305. [Google Scholar] [CrossRef]
- Tan, J.A.; Kuo, J.-L. Structure and Vibrational Spectra of ArnH+ (n = 2-3). J. Phys. Chem. A 2020, 124, 7726–7734. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, J.; Kauppi, E.; Lundell, J.; Kunttu, H. Potential Energy Surface and Vibrational Analysis along the Stretching Vibrations of XeHXe+ Ion. J. Chem. Phys. 1993, 98, 8698–8703. [Google Scholar] [CrossRef]
- Moncada, F.; Uribe, L.S.; Romero, J.; Reyes, A. Hydrogen Isotope Effects on Covalent and Noncovalent Interactions: The Case of Protonated Rare Gas Clusters. Int. J. Quantum Chem. 2013, 113, 1556–1561. [Google Scholar] [CrossRef]
- Grabowski, S.J.; Ugalde, J.M.; Andrada, D.M.; Frenking, G. Comparison of Hydrogen and Gold Bonding in [XHX]−, [XAuX]−, and Isoelectronic [NgHNg]+, [NgAuNg]+ (X = Halogen, Ng = Noble Gas). Chem. Eur. J. 2016, 22, 11317–11328. [Google Scholar] [CrossRef]
- Lundell, J. Density Functional Approach on Ground State RgH+ and RgHRg+ (Rg = Ar, Kr, Xe) Ions. J. Mol. Struct. 1995, 355, 291–297. [Google Scholar] [CrossRef]
- Fridgen, T.D.; Parnis, J.M. Density Functional Theory Study of the Proton-Bound Rare-Gas Dimers Rg2H+ and (RgHRg’)+ (Rg = Ar, Kr, Xe): Interpretation of Experimental Matrix Isolation Infrared Data. J. Chem. Phys. 1998, 109, 2162–2168. [Google Scholar] [CrossRef]
- Beyer, M.; Lammers, A.; Savchenko, E.V.; Niedner-Schatteburg, G.; Bondybey, V.E. Proton Solvated by Noble-Gas Atoms: Simplest Case of a Solvated Ion. Phys. Chem. Chem. Phys. 1999, 1, 2213–2221. [Google Scholar] [CrossRef]
- Matcha, R.L.; Pettitt, B.M.; Meier, P.; Pendegast, P. Potential Energy Surface for the Collinear Reaction of Ne and HeH+. J. Chem. Phys. 1978, 69, 2264–2265. [Google Scholar] [CrossRef]
- Koner, D.; Vats, A.; Vashishta, M.; Panda, A.N. Ab Initio Electronic Structure Investigation of Protonated Mixed Rare Gas Dimers [NeHHe]+, [ArHHe]+ and [ArHNe]+. Comput. Theor. Chem. 2012, 1000, 19–25. [Google Scholar] [CrossRef]
- Koner, D.; Panda, A.N. Quantum Dynamical Study of the He + NeH+ Reaction on a New Analytical Potential Energy Surface. J. Phys. Chem. A 2013, 117, 13070–13078. [Google Scholar] [CrossRef] [PubMed]
- Koner, D.; Barrios, L.; González-Lezana, T.; Panda, A.N. Wave Packet and Statistical Quantum Calculations for the He + NeH+→HeH+ + Ne Reaction on the Ground Electronic State. J. Chem. Phys. 2014, 141, 114302. [Google Scholar] [CrossRef]
- Koner, D.; Barrios, L.; González-Lezana, T.; Panda, A.N. Quantum, Statistical, and Quasiclassical Trajectory Studies For the Ne + HeH+→NeH+ + He Reaction on the Ground Electronic State. J. Phys. Chem. A 2015, 119, 12052–12061. [Google Scholar] [CrossRef] [PubMed]
- Koner, D.; Barrios, L.; González-Lezana, T.; Panda, A.N. State-to-State Dynamics of the Ne + HeH+ (v = 0, j = 0) →NeH+ (v’, j’) + He Reaction. J. Phys. Chem. A 2016, 120, 4731–4741. [Google Scholar] [CrossRef] [PubMed]
- Bop, C.T.; Hammami, K.; Faye, N.A.B. Collisional Rates Based on the First Potential Energy Surface of the NeH+−He System. Mon. Not. R. Astron. Soc. 2017, 470, 2911–2917. [Google Scholar] [CrossRef]
- Dallas, J.; Flint, A.; Fortenberry, R.C. Solvation of HeH+ in Neon Atoms: Proton-Bound Complexes of Mixed He and Ne. Chem. Phys. 2020, 539, 110927. [Google Scholar] [CrossRef]
- Bop, C.T.; Hammami, K.; Niane, A.; Faye, N.A.B.; Jaïdane, N. Rotational Excitation of 36ArH+ by He at Low Temperature. Mon. Not. R. Astron. Soc. 2017, 465, 1137–1143. [Google Scholar] [CrossRef]
- García-Vázquez, R.M.; Márquez-Mijares, M.; Rubayo-Soneira, J.; Denis-Alpizar, O. Relaxation of ArH+ by Collision with He: Isotopic Effects. Astron. Astrophys. 2019, 631, A86. [Google Scholar] [CrossRef]
- Linstrom, P.J.; Mallard, W.G. (Eds.) NIST Chemistry WebBook, NIST Standard Reference Database Number 69; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1998. [Google Scholar] [CrossRef]
- Borocci, S.; Giordani, M.; Grandinetti, F. Bonding Motifs of Noble-Gas Compounds as Described by the Local Electron Energy Density. J. Phys. Chem. A 2015, 119, 6528–6541. [Google Scholar] [CrossRef] [PubMed]
- Borocci, S.; Grandinetti, F.; Sanna, N.; Antoniotti, P.; Nunzi, F. Non-Covalent Complexes of the Noble-Gas Atoms: Analyzing the Transition from Physical to Chemical Interactions. J. Comput. Chem. 2019, 40, 2318–2328. [Google Scholar] [CrossRef] [PubMed]
- Borocci, S.; Grandinetti, F.; Sanna, N.; Nunzi, F. Classifying the Chemical Bonds Involving the Noble-Gas Atoms. New J. Chem. 2020, 44, 14536–14550. [Google Scholar] [CrossRef]
- Cremer, D.; Kraka, E. Chemical Bonds without Bonding Electron Density—Does the Difference Electron-Density Analysis Suffice for a Description of the Chemical Bond? Angew. Chem. Int. Ed. Engl. 1984, 23, 627–628. [Google Scholar] [CrossRef]
- Cremer, D.; Kraka, E. A Description of the Chemical Bond in Terms of Local Properties of Electron Density and Energy. Croat. Chem. Acta 1984, 57, 1259–1281. [Google Scholar]
- Johnson, E.R.; Keinan, S.; Mori-Sanchez, P.; Contreras-Garcia, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Narth, C.; Maroun, Z.; Boto, R.A.; Chaudret, R.; Bonnet, M.L.; Piquemal, J.-P.; Contreras-García, J. A Complete NCI Perspective: From New Bonds to Reactivity. In Applications of Topological Methods in Molecular Chemistry; Springer: Cham, Switzerland, 2016; pp. 491–527. [Google Scholar]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Espinosa, E.; Alkorta, E.; Elguero, J.; Molins, E. From Weak to Strong Interactions: A Comprehensive Analysis of the Topological and Energetic Properties of the Electron Density Distribution Involving XH···F-Y Systems. J. Chem. Phys. 2002, 117, 5529–5542. [Google Scholar] [CrossRef]
- Raghavachari, K.; Trucks, G.W.; Pople, J.A.; Head-Gordon, M. A Fifth-Order Perturbation Comparison of Electron Correlation Theories. Chem. Phys. Lett. 1989, 157, 479–483. [Google Scholar] [CrossRef]
- Pritchard, B.P.; Altarawy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. New Basis Set Exchange: An Open, Up-To-Date Resource for the Molecular Sciences Community. J. Chem. Inf. Model. 2019, 59, 4814–4820. Available online: https://www.basissetexchange.org (accessed on 1 October 2020). [CrossRef]
- Peterson, K.A.; Figgen, D.; Goll, E.; Stoll, H.; Dolg, M. Systematically Convergent Basis Sets with Relativistic Pseudopotentials. II. Small-Core Pseudopotentials and Correlation Consistent Basis Sets for the post-d group 16-18 elements. J. Chem. Phys. 2003, 119, 11113–11123. [Google Scholar] [CrossRef]
- Halkier, A.; Helgaker, T.; Jørgensen, P.; Klopper, W.; Koch, H.; Olsen, J.; Wilson, A.K. Basis-Set Convergence in Correlated Calculations on Ne, N2, and H2O. Chem. Phys. Lett. 1998, 286, 243–252. [Google Scholar] [CrossRef]
- Stanton, J.F.; Gauss, J.; Harding, M.E.; Szalay, P.G.; Auer, A.A.; Bartlett, R.J.; Benedikt, U.; Berger, C.; Bernholdt, D.E.; Bom-ble, Y.J.; et al. CFOUR, a Quantum Chemical Program Package and the Integral Packages Molecule, and ECP Routines. Available online: http://www.cfour.de (accessed on 1 October 2020).
- Saleh, G.; Gatti, C.; Lo Presti, L. Energetics of Non-Covalent Interactions from Electron and Energy Density Distributions. Comput. Theor. Chem. 2015, 1053, 53–59. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Zou, W. Molden2AIM. Available online: https://github.com/zorkzou/Molden2AIM (accessed on 1 October 2020).
- Stärck, J.; Meyer, W. Ab Initio Potential Energy Surface for the Collisional System H− + H2 and Properties of its van der Waals Complexes. Chem. Phys. 1993, 176, 83–95. [Google Scholar] [CrossRef]
- Wang, W.; Belyaev, A.K.; Xu, Y.; Zhu, A.; Xiao, C.; Yang, X.-F. Observations of H3− and D3− from Dielectric Barrier Discharge Plasmas. Chem. Phys. Lett. 2003, 377, 512–518. [Google Scholar] [CrossRef]
- Braïda, B.; Hiberty, P.C. Application of the Valence Bond Mixing Configuration Diagrams to Hypervalency in Trihalide Anions: A Challenge to the Rundle-Pimentel Model. J. Phys. Chem. A 2008, 112, 13045–13052. [Google Scholar] [CrossRef]
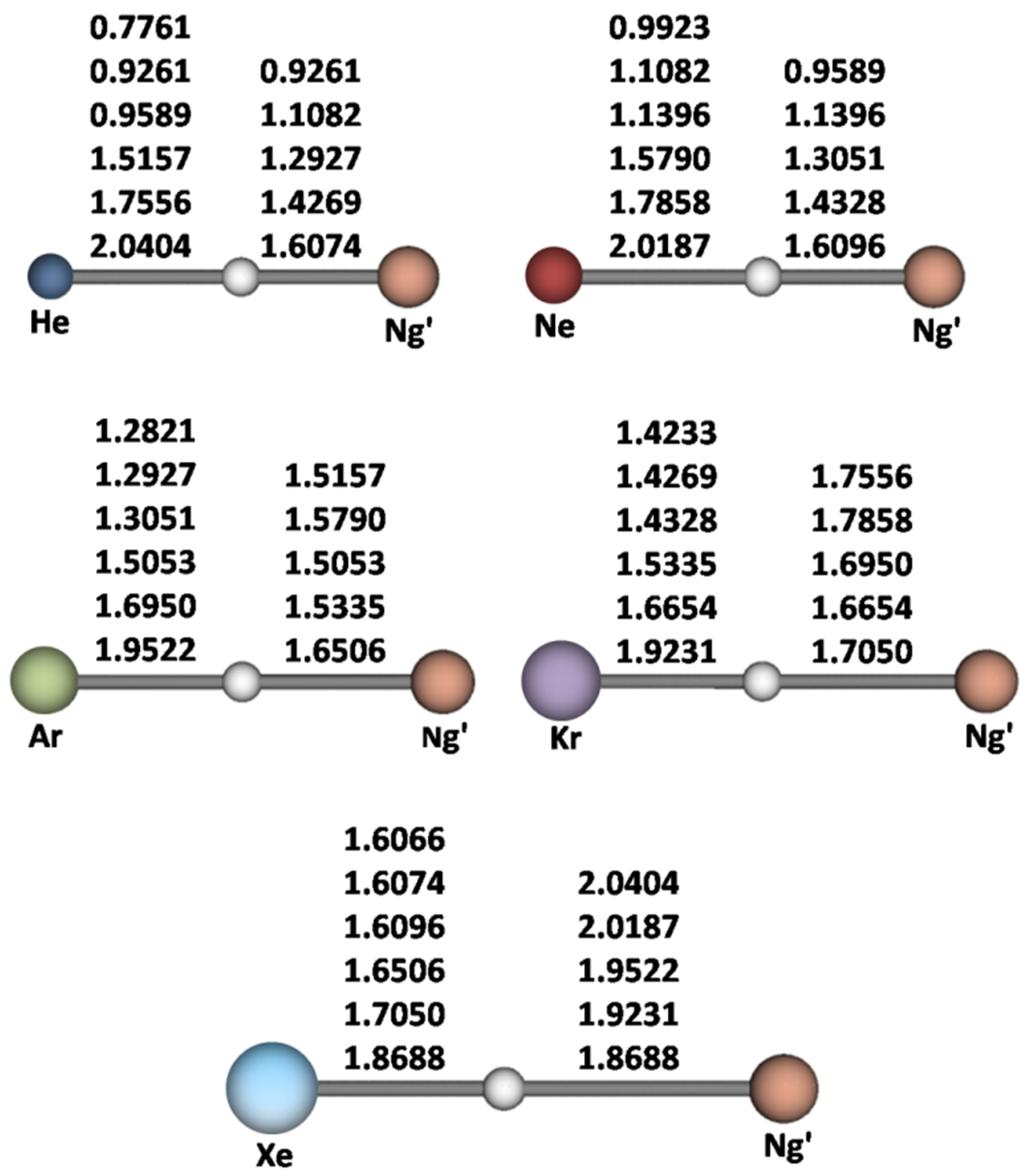
| (Ng-H-Ng’)+ | R(Ng-H) | R(Ng’-H) | ν11 | ν22 | ν33 | Ng’H+ + Ng 4 | NgH+ + Ng’ 5 | Ng + Ng’ + H+ 6 |
|---|---|---|---|---|---|---|---|---|
| (He-H-He)+ | 0.9261 | 0.9261 | 1138 | 958 | 1559 | 13.2 | 13.2 | 60.3 |
| (He-H-Ne)+ | 0.9589 | 1.1082 | 853 | 882 | 1645 | 11.9 | 17.6 | 64.7 |
| (He-H-Ar)+ | 1.5157 | 1.2927 | 234 | 333 | 2569 | 2.08 | 48.6 | 95.7 |
| (He-H-Kr)+ | 1.7556 | 1.4269 | 159 | 193 | 2482 | 1.18 | 59.1 | 106.2 |
| (He-H-Xe)+ | 2.0404 | 1.6074 | 109 | 102 | 2291 | 0.60 | 73.8 | 120.8 |
| (Ne-H-Ne)+ | 1.1396 | 1.1396 | 519 | 850 | 1616 | 15.8 | 15.8 | 68.5 |
| (Ne-H-Ar)+ | 1.5790 | 1.3051 | 169 | 431 | 2399 | 3.83 | 44.6 | 97.4 |
| (Ne-H-Kr)+ | 1.7858 | 1.4328 | 110 | 268 | 2408 | 2.36 | 54.6 | 107.4 |
| (Ne-H-Xe)+ | 2.0187 | 1.6096 | 81 | 188 | 2271 | 1.25 | 68.7 | 121.4 |
| (Ar-H-Ar)+ | 1.5053 | 1.5053 | 322 | 705 | 956 | 15.5 | 15.5 | 109.0 |
| (Ar-H-Kr)+ | 1.6950 | 1.5335 | 185 | 594 | 1390 | 10.4 | 21.8 | 115.4 |
| (Ar-H-Xe)+ | 1.9522 | 1.6506 | 112 | 395 | 1849 | 5.70 | 32.4 | 125.9 |
| (Kr-H-Kr)+ | 1.6654 | 1.6654 | 206 | 639 | 869 | 15.2 | 15.2 | 120.2 |
| (Kr-H-Xe)+ | 1.9231 | 1.7050 | 113 | 506 | 1410 | 8.68 | 23.9 | 128.9 |
| (Xe-H-Xe)+ | 1.8688 | 1.8688 | 150 | 575 | 846 | 14.4 | 14.4 | 134.6 |
| HeH+ | 0.7761 | 3206 | 47.1 | |||||
| NeH+ | 0.9923 | 2946 | 52.8 | |||||
| ArH+ | 1.2821 | 2729 | 93.6 | |||||
| KrH+ | 1.4233 | 2528 | 105.0 | |||||
| XeH+ | 1.6066 | 2297 | 120.2 |
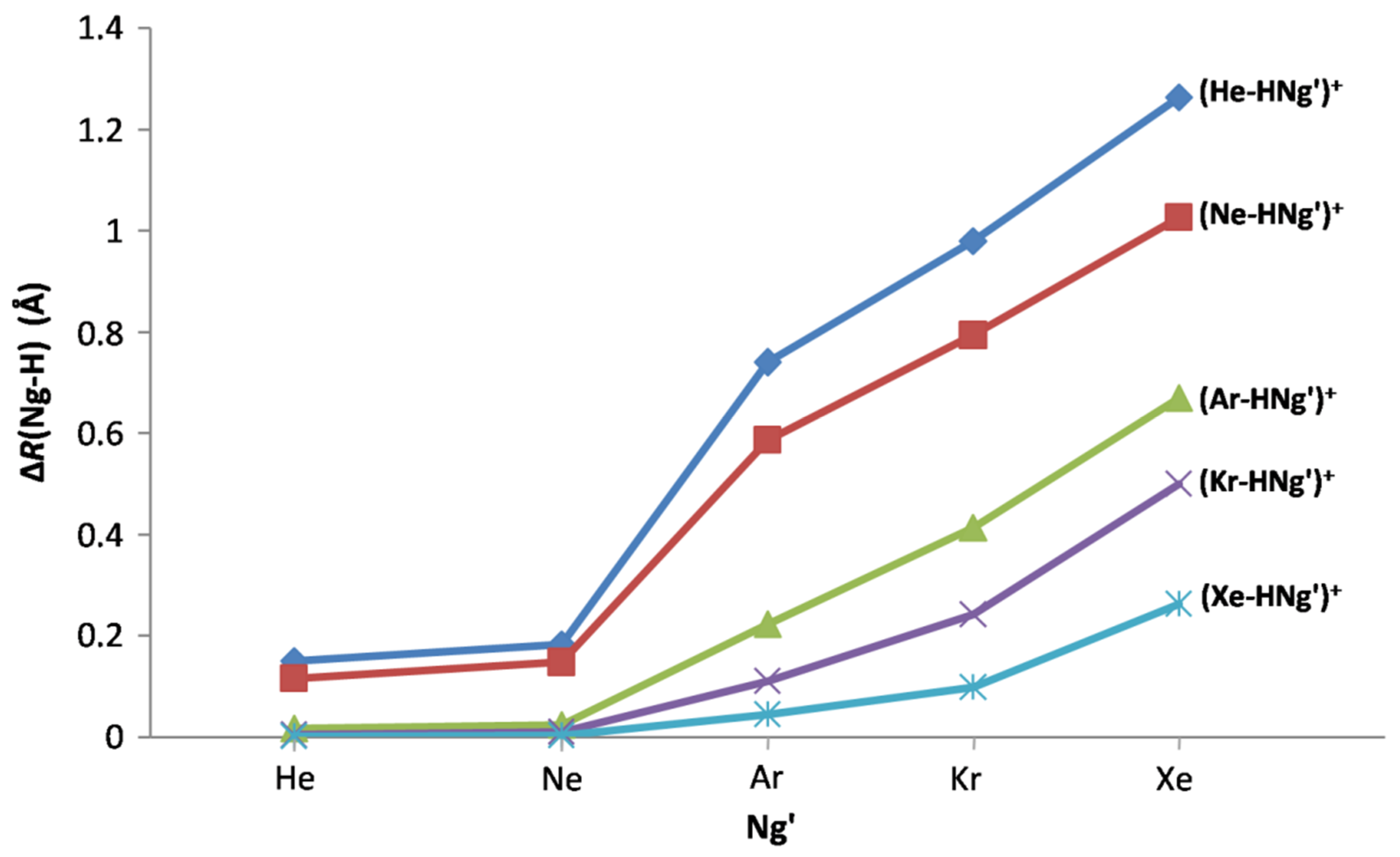
| Ng’ | (He-HNg’)+ | (Ne-HNg’)+ | (Ar-HNg’)+ | (Kr-HNg’)+ | (Xe-HNg’)+ |
|---|---|---|---|---|---|
| He | 0.1500 | 0.1159 | 0.0106 | 0.0036 | 0.0008 |
| Ne | 0.1828 | 0.1473 | 0.0230 | 0.0095 | 0.0030 |
| Ar | 0.7396 | 0.5867 | 0.2232 | 0.1102 | 0.0440 |
| Kr | 0.9795 | 0.7935 | 0.4129 | 0.2421 | 0.0984 |
| Xe | 1.2643 | 1.0264 | 0.6701 | 0.4998 | 0.2622 |
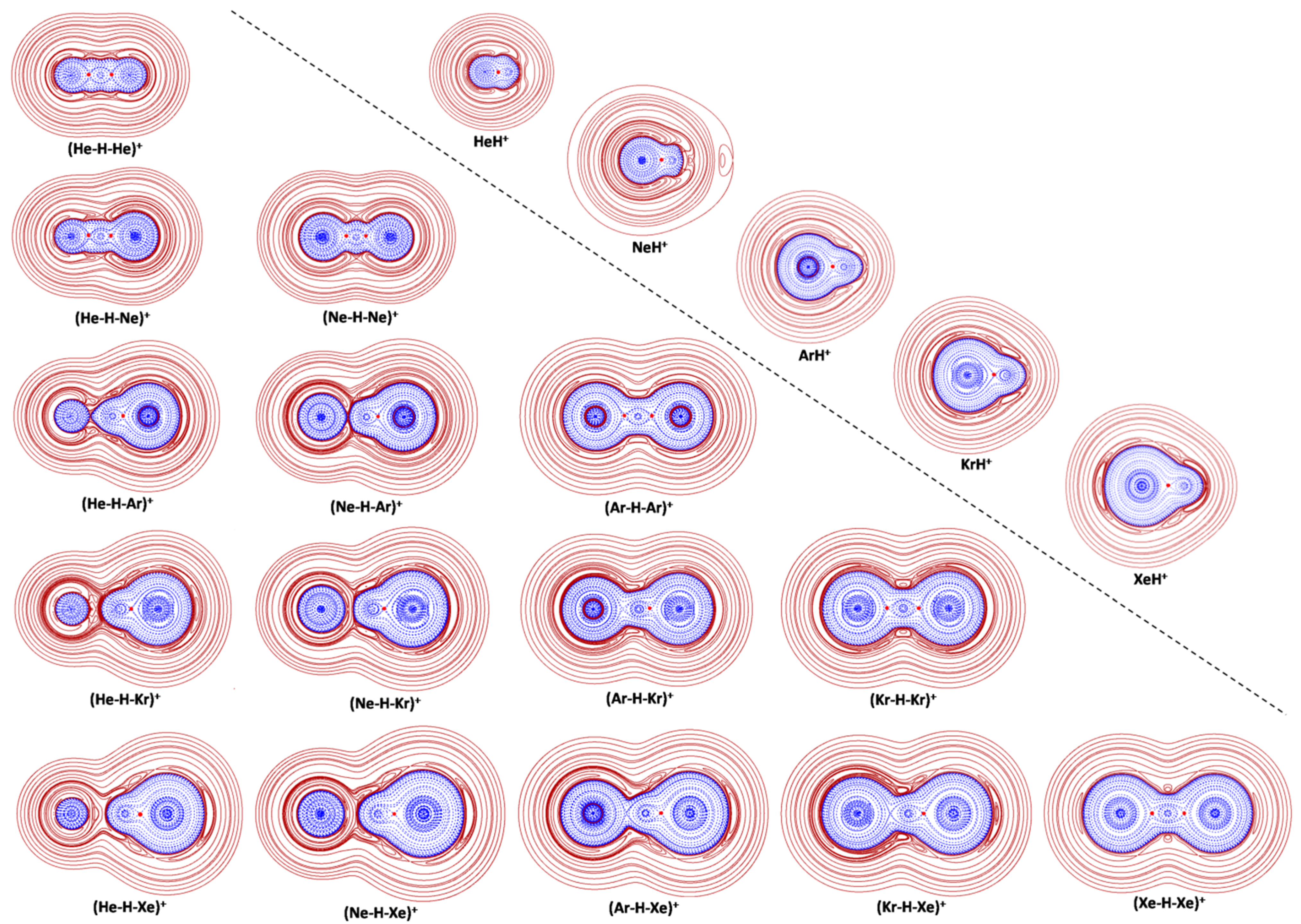
| ρ(BCP) | ρ(HCP) | H(HCP) | BD | ||
| He-H+ | Cov | 0.2057 | 0.2715 | −0.3305 | 1.217 |
| Ne-H+ | Cov | 0.2135 | 0.3083 | −0.3293 | 1.068 |
| Ar-H+ | Cov | 0.2324 | 0.2445 | −0.2387 | 0.976 |
| Kr-H+ | Cov | 0.2057 | 0.2079 | −0.1948 | 0.937 |
| Xe-H+ | Cov | 0.1677 | 0.1690 | −0.1393 | 0.824 |
| (He-HHe)+ | Cov | 0.1299 | 0.1547 | −0.1136 | 0.734 |
| (He-HNe)+ | Cov | 0.1187 | 0.1381 | −0.0897 | 0.650 |
| (HeH-Ne)+ | Cov | 0.1481 | 0.1812 | −0.1627 | 0.898 |
| (HeH-Ar)+ | Cov | 0.2246 | 0.2375 | −0.2289 | 0.964 |
| (HeH-Kr)+ | Cov | 0.2037 | 0.2063 | −0.1924 | 0.933 |
| (HeH-Xe)+ | Cov | 0.1677 | 0.1689 | −0.1392 | 0.824 |
| (Ne-HNe)+ | Cov | 0.1359 | 0.1602 | −0.1317 | 0.822 |
| (NeH-Ar)+ | Cov | 0.2163 | 0.2294 | −0.2166 | 0.944 |
| (NeH-Kr)+ | Cov | 0.2006 | 0.2034 | −0.1881 | 0.925 |
| (NeH-Xe)+ | Cov | 0.1671 | 0.1684 | −0.1386 | 0.823 |
| (Ar-HAr)+ | Cov | 0.1235 | 0.1325 | −0.0870 | 0.657 |
| (ArH-Kr)+ | Cov | 0.1538 | 0.1586 | −0.1241 | 0.782 |
| (ArH-Xe)+ | Cov | 0.1536 | 0.1554 | −0.1206 | 0.776 |
| (Kr-HKr)+ | Cov | 0.1103 | 0.1175 | −0.0691 | 0.588 |
| (KrH-Xe)+ | Cov | 0.1361 | 0.1387 | −0.0983 | 0.709 |
| (Xe-HXe)+ | Cov | 0.0940 | 0.0973 | −0.0494 | 0.508 |
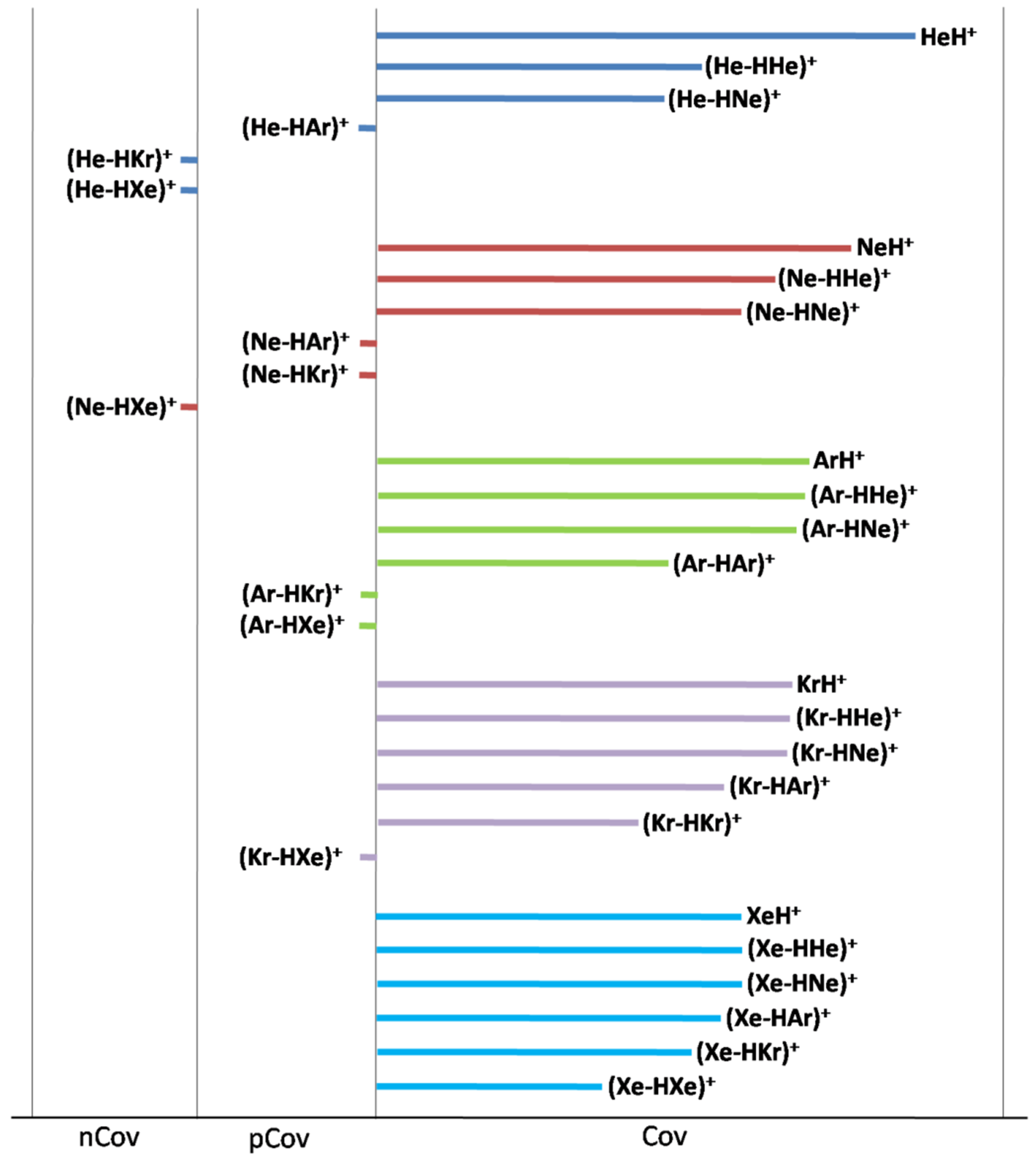
| Ωs | N(Ωs) | ρs(ave) | Hs(ave/max/min) | ||
| (He-HAr)+ | pCov (C/H−) | 0.0576 | 1.44 | 0.0249 | −0.0024/−0.0012/−0.0043 |
| (He-HKr)+ | nCov (C) | 0.0428 | 0.59 | 0.0138 | 0.0016/0.0020/0.0009 |
| (He-HXe)+ | nCov (C) | 0.0320 | 0.23 | 0.0070 | 0.0021/0.0023/0.0020 |
| (Ne-HAr)+ | pCov (A/H−) | 0.0831 | 3.18 | 0.0383 | −0.0062/−0.0025/−0.0121 |
| (Ne-HKr)+ | pCov (C/H+/−) | 0.0759 | 1.73 | 0.0228 | 0.0001/0.0025/−0.0029 |
| (Ne-HXe)+ | nCov | 0.0651 | 0.87 | 0.0134 | 0.0018/0.0025/0.0012 |
| (Ar-HKr)+ | pCov (A/H−) | 0.3511 | 25.1 | 0.0715 | −0.0333/−0.0224/−0.0703 |
| (Ar-HXe)+ | pCov (A/H−) | 0.2523 | 9.78 | 0.0387 | −0.0086/−0.0047/−0.0173 |
| (Kr-HXe)+ | pCov (A/H−) | 0.4932 | 27.4 | 0.0556 | −0.0192/−0.0111/−0.0409 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borocci, S.; Grandinetti, F.; Sanna, N. On the Proton-Bound Noble Gas Dimers (Ng-H-Ng)+ and (Ng-H-Ng’)+ (Ng, Ng’ = He-Xe): Relationships between Structure, Stability, and Bonding Character. Molecules 2021, 26, 1305. https://doi.org/10.3390/molecules26051305
Borocci S, Grandinetti F, Sanna N. On the Proton-Bound Noble Gas Dimers (Ng-H-Ng)+ and (Ng-H-Ng’)+ (Ng, Ng’ = He-Xe): Relationships between Structure, Stability, and Bonding Character. Molecules. 2021; 26(5):1305. https://doi.org/10.3390/molecules26051305
Chicago/Turabian StyleBorocci, Stefano, Felice Grandinetti, and Nico Sanna. 2021. "On the Proton-Bound Noble Gas Dimers (Ng-H-Ng)+ and (Ng-H-Ng’)+ (Ng, Ng’ = He-Xe): Relationships between Structure, Stability, and Bonding Character" Molecules 26, no. 5: 1305. https://doi.org/10.3390/molecules26051305
APA StyleBorocci, S., Grandinetti, F., & Sanna, N. (2021). On the Proton-Bound Noble Gas Dimers (Ng-H-Ng)+ and (Ng-H-Ng’)+ (Ng, Ng’ = He-Xe): Relationships between Structure, Stability, and Bonding Character. Molecules, 26(5), 1305. https://doi.org/10.3390/molecules26051305






