Stabilization of β-Galactosidase on Modified Gold Nanoparticles: A Preliminary Biochemical Study to Obtain Lactose-Free Dairy Products for Lactose-Intolerant Individuals
Abstract
1. Introduction
2. Results and Discussion
2.1. Selection of AuNPs for the Current Study
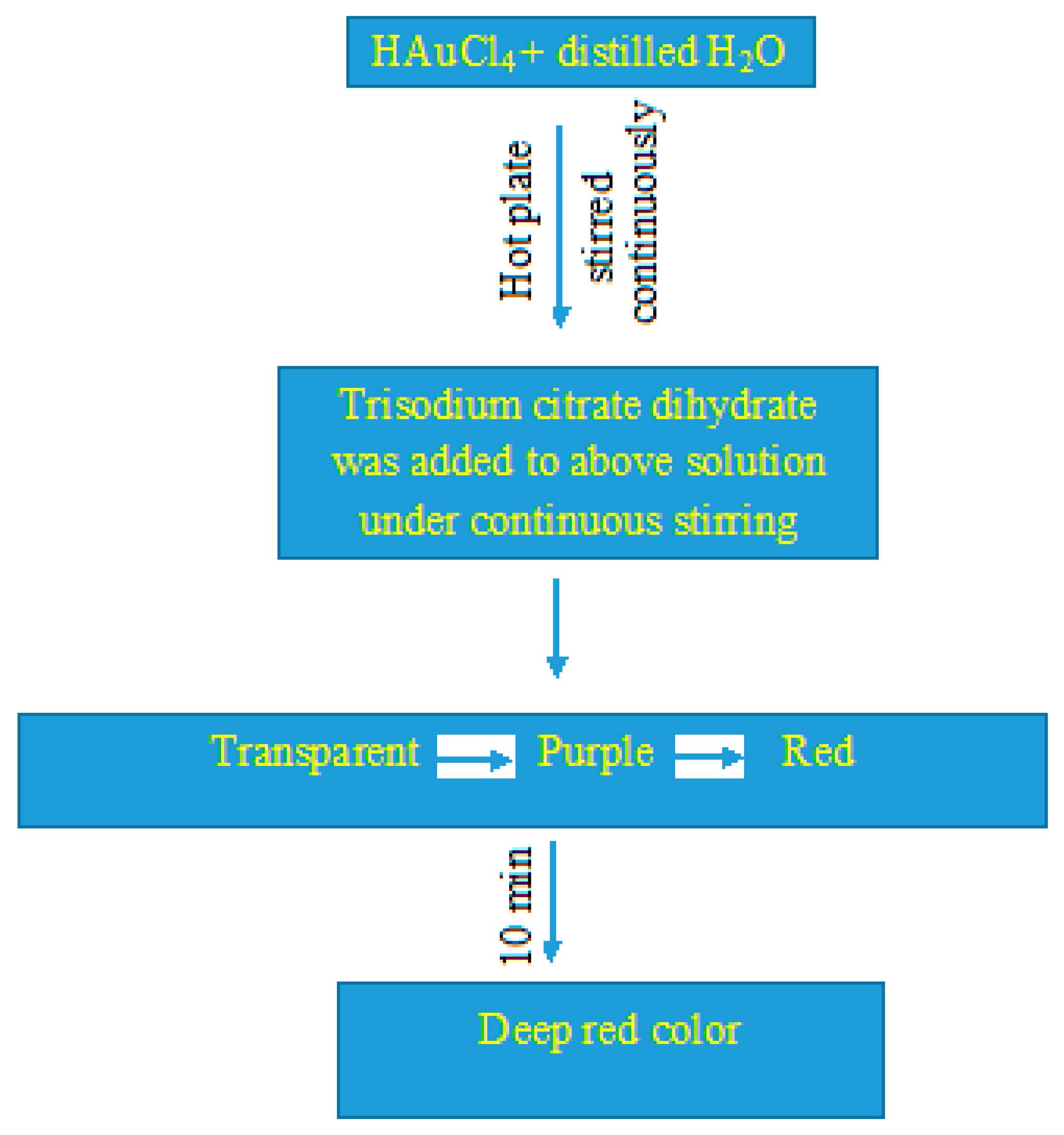
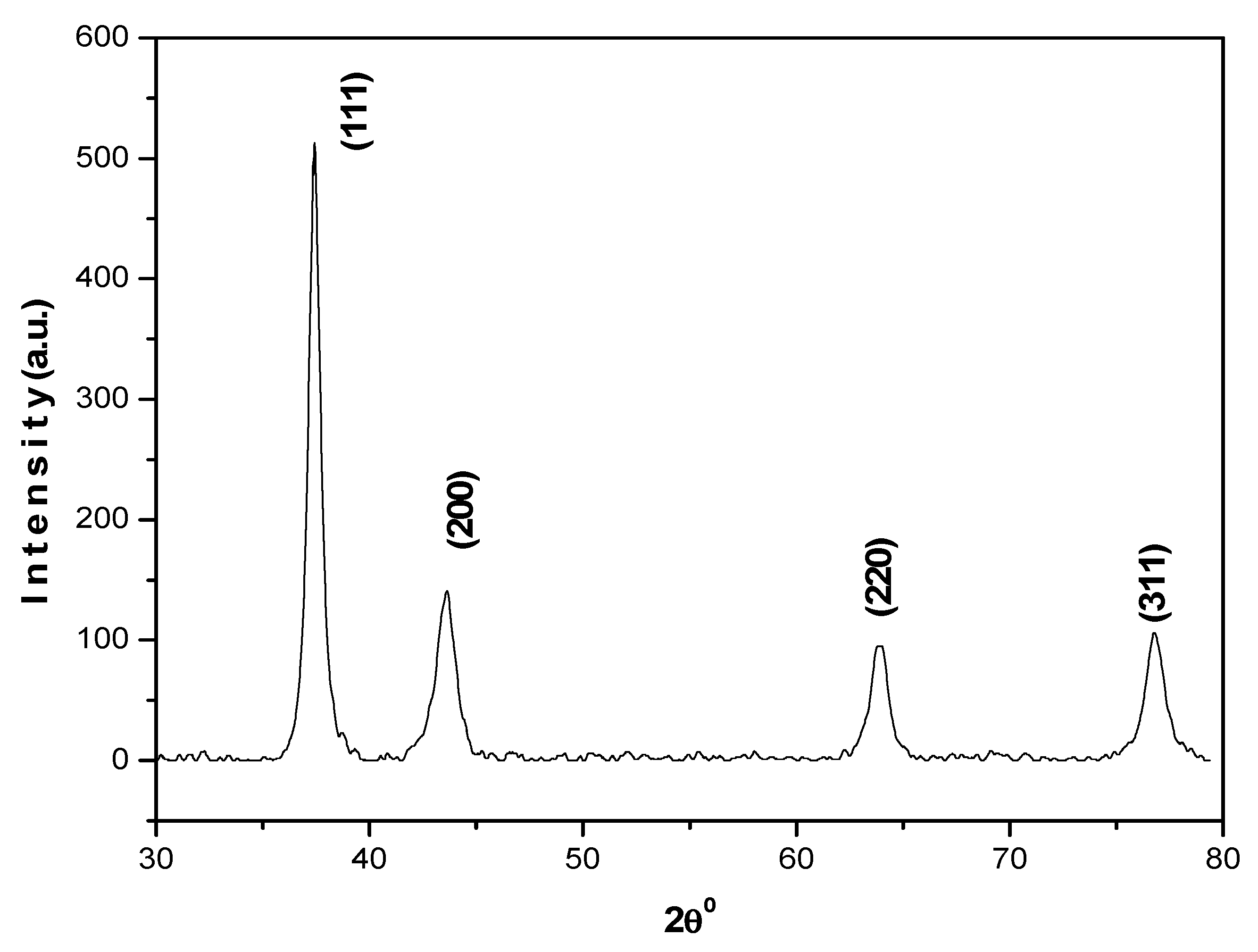
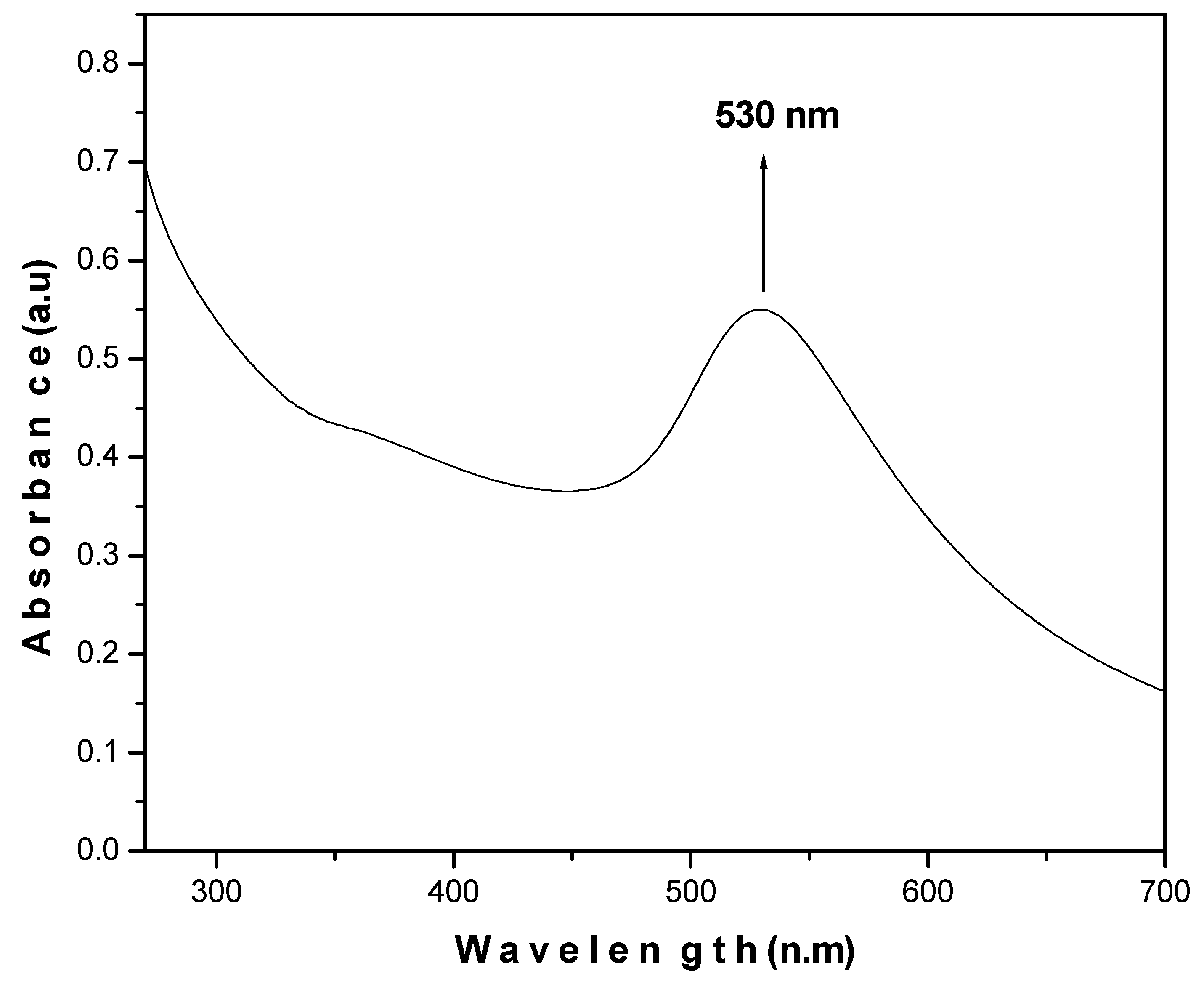
2.2. Synthesis and Characterization of AuNPs
2.3. Surface Functionalization of AuNPs via PVA for Stabilizing β-Galactosidase
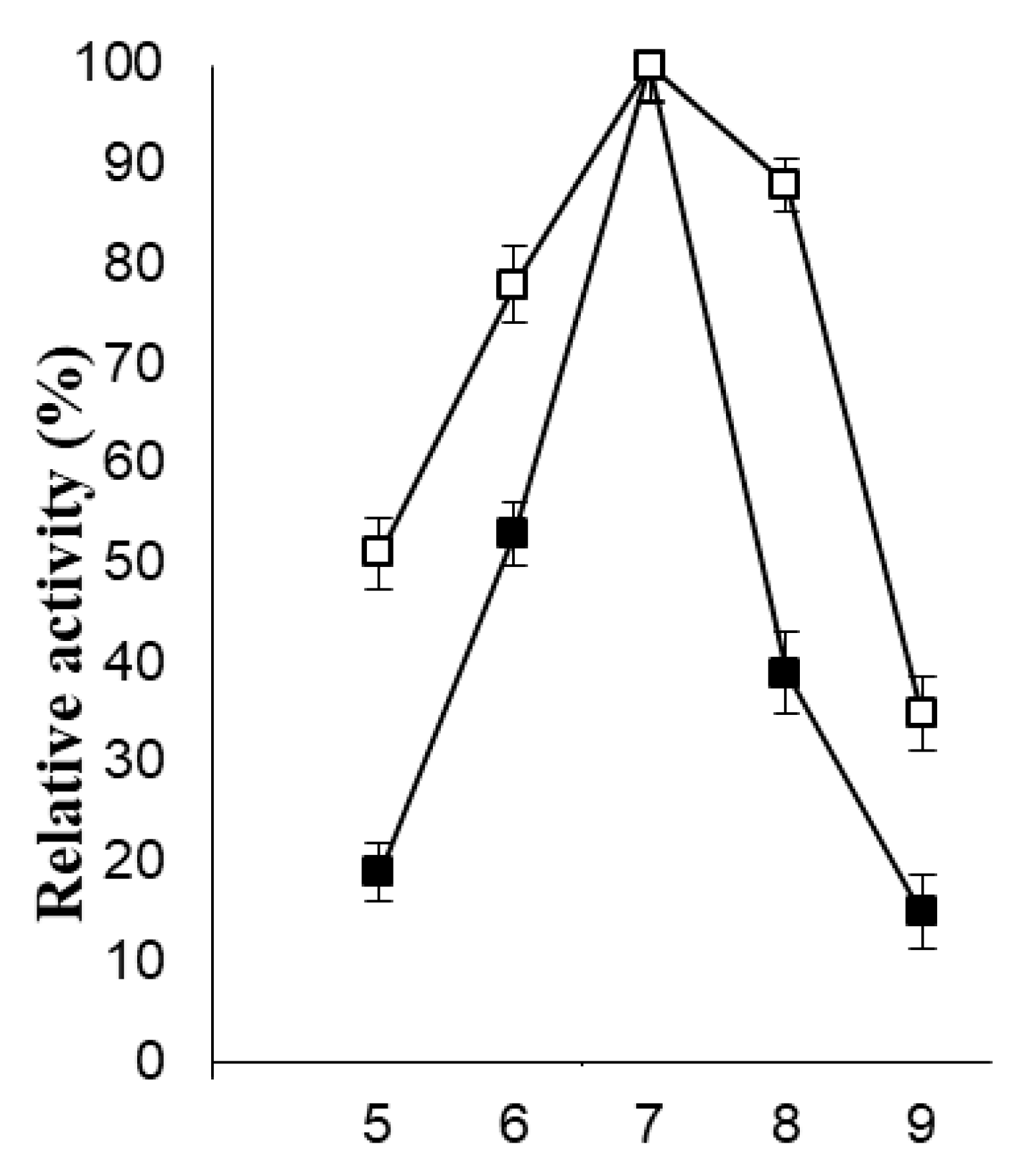
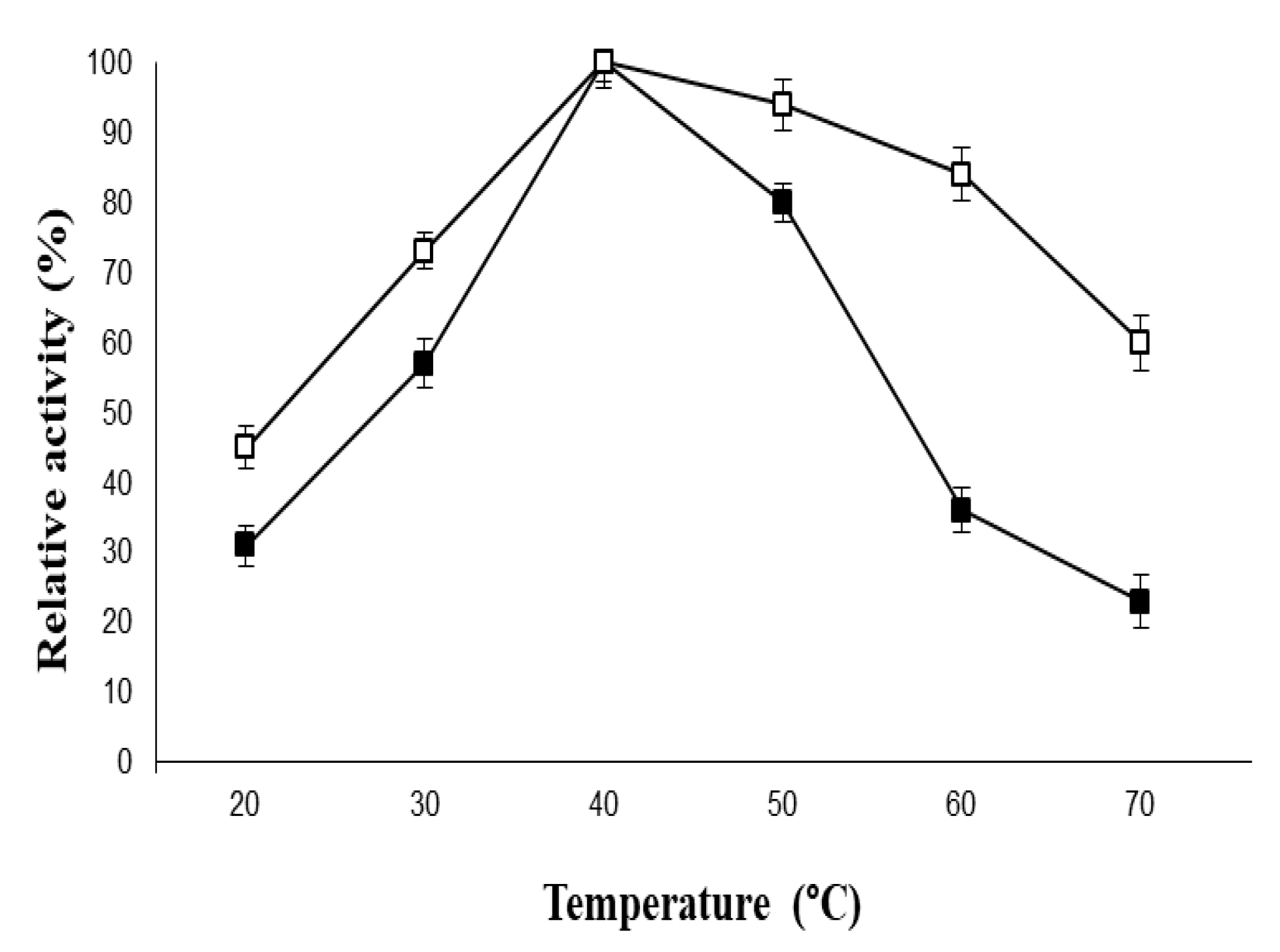
2.4. Stabilization of PVA-AuNPs-β-Galactosidase Against Physical and Chemical Denaturation
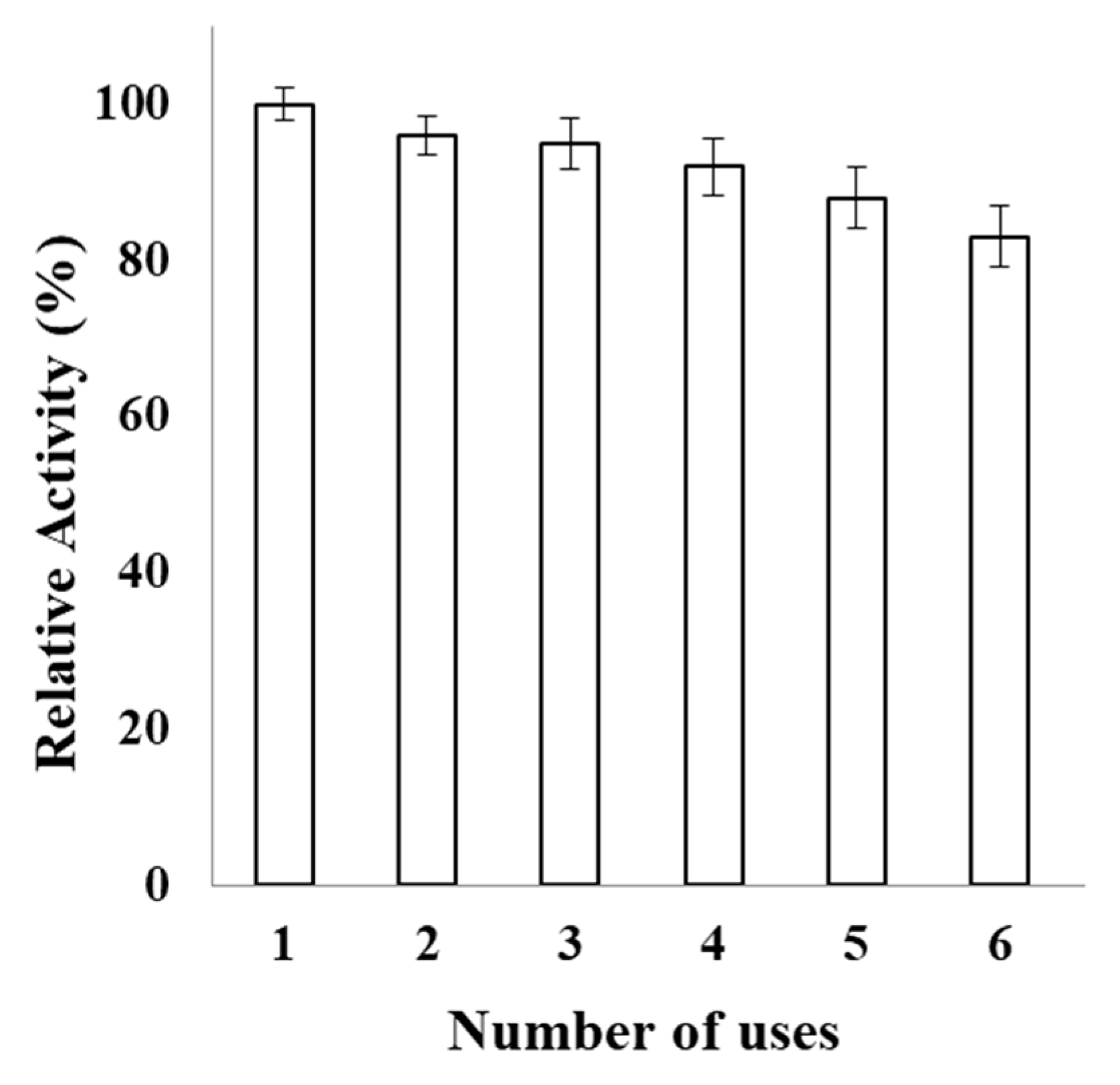
2.5. Operational Stability of PVA-AuNPs-β-Galactosidase
3. Materials and Methods
3.1. Synthesis and Characterization of AuNPs
3.2. Surface Modification of AuNPs by PVA
3.3. β-Galactosidase Binding on PVA-Modified AuNPs
3.4. β-Galactosidase Assay
3.5. Physical and Chemical Stability of PVA-AuNPs-β-Galactosidase
3.6. Operational Stability of PVA-AuNPs-β-Galactosidase
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.I.; Fiore, V.; et al. Enzyme biosensors for biomedical applications: Strategies for safeguarding analytical performances in biological fluids. Sensors 2016, 16, 780. [Google Scholar] [CrossRef]
- Dicosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Betancor, L.; Luckarift, H.R.; Seo, J.H.; Brand, O.; Spain, J.C. Three-dimensional immobilization of β-galactosidase on a silicon surface. Biotechnol. Bioeng. 2008, 99, 261–267. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, S.; Gao, G. Immobilization of β-galactosidase onto magnetic beads. Appl. Biochem. Biotechnol. 2010, 160, 1386–1393. [Google Scholar] [CrossRef]
- Ansari, S.A.; Husain, Q. Immobilization of Kluyveromyces lactis β galactosidase on concanavalin A layered aluminium oxide nanoparticles-its future aspects in biosensor applications. J. Mol. Cat. B Enz. 2011, 70, 119–126. [Google Scholar] [CrossRef]
- Ansari, S.A.; Husain, Q. Lactose hydrolysis by β galactosidase immobilized on concanavalin A-cellulose in batch and continuous mode. J. Mol. Cat. B Enz. 2010, 63, 68–74. [Google Scholar] [CrossRef]
- Sardar, M.; Gupta, M.N. Immobilization of tomato pectinase on Con A–Seralose 4B by bioaffinity layering. Enz. Microb. Technol. 2005, 37, 355–359. [Google Scholar] [CrossRef]
- Ellis, J.L.; Tomasko, D.L.; Dehghani, F. Novel dense CO2 technique for beta-galactosidase immobilization in polystyrene microchannels. Biomacromolecules 2008, 9, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Hu, B.; Li, W.; Sun, Y. Novel and efficient method for immobilization and stabilization of β-d-galactosidase by covalent attachment onto magnetic Fe3O4-chitosan nanoparticles. J. Mol. Catal. B Enzym. 2009, 61, 208–215. [Google Scholar] [CrossRef]
- Reis, C.L.B.; de Sousa, E.Y.A.; Serpa, J.F.; Santos, J.C.S. Design of immobilized enzyme biocatalysts: Drawbacks and opportunities. Quim. Nov. 2019, 42, 768–783. [Google Scholar] [CrossRef]
- Mateo, C.; Abia, A.; Fernandes-Lafuente, G.; Pessela, B.C.; Grazu, V.; Guisan, J.M.; Fernandes-Lafuente, R. Multi-point covalent immobilization of enzymes on supports activated with epoxy groups: Stabilization of industrial enzymes. Meth. Mol. Biol. 2020, 2100, 109–117. [Google Scholar]
- Ansari, S.A.; Satar, R.; Zaidi, S.K.; Naseer, M.I.; Karim, S.; Alqahtani, M.H.; Rasool, M. Nanodiamonds as an effective and novel matrix for immobilizing β galactosidase. Food Bioprod. Proc. 2015, 95, 298–303. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Manoel, E.A.; Fernandez-Lafuente, R.; Freire, D.M.G. Support engineering: Relation between development of new supports for immobilization of lipases and their applications. Biotechnol. Res. Innov. 2017, 1, 26–34. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Silva, M.J.; Klein, M.; Feddern, V.; Feltes, M.M.C.; Oliveira, J.V.; Ninow, J.L.; de Oliveira, D. Current status and trends in enzymatic nanoimmobilization. J. Mol. Catal. B Enz. 2014, 99, 56–67. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Valerio, A.; Henriques, R.O.; Moritz, D.E.; Ninow, J.K.; Freire, D.M.G.; Manoel, E.A.; Fernandez-Lafuente, R.; de Oliveira, D. Nanomaterials for biocatalyst immobilization—State of the art and future trends. RSC Adv. 2016, 106, 104675–104692. [Google Scholar] [CrossRef]
- Mokhtar, N.M.; Rahman, R.N.Z.R.; Noor, N.D.M.; Shariff, F.M.; Ali, M.S.M. The immobilization of lipases on porous support by adsorption and hydrophobic interaction method. Catalysts 2020, 10, 744. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Rios, N.S.; Carballares, D.; Mendez-Sanchez, C.; Lokha, Y.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Effects of Enzyme Loading and Immobilization Conditions on the Catalytic Features of Lipase From Pseudomonas fluorescens Immobilized on Octyl-Agarose Beads. Front. Bioeng. Biotechnol. 2020, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- An, J.; Li, G.; Zhang, Y.; Zhang, T.; Liu, X.; Gao, F.; Peng, M.; He, Y.; Fan, H. Recent advances in enzyme-nanostructure biocatalysts with enhanced activity. Catalysts 2020, 10, 338. [Google Scholar] [CrossRef]
- Ealias, A.M.; Saravanakumar, M.P. A review on the classification, characterization, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 1–15. [Google Scholar]
- Kockmann, A.; Porsiel, J.C.; Saadat, R.; Garnweitner, G. Impact of nanoparticle surface modification on the mechanical properties of polystyrene-based nanocomposites. RSC Adv. 2018, 8, 11109–11118. [Google Scholar] [CrossRef]
- Studart, A.R.; Amstad, E.; Gauckler, L.J. Colloidal stabilization of nanoparticles in concentrated suspensions. Langmuir 2007, 23, 1081–1090. [Google Scholar] [CrossRef]
- Cabuzu, D.; Cirja, A.; Puiu, R.; Grumezescu, A.M. Biomedical applications of gold nanoparticles. Curr. Top. Med. Chem. 2015, 15, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xianyu, Y.; Jiang, X. Surface modification of gold nanoparticles with small molecules for biochemical analysis. Acc. Chem. Res. 2017, 50, 310–319. [Google Scholar] [CrossRef]
- Saqib, S.; Akram, A.; Halim, S.A.; Tassaduq, R. Sources of β-galactosidase and its applications in food industry. 3 Biotech. 2017, 7, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Zibrat, N.; Skrt, M.; Jamnik, P. Potential application of β-galactosidase in food science and nutrition. Acta. Agric. Slov. 2017, 110, 258–269. [Google Scholar]
- Dykman, L.A.; Khlebtsov, N.G. Gold nanoparticles in biology and medicine: Recent advances and prospects. Act. Nat. 2011, 3, 34–55. [Google Scholar]
- Gupta, A.; Landis, R.F.; Rotelloa, V.M. Nanoparticle-based antimicrobials: Surface functionality is critical. F1000Research 2016, 5, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Chi, J.; Zhang, C.; Wang, M.; Liang, H.; Hou, J.; Ai, S.; Li, X. A simple and sensitive sensor for lactose based on cascade reactions in Au nanoclusters and enzymes co-encapsulated metal-organic frameworks. Food Chem. 2021, 339, 127863. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Husain, Q. Potential applications of enzymes immobilized on/in nanoparticles: A review. Biotechnol. Adv. 2012, 30, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.M.; Zhou, H.S.; Matsuda, N.; Honma, I.; Shimada, K.; Takatsu, A.; Kato, K. Characterization of gold nanoparticles synthesized using sucrose by seeding formation in the solid phase and seeding growth in aqueous solution. J. Phys. Chem. B 2004, 108, 7006–7011. [Google Scholar] [CrossRef]
- Zarabi, M.F.; Arshadi, N.; Farhangi, A.; Akbarzadeh, A. Preparation and characterization of gold nanoparticles with amino acids, examination of their stability. Ind. J. Clin. Biochem. 2014, 29, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Dincer, A.; Telefoncu, A. Improving the stability of cellulase by immobilization on modified polyvinyl alcohol coated chitosan beads. J. Mol. Catal. B Enzym. 2007, 45, 10–14. [Google Scholar] [CrossRef]
- Satar, R.; Ismail, S.A.; Rehan, M.; Ansari, S.A. Elucidating the binding efficacy of β-galactosidase on graphene by docking approach and its potential application in galacto-oligosaccharide production. Bioproc. Biosyst. Eng. 2016, 39, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Talebi, M.; Vaezifar, S.; Jafary, F.; Fazilati, M.; Motamedi, S. Stability improvement of immobilized α-amylase using nano pore zeolite. Iran. J. Biotech. 2016, 14, 33–38. [Google Scholar] [CrossRef]
- Guven, R.G.; Kaplan, A.; Guven, K.; Matpan, F.; Dogru, M. Effects of various inhibitors on β-galactosidase purified from the thermoacidophilic Alicyclobacillus acidocaldarius subsp. Rittmannii isolated from Antarctica. Biotechnol. Bioproc. Eng. 2011, 16, 114–119. [Google Scholar] [CrossRef]
- Ansari, S.A.; Al-shaeri, M. Biotechnological application of surface modified cerium oxide nanoparticles. Braz. J. Chem. Eng. 2019, 36, 109–115. [Google Scholar] [CrossRef]
- McFarland, A.D.; Haynes, C.L.; Mirkin, C.A.; Van Duyne, R.P.; Godwin, H.A. Color my nanoworld. J. Chem. Educ. 2004, 81, 544–550. [Google Scholar] [CrossRef]
- Nugraha, A.D.; Wulandari, I.O.; Rahayu, L.B.H.; Rivai, I.; Santojo, D.J.D.H.; Sabarudin, A. One-pot synthesis and surface modification of Fe3O4 nanoparticles using polyvinyl alcohol by coprecipitation and ultrasonication methods. IOP Conf. Ser. Mat. Sci. Eng. 2018, 299, 1–7. [Google Scholar] [CrossRef]
- Ansari, S.A.; Ahmad, S.I.; Jafri, M.A.; Naseer, M.I.; Satar, R. Utility of functionalized agarose nanoparticles in hydrolyzing lactose in batch reactors for dairy industries. Quim. Nov. 2018, 41, 429–433. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshanberi, A.M.; Satar, R.; Ansari, S.A. Stabilization of β-Galactosidase on Modified Gold Nanoparticles: A Preliminary Biochemical Study to Obtain Lactose-Free Dairy Products for Lactose-Intolerant Individuals. Molecules 2021, 26, 1226. https://doi.org/10.3390/molecules26051226
Alshanberi AM, Satar R, Ansari SA. Stabilization of β-Galactosidase on Modified Gold Nanoparticles: A Preliminary Biochemical Study to Obtain Lactose-Free Dairy Products for Lactose-Intolerant Individuals. Molecules. 2021; 26(5):1226. https://doi.org/10.3390/molecules26051226
Chicago/Turabian StyleAlshanberi, Asim Muhammed, Rukhsana Satar, and Shakeel Ahmed Ansari. 2021. "Stabilization of β-Galactosidase on Modified Gold Nanoparticles: A Preliminary Biochemical Study to Obtain Lactose-Free Dairy Products for Lactose-Intolerant Individuals" Molecules 26, no. 5: 1226. https://doi.org/10.3390/molecules26051226
APA StyleAlshanberi, A. M., Satar, R., & Ansari, S. A. (2021). Stabilization of β-Galactosidase on Modified Gold Nanoparticles: A Preliminary Biochemical Study to Obtain Lactose-Free Dairy Products for Lactose-Intolerant Individuals. Molecules, 26(5), 1226. https://doi.org/10.3390/molecules26051226




