Structural and Spectral Investigation of a Series of Flavanone Derivatives
Abstract
1. Introduction
2. Results and Discussion
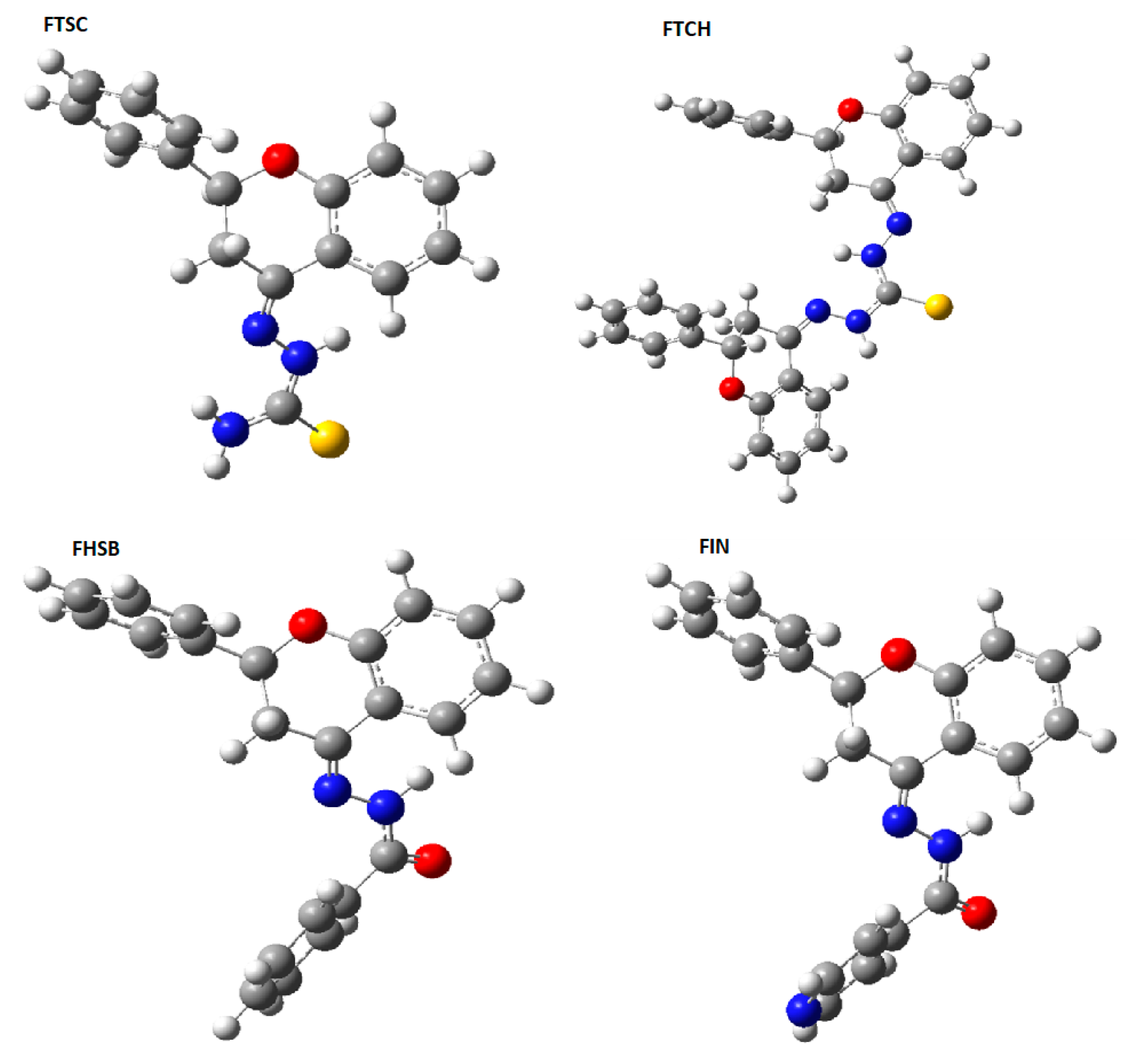
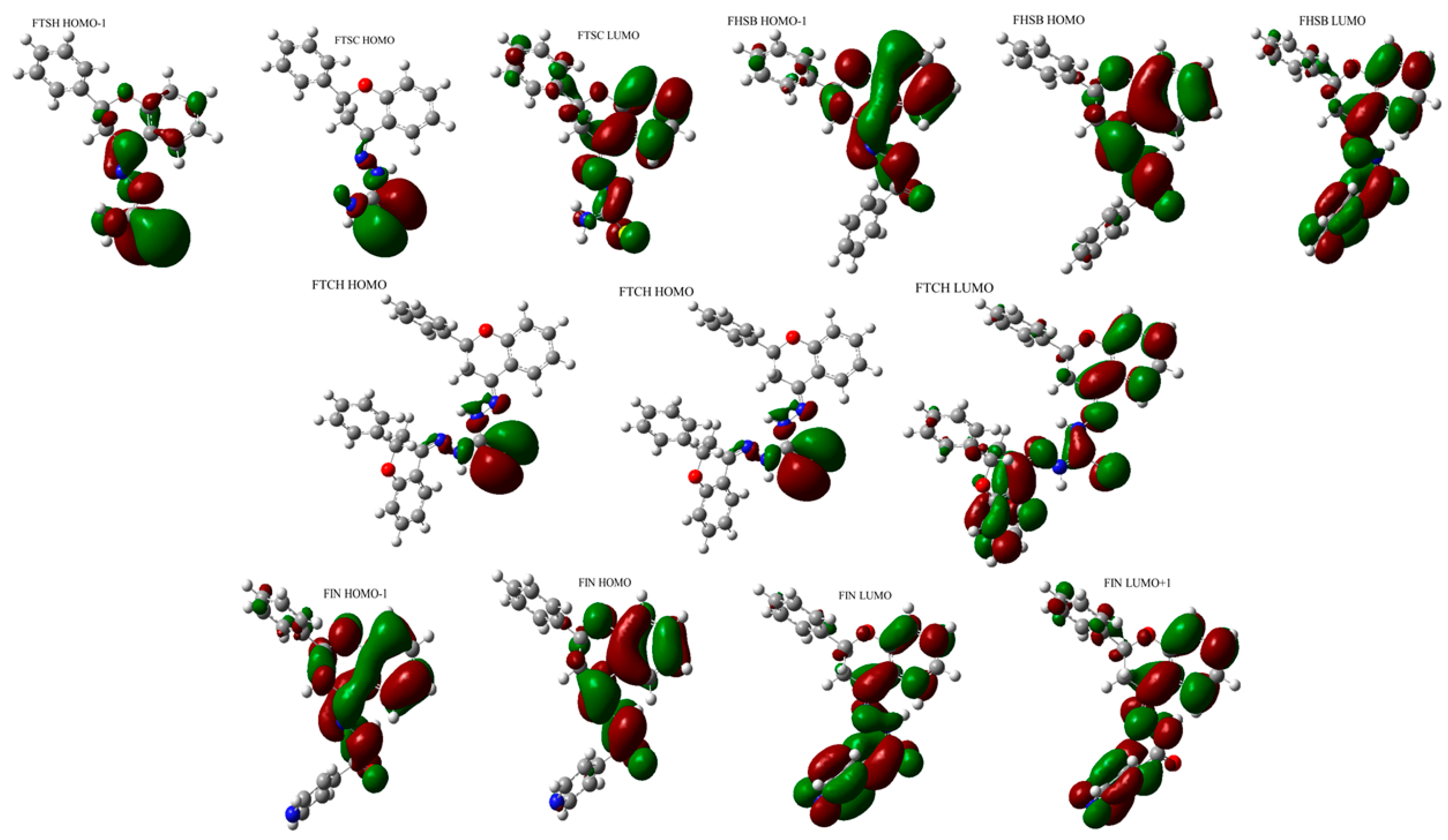
2.1. The Optimized Electronic Structures of the Studied Molecules
2.2. Spectral Profiles of Flavanone Schiff Bases
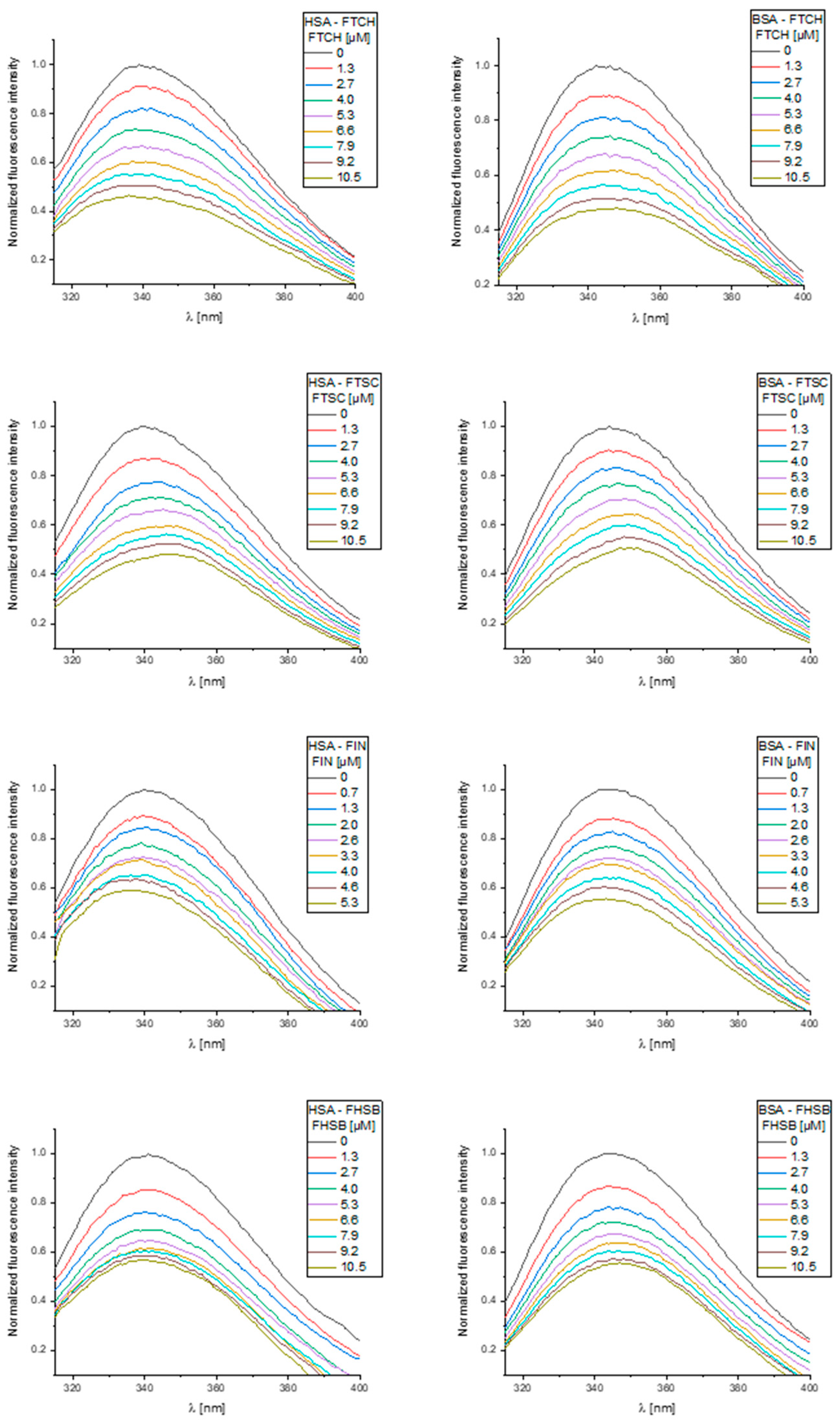
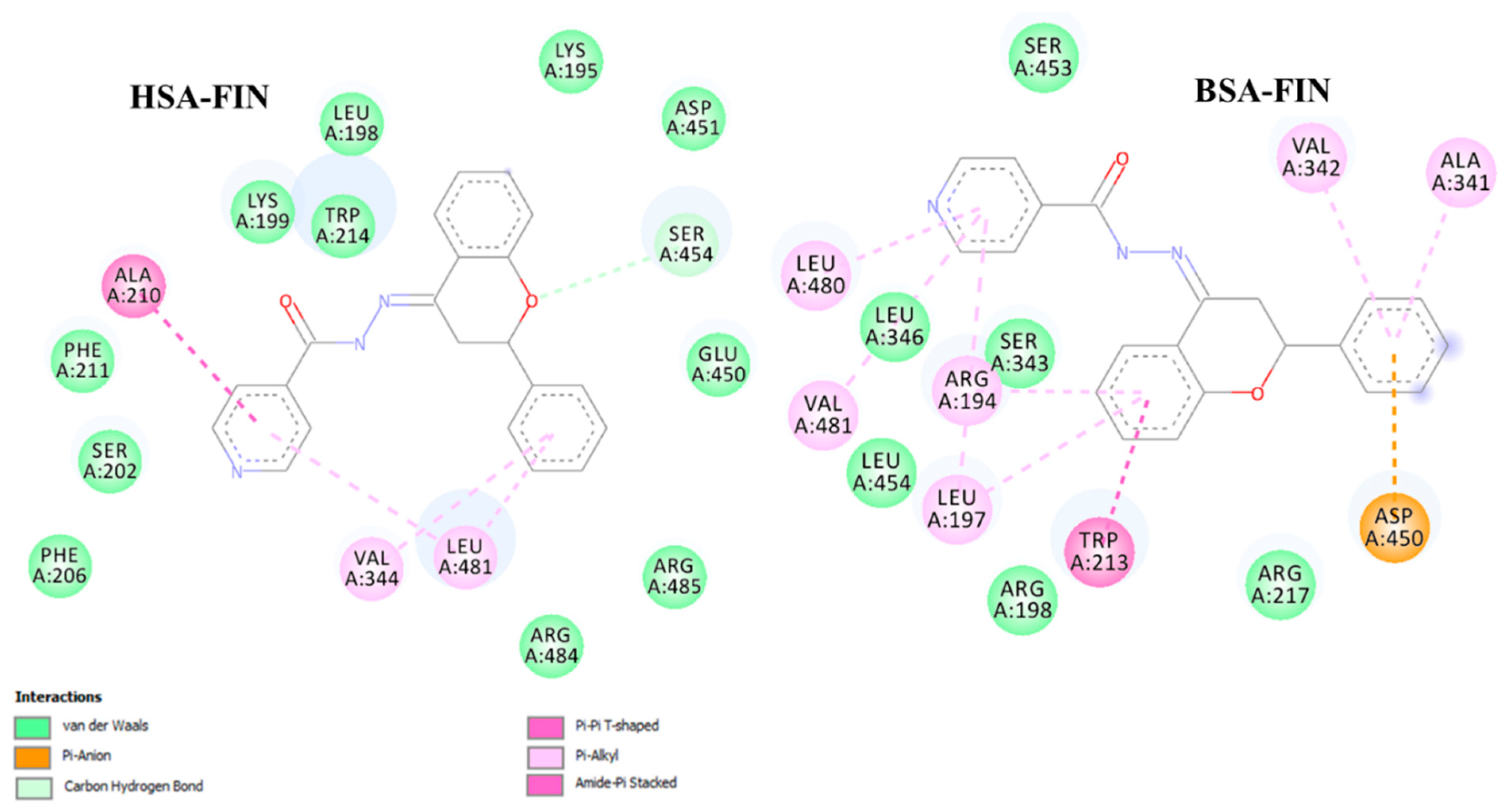
2.3. Binding Interactions with Human and Bovine Serum Albumins
3. Materials and Methods
3.1. Reagents
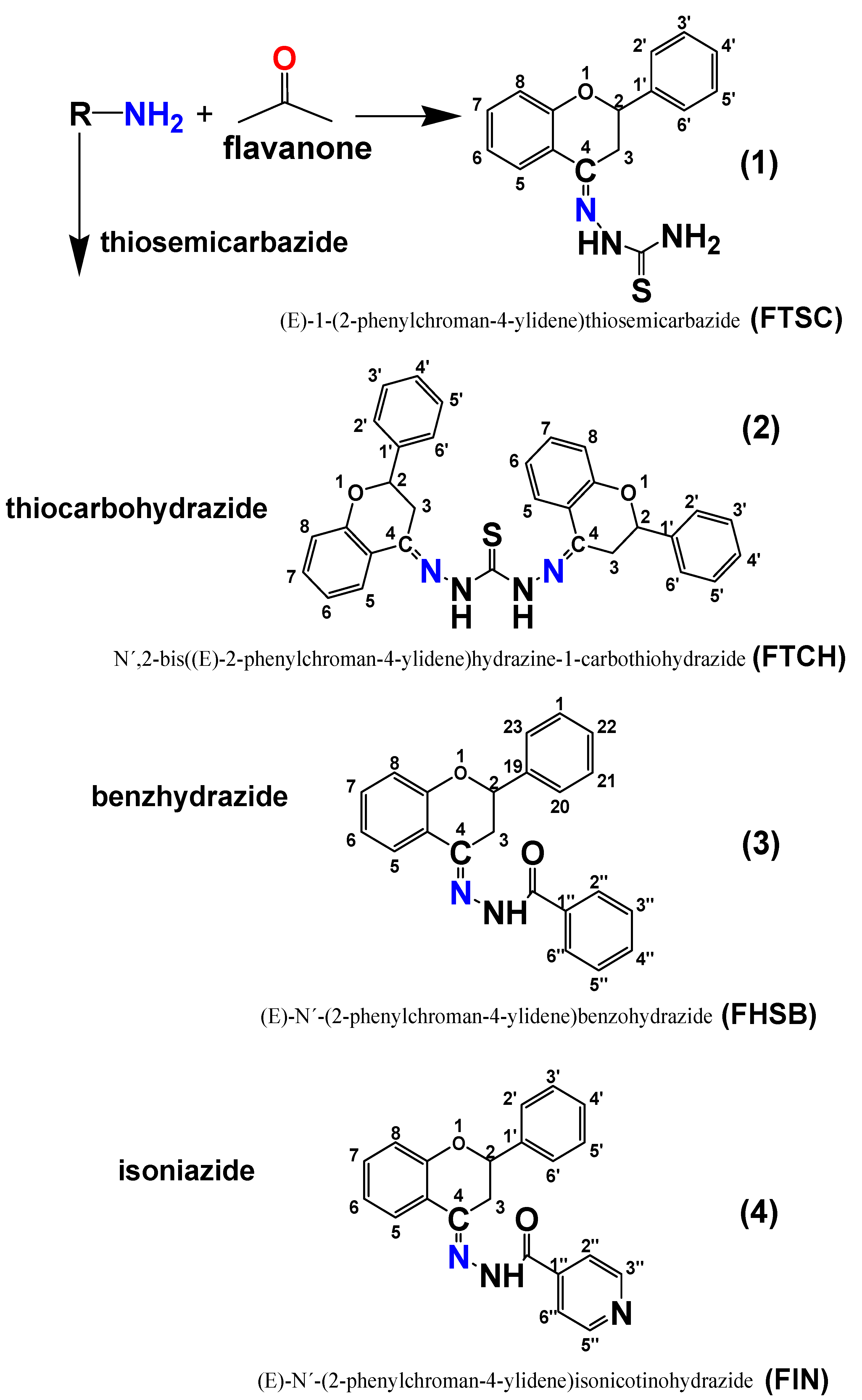
3.2. Synthesis of Ligands
3.3. Theoretical Methods
3.4. Absorption and Fluorescence Measurements
3.4.1. Absorption Measurements
3.4.2. Steady-State Fluorescence Measurements
3.4.3. Time-Resolved Fluorescence Measurements
3.4.4. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Vardhan, H.; Mehta, A.; Nath, I.; Verpoort, F. Dynamic imine chemistry in metal-organic polyhedra. RSC Adv. 2015, 5, 67011–67030. [Google Scholar] [CrossRef]
- Selvan, G.T.; Chitra, C.; Israel, E.V.M.V.; Selvakumar, P.M. Development of a fluorescent chemosensor towards sensing and separation of Mg2+ ions in chlorophyll and hard water. New J. Chem. 2018, 42, 902–909. [Google Scholar] [CrossRef]
- Curran, D.J.; Siggia, S. Analysis of azomethines. In Carbon-Nitrogen Double Bonds (1970); John Wiley & Sons, Ltd.: Chichester, UK, 2010; pp. 149–180. [Google Scholar]
- Kállay, F.; Janzsó, G.; Koczor, I. The reactions of flavanone with substituted hydrazines. Tetrahedron 1967, 23, 4317–4321. [Google Scholar] [CrossRef]
- Somogyi, L. Reactions of flavonoid thiosemicarbazones under acetylating conditions. Tetrahedron 1991, 47, 9305–9316. [Google Scholar] [CrossRef]
- Nie, A.; Huang, Z. Microwave-assisted reaction of 2′-hydroxychalcones with hydrazides to synthesize flavanone hydrazone and 4,5-dihydropyrazole derivatives. J. Comb. Chem. 2006, 8, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Martens, S.; Mithöfer, A. Flavones and flavone synthases. Phytochemistry 2005, 66, 2399–2407. [Google Scholar] [CrossRef]
- Schijlen, E.G.W.M.; Ric De Vos, C.H.; Van Tunen, A.J.; Bovy, A.G. Modification of flavonoid biosynthesis in crop plants. Phytochemistry 2004, 65, 2631–2648. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.C.; Hsieh, Y.S.; Kuo, W.H.; Chiou, H.L.; Yang, S.F.; Chiang, W.L.; Chu, S.C. The tumor-growth inhibitory activity of flavanone and 2′-OH flavanone in vitro and in vivo through induction of cell cycle arrest and suppression of cyclins and CDKs. J. Biomed. Sci. 2007, 14, 107–119. [Google Scholar] [CrossRef]
- Hsiao, Y.C.; Kuo, W.H.; Chen, P.N.; Chang, H.R.; Lin, T.H.; Yang, W.E.; Hsieh, Y.S.; Chu, S.C. Flavanone and 2′-OH flavanone inhibit metastasis of lung cancer cells via down-regulation of proteinases activities and MAPK pathway. Chem. Biol. Interact. 2007, 167, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.C.; Ko, C.H.; Tseng, S.W.; Tsai, S.H.; Chen, Y.C. Structurally related antitumor effects of flavanones in vitro and in vivo: Involvement of caspase 3 activation, p21 gene expression, and reactive oxygen species production. Toxicol. Appl. Pharmacol. 2004, 197, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Ding, Y.; Li, X.C.; Ferreira, D. Theoretical calculation of electronic circular dichroism of a hexahydroxydiphenoyl-containing flavanone glycoside. J. Nat. Prod. 2009, 72, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Anouar, E.H.; Gierschner, J.; Duroux, J.L.; Trouillas, P. UV/Visible spectra of natural polyphenols: A time-dependent density functional theory study. Food Chem. 2012, 131, 79–89. [Google Scholar] [CrossRef]
- Wróblewski, T.; Ushakou, D.V. Photophysical properties of 7-hydroxyflavanone: Quantum chemical calculations and experimental studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 215, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Serdiuk, I.E.; Wera, M.; Roshal, A.D. Structural and Spectral Features of 4′-Substituted 2′-Hydroxychalcones in Solutions and Crystals: Spectroscopic and Theoretical Investigations. J. Phys. Chem. A 2018, 122, 2030–2038. [Google Scholar] [CrossRef] [PubMed]
- Amat, A.; Dementia, C.; De Angelis, F.; Sgamellotti, A.; Fantacci, S. Absorption and emission of the apigenin and luteolin flavonoids: A TDDFT investigation. J. Phys. Chem. A 2009, 113, 15118–15126. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, P.; Priyadarsini, K.I.; Naumov, S.; Rao, B.S.M. Radiation and quantum chemical studies of chalcone derivatives. J. Phys. Chem. A 2010, 114, 7877–7885. [Google Scholar] [CrossRef]
- Casida, M.E. Time-dependent density-functional theory for molecules and molecular solids. J. Mol. Struct. THEOCHEM 2009, 914, 3–18. [Google Scholar] [CrossRef]
- Dreuw, A.; Head-Gordon, M. Single-reference ab initio methods for the calculation of excited states of large molecules. Chem. Rev. 2005, 105, 4009–4037. [Google Scholar] [CrossRef] [PubMed]
- Scalmani, G.; Frisch, M.J.; Mennucci, B.; Tomasi, J.; Cammi, R.; Barone, V. Geometries and properties of excited states in the gas phase and in solution: Theory and application of a time-dependent density functional theory polarizable continuum model. J. Chem. Phys. 2006, 124, 094107. [Google Scholar] [CrossRef] [PubMed]
- Scalmani, G.; Frisch, M.J. Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 2010, 132, 114110. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, G.; Birkett, D.J.; Wade, D.N. The Characterization of Two Specific Drug Binding Sites on Human Serum Albumin. Mol. Pharmacol. 1975, 11, 824–832. [Google Scholar] [PubMed]
- Mendoza-Wilson, A.M.; Glossman-Mitnik, D. CHIH-DFT study of the electronic properties and chemical reactivity of quercetin. J. Mol. Struct. THEOCHEM 2005, 716, 67–72. [Google Scholar] [CrossRef]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B.; Mabry, T.J.; Markham, K.R.; Thomas, M.B. The Ultraviolet Spectra of Isoflavones, Flavanones and Dihydroflavonols. In The Systematic Identification of Flavonoids; Springer: Berlin/Heidelberg, Germany, 1970; pp. 165–226. [Google Scholar]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid Composition of Citrus Juices. Molecules 2007, 12, 1641. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Li, Y.G.; Wang, T.Y.; Liu, M.H.; Li, Z.S. Effect of solvent and molecular structure on the enhanced fluorescence and supramolecular chirality of Schiff bases in organogels. Wuli Huaxue Xuebao/ Acta Phys. Chim. Sin. 2008, 24, 1535–1539. [Google Scholar] [CrossRef]
- Sykula, A.; Kowalska-Baron, A.; Dzeikala, A.; Bodzioch, A.; Lodyga-Chruscinska, E. An experimental and DFT study on free radical scavenging activity of hesperetin Schiff bases. Chem. Phys. 2019, 517, 91–103. [Google Scholar] [CrossRef]
- Łodyga-Chruścińska, E.; Kowalska-Baron, A.; Błazińska, P.; Pilo, M.; Zucca, A.; Korolevich, V.M.; Cheshchevik, V.T. Position impact of hydroxy groups on spectral, acid-base profiles and DNA interactions of several monohydroxy flavanones. Molecules 2019, 24, 3049. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, M.; Oliveri, I.P.; Fragalà, M.E.; Failla, S.; D’Urso, A.; Di Bella, S.; Purrello, R. Chirality of self-assembled achiral porphyrins induced by chiral Zn(II) Schiff-base complexes and maintained after spontaneous dissociation of the templates: A new case of chiral memory. Chem. Commun. 2016, 52, 8518–8521. [Google Scholar] [CrossRef]
- Salassa, G.; Castilla, A.M.; Kleij, A.W. Cooperative self-assembly of a macrocyclic Schiff base complex. Dalt. Trans. 2011, 40, 5236–5243. [Google Scholar] [CrossRef]
- Strambini, G.B.; Gonnelli, M. Fluorescence quenching of buried Trp residues by acrylamide does not require penetration of the protein fold. J. Phys. Chem. B 2010, 114, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Kragh-Hansen, U.; Chuang, V.T.G.; Otagiri, M. Practical Aspects of the Ligand-Binding and Enzymatic Properties of Human Serum Albumin. Biol. Pharm. Bull. 2002, 25, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Pantsar, T.; Poso, A. Binding Affinity via Docking: Fact and Fiction. Molecules 2018, 23, 1899. [Google Scholar] [CrossRef] [PubMed]
- Mobley, D.L.; Dill, K.A. Binding of Small-Molecule Ligands to Proteins: “What You See” Is Not Always “What You Get.”. Structure 2009, 17, 489–498. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09. Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Dennington, R.; Keith, T.; Millam, J.E. GaussView 05 Software Package; Version 5; GaussView; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results obtained with the correlation energy density functionals of becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- CCCBDB Listing of Precalculated Vibrational Scaling Factors. Available online: https://cccbdb.nist.gov/vibscalejust.asp (accessed on 7 January 2021).
- Parr, R.G.; Yang, W. Chemical potential derivatives. In Density-Functional Theory of Atoms and Molecules; Oxford University Press: Oxford, UK, 1989; pp. 87–99. [Google Scholar]
- Parr, R.G.; Szentpály, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Young, G. Density Functional Calculations—Recent Progresses of Theory and Application; IntechOpen: London, UK, 2018. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; ISBN 0387312781. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- RCSB PDB: Homepage. Available online: http://www.rcsb.org/ (accessed on 7 January 2021).
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- BIOVIA; Dassault Systèmes. Discovery Studio Visualizer Software, Version 4.0; Dassault Systèmes: San Diego, CA, USA, 2019.
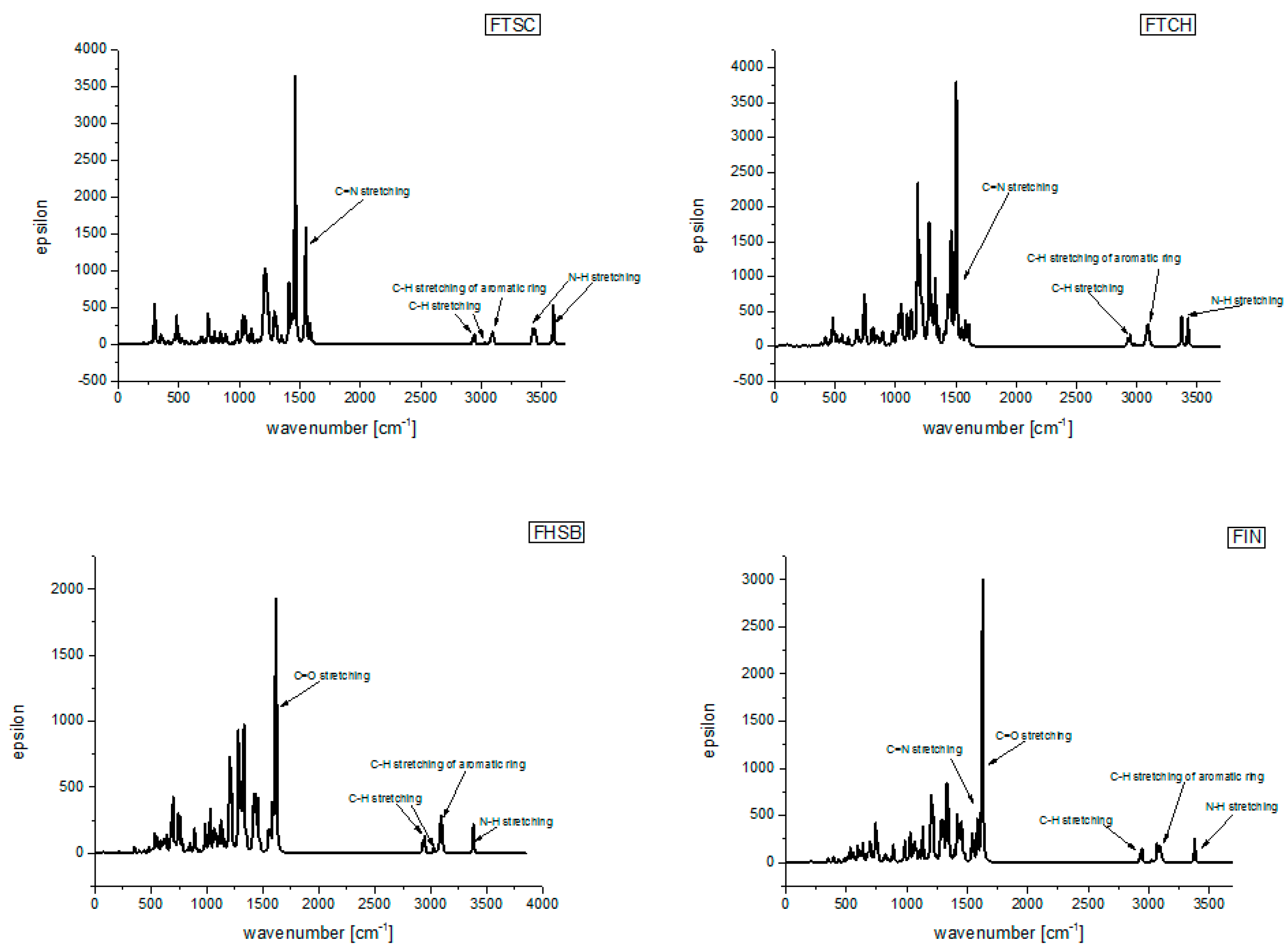



| ν [cm−1] | Scaled * ν [cm−1] | νex [cm−1] | Description | |
|---|---|---|---|---|
| FIN | 3506 | 3380 | 3225 | N-H stretching |
| 3236–3180 | 3119–3066 | 3035 | CH stretching of aromatic ring | |
| 3055 | 2945 | 2867 | C-H stretching | |
| 1685 | 1625 | 1650 | C=O stretching | |
| 1674 | 1614 | 1525 | C=N stretching | |
| 1650–1474 | 1591–1421 | 1450 | deforming of aromatic rings | |
| FHSB | 3506 | 3380 | very weak | N-H stretching |
| 3229–3180 | 3113–3065 | 3275 | CH stretching of aromatic ring | |
| 3137 | 3024 | 3165 | C-H stretching | |
| 3053 | 2943 | 3071 | C-H stretching | |
| 1675 | 1615 | C=O stretching | ||
| 1672 | 1612 | 1621 | C=N stretching | |
| 1527–1404 | 1472–1353 | deforming of aromatic rings | ||
| FTSC | 3728 | 3594 | 3495 | N-H stretching |
| 3572–3554 | 3444–3426 | 3433–3376 | ||
| 3222–3181 | 3106–3066 | 3215–3145 | CH stretching of aromatic ring | |
| 3138 | 3025 | 3066 | C-H stretching | |
| 3054 | 2944 | C-H stretching | ||
| 1673 | 1613 | 2100 | C=N stretching | |
| 1649–1105 | 1590–1065 | 1500–2000 | deforming of aromatic rings | |
| FTCH | 3551–3494 | 3424–3368 | 3290 | N-H stretching |
| 3222–3180 | 3106–3066 | 3155 | CH stretching of aromatic ring | |
| 3140–3031 | 3027–2922 | 3060–2974 | C-H stretching | |
| 1667 | 1607 | 2000 | C=N stretching | |
| 1558 | 1502 | 1605 | C=N stretching | |
| 1513–1336 | 1459–1288 | 1525 | deforming of aromatic rings |
| Compound | HOMO [eV] | LUMO [eV] | HOMO-LUMO gap [eV] | IP [eV] | EA [eV] | η | χ | µ | ω | S |
|---|---|---|---|---|---|---|---|---|---|---|
| FHSB | −6.416 | −2.067 | 4.349 | 6.416 | 2.067 | 2.175 | 4.242 | −4.242 | 4.137 | 0.230 |
| FIN | −6.359 | −1.850 | 4.509 | 6.359 | 1.850 | 2.255 | 4.104 | −4.104 | 3.736 | 0.222 |
| FTSC | −6.173 | −2.166 | 4.007 | 6.173 | 2.166 | 2.003 | 4.169 | −4.169 | 4.338 | 0.250 |
| FTCH | −6.197 | −1.930 | 4.268 | 6.197 | 1.930 | 2.134 | 4.064 | −4.064 | 3.869 | 0.234 |
| Compound | S0→S1 | S0→S2 | S0→S3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| μ [D] | λ [nm] | f | μ [D] | λ [nm] | f | μ [D] | λ [nm] | f | |
| FHSB | 4.1484 | 316.29 | 0.3984 | 1.9310 | 290.84 | 0.2017 | 0.2152 | 275.94 | 0.0237 |
| FIN | 3.0648 | 326.39 | 0.2852 | 0.4040 | 300.26 | 0.0409 | 1.7873 | 289.25 | 0.1877 |
| FTSC | 0.0433 | 334.19 | 0.0039 | 5.4252 | 332.72 | 0.4953 | 0.2457 1.5365 | 307.25 270.47 | 0.0243 0.1726 |
| FTCH | 0.0405 | 372.67 | 0.0033 | 11.0420 | 357.90 | 0.9372 | 2.2967 | 346.30 | 0.2015 |
| Ka [M−1] | ∆G [kcal/mol] * | |||
|---|---|---|---|---|
| Experiment | Experiment | Docking | ||
| HSA | FTCH | (1.2 ± 0.1) × 105 | −6.8 | −8.8 |
| FTSC | (1.6 ± 0.1) × 105 | −7.0 | −8.9 | |
| FIN | (3.3 ± 0.1) × 105 | −7.3 | −9.5 | |
| FHSB | (1.8 ± 0.1) × 105 | −7.0 | −8.9 | |
| BSA | FTCH | (1.1 ± 0.1) × 105 | −6.8 | −8.9 |
| FTSC | (1.2 ± 0.1) × 105 | −6.8 | −8.5 | |
| FIN | (2.5 ± 0.1) × 105 | −7.2 | −9.4 | |
| FHSB | (1.7 ± 0.1) × 105 | −7.0 | −9.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sykuła, A.; Kowalska-Baron, A.; Gałęcki, K.; Błazińska, P.; Łodyga-Chruścińska, E. Structural and Spectral Investigation of a Series of Flavanone Derivatives. Molecules 2021, 26, 1298. https://doi.org/10.3390/molecules26051298
Sykuła A, Kowalska-Baron A, Gałęcki K, Błazińska P, Łodyga-Chruścińska E. Structural and Spectral Investigation of a Series of Flavanone Derivatives. Molecules. 2021; 26(5):1298. https://doi.org/10.3390/molecules26051298
Chicago/Turabian StyleSykuła, Anna, Agnieszka Kowalska-Baron, Krystian Gałęcki, Paulina Błazińska, and Elżbieta Łodyga-Chruścińska. 2021. "Structural and Spectral Investigation of a Series of Flavanone Derivatives" Molecules 26, no. 5: 1298. https://doi.org/10.3390/molecules26051298
APA StyleSykuła, A., Kowalska-Baron, A., Gałęcki, K., Błazińska, P., & Łodyga-Chruścińska, E. (2021). Structural and Spectral Investigation of a Series of Flavanone Derivatives. Molecules, 26(5), 1298. https://doi.org/10.3390/molecules26051298






