2,1,3-Benzothiadiazole Small Donor Molecules: A DFT Study, Synthesis, and Optoelectronic Properties
Abstract
1. Introduction
2. Results
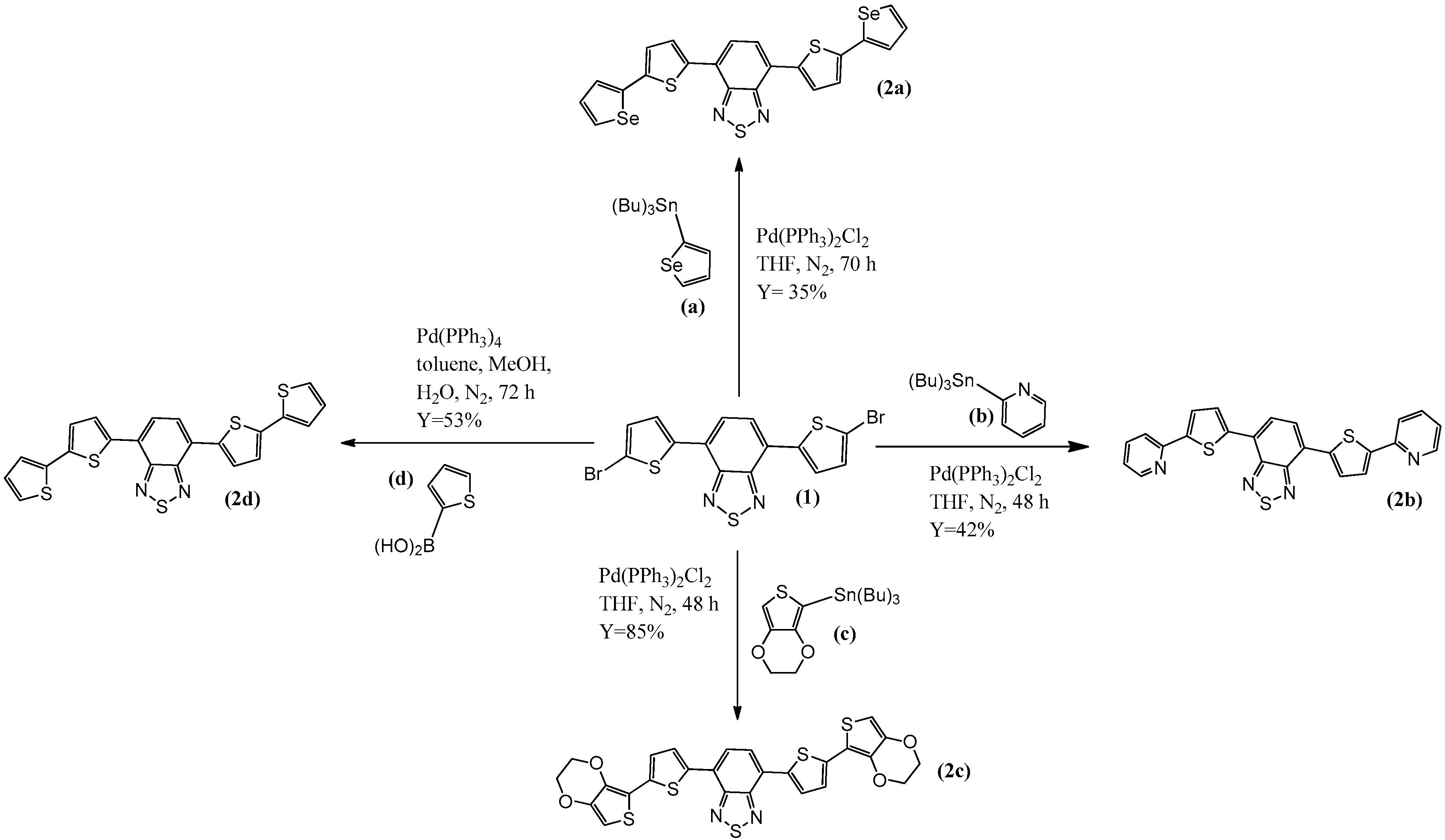
2.1. Synthesis
2.2. Theoretical Studies
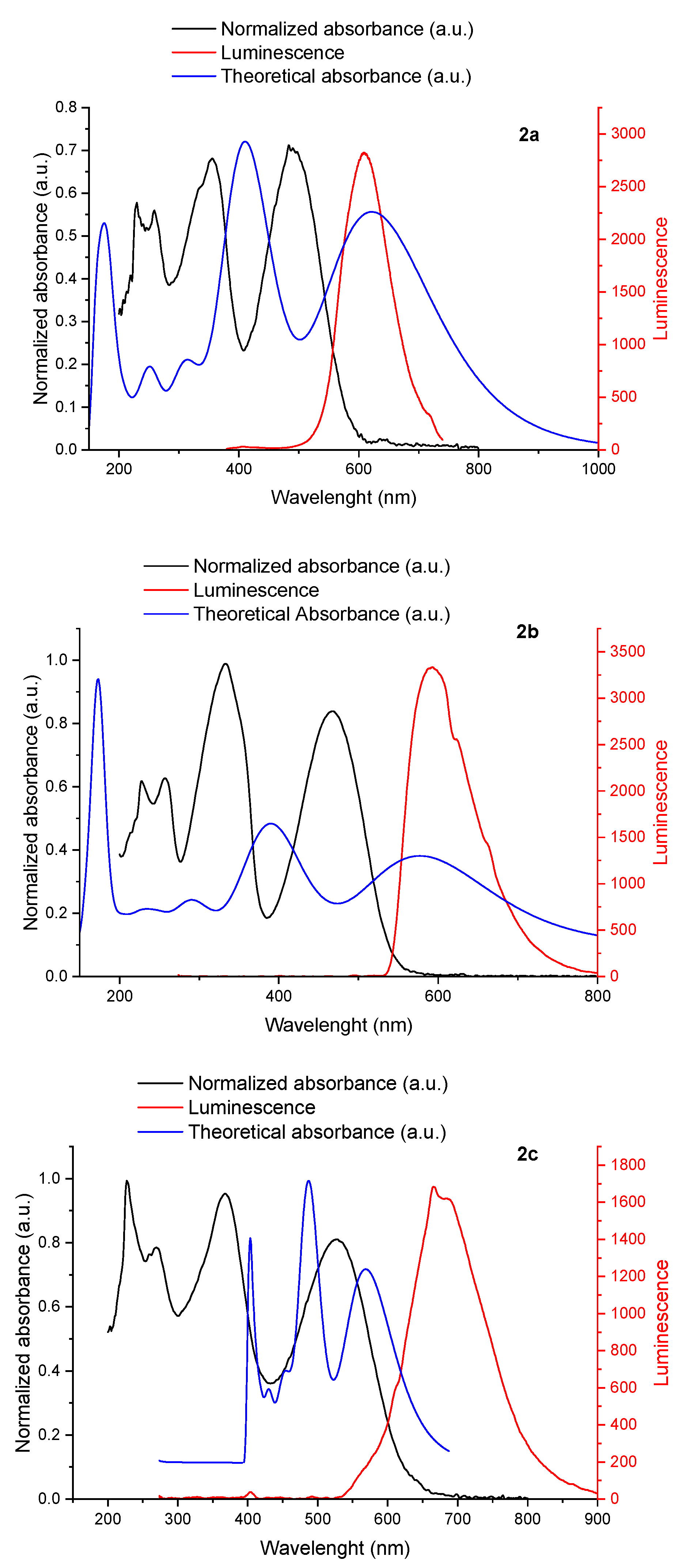
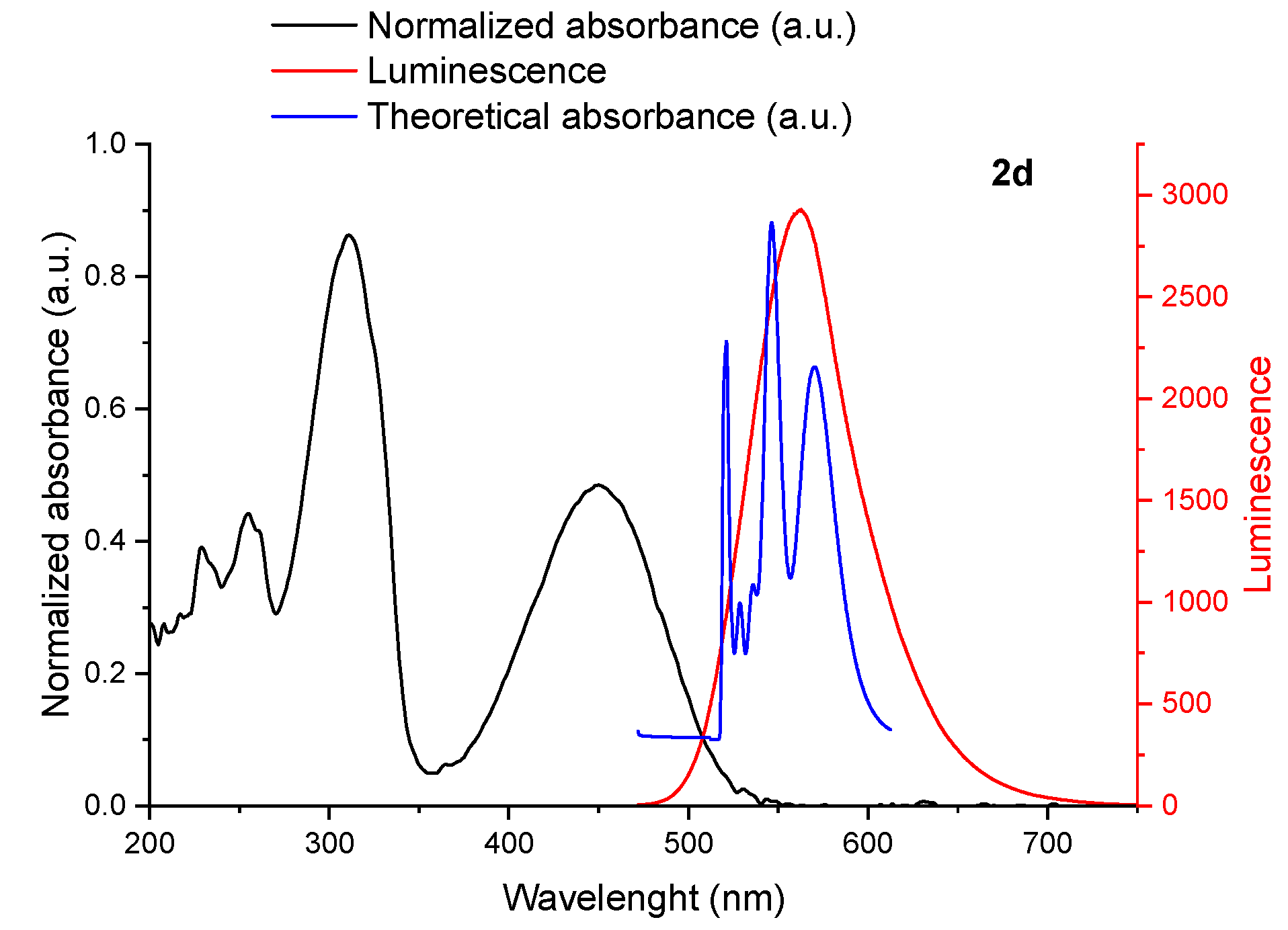
2.3. Photophysical Studies
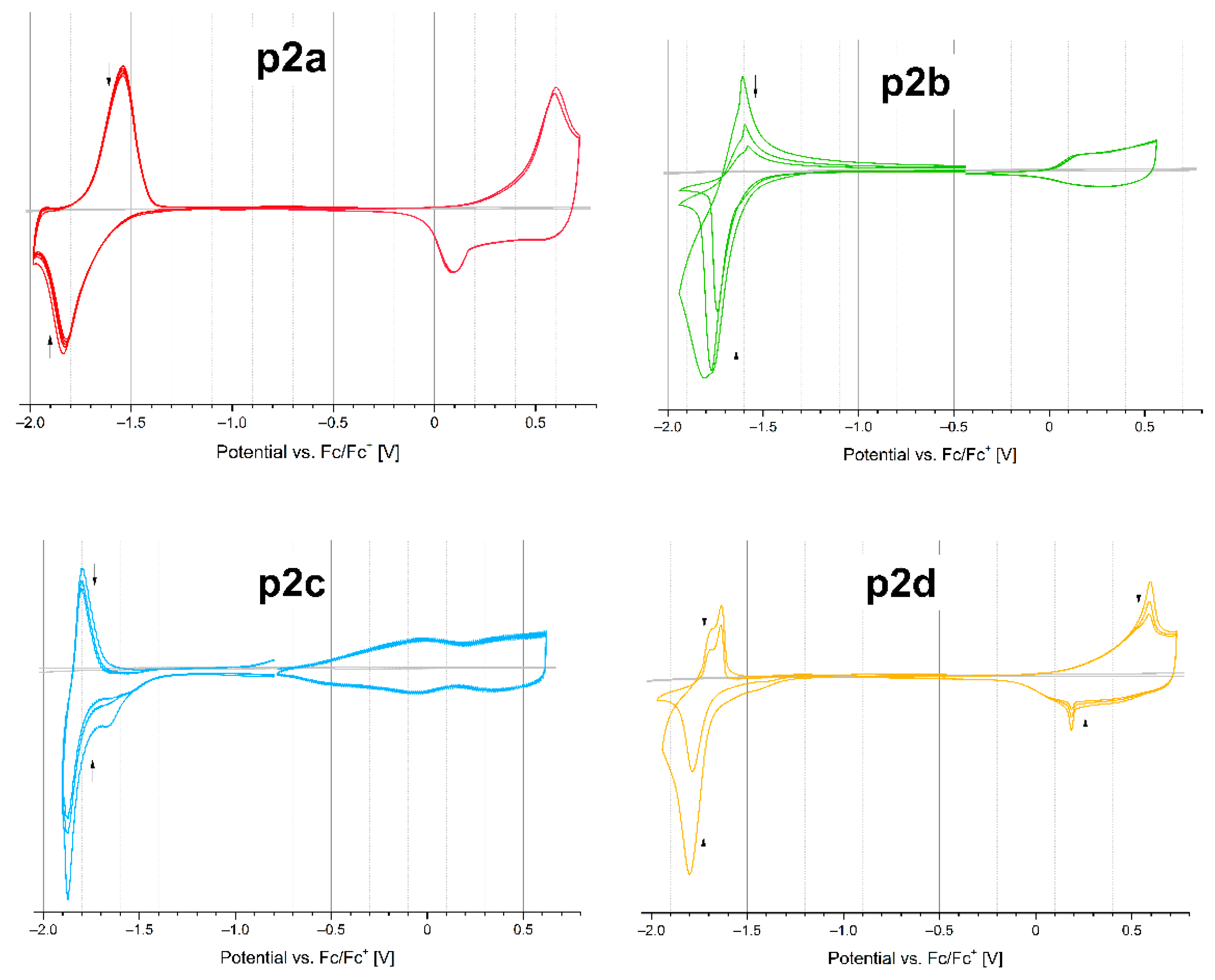
2.4. Electrochemical Properties
3. Materials and Methods
3.1. Computational Details
3.2. Chemistry
3.2.1. Preparation of 4,7-bis(5-(selenophen-2-yl)thiophen-2-yl)benzothiadiazole (2a)
3.2.2. Preparation of 4,7-bis(5-(pyridin-2-yl)thiophen-2-yl)benzothiadiazole (2b)
3.2.3. Preparation of 4,7-bis(5-(3,4-ethylenedioxythiophene)thiophen-2-yl)benzothiadiazole (2c)
3.2.4. Preparation of 4,7-di([2,2′-bithiophen]-5-yl)benzothiadiazole (2d)
3.3. Optical Measurements
3.4. Cyclic Voltammetry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Baldo, M.A.; Thompson, M.E.; Forrest, S.R. High-efficiency fluorescent organic light emitting devices using a phosphorescent sensitizer. Nature 2000, 403, 750–753. [Google Scholar] [CrossRef]
- Shu, Y.C.; Gong, Z.H.; Shu, C.F.; Breitung, E.M.; McMahon, R.J.; Lee, G.H.; Jen, A.K.Y. Synthesis and characterization of nonlinear optical chromophores with conformationally locked polyenes possessing enhanced thermal stability. Chem. Mater. 1999, 11, 1628–1632. [Google Scholar] [CrossRef]
- van Franeker, J.J.; Bruijnaers, B.J.; Wienk, M.M.; Janssen, R.A.J. Structure–property relationships for bis-diketopyrrolopyrrole molecules in organic photovoltaics. J. Mater. Chem. 2016, 4, 10532–10541. [Google Scholar] [CrossRef]
- Mahadik, F.S.S.; Garud, D.R.; Ware, A.P.; Pingale, S.S.; Kamble, R.M. Design, Synthesis and Opto-electrochemical Properties of Novel Donor–Acceptor Based 2,3-di(hetero-2-yl)pyrido [2,3-b]pyrazine amine derivatives as Blue-Orange Fluorescent Materials. Dye. Pigment. 2021, 184, 108742. [Google Scholar] [CrossRef]
- Liu, Z.; Hao, F.; Xu, H.; Wang, H.; Wu, J.; Tian, Y. A-π-D-π-A pyridinium salts: Synthesis, crystal structures, two-photon absorption properties and application to biological imaging. CrystEngComm 2015, 17, 5562–5568. [Google Scholar] [CrossRef]
- Renkert, S.; Fall, S.; Motamen, S.; Jarrosson, T.; Serein-Spirau, F.; Heiser, T.; Simon, L.; Reiter, G.; Bubendorff, J.L. A new growth process for crystalline ultra-thin layers of conjugated oligomers used in field-effect transistor applications. Appl. Surf. Sci. 2021, 539, 148024. [Google Scholar] [CrossRef]
- He, J.; Liang, B.; Yan, X.; Liu, F.; Wang, J.; Yang, Z.; You, R.; Wang, C.; Sun, P.; Yan, X.; et al. A TPA-DCPP organic semiconductor film-based room temperature NH3 sensor for insight into the sensing properties. Sens. Actuators B Chem. 2021, 327, 128940. [Google Scholar] [CrossRef]
- Lin, Y.; He, Q.; Zhao, F.; Huo, L.; Mai, J.; Lu, X.; Su, C.-J.; Li, T.; Wang, J.; Zhu, J.; et al. A Facile Planar Fused-Ring Electron Acceptor for As-Cast Polymer Solar Cells with 8.71% Efficiency. J. Am. Chem. Soc. 2016, 138, 2973–2976. [Google Scholar] [CrossRef]
- Chen, F.-X.; Xu, J.-Q.; Liu, Z.-X.; Chen, M.; Xia, R.; Yang, Y.; Lau, T.-K.; Zhang, Y.; Lu, X.; Yip, H.-L.; et al. Near-infrared electron acceptors with fluorinated regioisomeric backbone for highly efficient polymer solar cells. Adv. Mater. 2018, 30, 1803769. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Y.; Zhang, W.; Li, E.; Shen, C.; Jiang, H.; Tian, H.; Zhu, W.H. Low cost and stable quinoxaline-based hole-transporting materials with a D-A-D molecular configuration for efficient perovskite solar cells. Chem. Sci. 2018, 9, 5919–5928. [Google Scholar] [CrossRef]
- Ding, X.; Chen, C.; Sun, L.; Li, H.; Chen, H.; Su, J.; Li, H.; Li, H.; Xu, L.; Cheng, M. Highly efficient phenothiazine 5,5-dioxide-based hole transport materials for planar perovskite solar cells with a PCE exceeding 20%. J. Mater. Chem. A 2019, 7, 9510–9516. [Google Scholar] [CrossRef]
- Liu, M.; Yang, J.; Yin, Y.; Zhang, Y.; Zhou, E.; Guo, F.; Zhao, L. Novel perylene diimide-based polymers with electron-deficient segments as the comonomer for efficient all-polymer solar cells. J. Mater. Chem. A 2018, 6, 414–422. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, W.; Xu, X.; Genene, Z.; Di Carlo Rasi, D.; Mammo, W.; Yartsev, A.; Andersson, M.R.; Janssen, R.A.J.; Wang, E. High-Performance and Stable All-Polymer Solar Cells Using Donor and Acceptor Polymers with Complementary Absorption. Adv. Energy Mater. 2017, 7, 1602722. [Google Scholar] [CrossRef]
- Zhao, R.; Lin, B.; Feng, J.; Dou, C.; Ding, Z.; Ma, W.; Liu, J.; Wang, L. Amorphous Polymer Acceptor Containing B ← N Units Matches Various Polymer Donors for All-Polymer Solar Cells. Macromolecules 2019, 52, 7081–7088. [Google Scholar] [CrossRef]
- Chen, Y.S.; Wan, X.J.; Long, G.K. High Performance Photovoltaic Applications Using Solution-Processed Small Molecules. Acc. Chem. Res. 2013, 46, 2645–2655. [Google Scholar] [CrossRef]
- Sun, Y.; Welch, G.C.; Leong, W.L.; Takacs, C.J.; Bazan, G.C.; Heeger, A.J. Solution-Processed Small-Molecule Solar Cells with 6.7% Efficiency. Nat. Mater. 2012, 11, 44–48. [Google Scholar] [CrossRef]
- Zając, D.; Honisz, D.; Łapkowski, M.; Sołoducho, J. Synthesis and Properties of New Dithienosilole Derivatives as Luminescent Materials. Molecules 2019, 24, 2259. [Google Scholar] [CrossRef]
- Zhu, L.; Zhong, W.K.; Qiu, C.Q.; Lyu, B.S.; Zhou, Z.C.; Zhang, M.J.; Song, N.; Xu, J.Q.; Wang, J.; Ali, J.; et al. Aggregation-Induced Multilength Scaled Morphology Enabling 11.76% Efficiency in All-Polymer Solar Cells Using Printing Fabrication. Adv. Mater. 2019, 31, 1902899. [Google Scholar] [CrossRef]
- Yan, H.; Chen, Z.; Zheng, Y.; Newman, C.; Quinn, J.R.; Dotz, F.; Kastler, M.; Facchetti, A. A high-mobility electron-transporting polymer for printed transistors. Nature 2009, 457, 679–686. [Google Scholar] [CrossRef]
- Kim, T.; Kim, J.H.; Kang, T.E.; Lee, C.; Kang, H.; Shin, M.; Wang, C.; Ma, B.; Jeong, U.; Kim, T.S.; et al. Flexible, highly efficient all-polymer solar cells. Nat. Commun. 2015, 6, 8547. [Google Scholar] [CrossRef]
- Benten, H.; Nishida, T.; Mori, D.; Xu, H.; Ohkita, H.; Ito, S. High-performance ternary blend all-polymer solar cells with complementary absorption bands from visible to near-infrared wavelengths. Energy Environ. Sci. 2016, 9, 135. [Google Scholar] [CrossRef]
- Walker, B.; Tamayo, A.B.; Dang, X.D.; Zalar, P.; Seo, J.H.; Garcia, A.; Tantiwiwat, M.; Nguyen, T.Q. Nanoscale Phase Separation and High Photovoltaic Efficiency in Solution-Processed, Small-Molecule Bulk Heterojunction Solar Cells. Adv. Funct. Mater. 2009, 19, 3063. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.-L.; Lau, T.-K.; Lu, X.; Zhu, C.; Peng, H.; Johnson, P.A.; et al. Single-Junction Organic Solar Cell with over 15% Efficiency Using Fused-Ring Acceptor with Electron-Deficient Core. Joule 2019, 3, 1140–1151. [Google Scholar] [CrossRef]
- Liu, T.; Luo, Z.; Chen, Y.; Yang, T.; Xiao, Y.; Zhang, G.; Ma, R.; Lu, X.; Zhan, C.; Zhang, M.; et al. A nonfullerene acceptor with a 1000 nm absorption edge enables ternary organic solar cells with improved optical and morphological properties and efficiencies over 15%. Energy Environ. Sci. 2019, 12, 2529–2536. [Google Scholar] [CrossRef]
- Xu, C.; Wang, J.; An, Q.; Ma, X.; Hu, Z.; Gao, J.; Zhang, J.; Zhang, F. Ternary small molecules organic photovoltaics exhibiting 12.84% efficiency. Nano Energy 2019, 66, 104119. [Google Scholar] [CrossRef]
- Chen, H.; Hu, D.; Yang, Q.; Gao, J.; Fu, J.; Yang, K.; He, H.; Chen, S.; Kan, Z.; Duan, T.; et al. All-Small-Molecule Organic Solar Cells with an Ordered Liquid Crystalline Donor. Joule 2019, 3, 3034–3047. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, S.; He, C.; Zhang, J.; Yao, H.; Yang, Y.; Zhang, Y.; Zhao, W.; Hou, J. New wide band gap donor for efficient fullerene-free all-small-molecule organic solar cells. J. Am. Chem. Soc. 2017, 139, 1958–1966. [Google Scholar] [CrossRef]
- Deng, D.; Zhang, Y.; Zhang, J.; Wang, Z.; Zhu, L.; Fang, J.; Xia, B.; Wang, Z.; Lu, K.; Ma, W.; et al. Fluorination-enabled Optimal Morphology Leads to over 11% Efficiency for Inverted Small-molecule Organic Solar Cells. Nat Commun. 2016, 7, 13740. [Google Scholar] [CrossRef]
- Gao, K.; Jo, S.B.; Shi, X.; Nian, L.; Zhang, M.; Kan, Y.; Lin, F.; Kan, B.; Xu, B.; Rong, Q.; et al. Over 12% efficiency nonfullerene all-small-molecule organic solar cells with sequentially evolved multilength scale morphologies. Adv. Mater. 2019, 31, 1807842. [Google Scholar] [CrossRef]
- Spychalska, K.; Zając, D.; Cabaj, J. Electrochemical biosensor for detection of 17β-estradiol using semi-conducting polymer and horseradish peroxidase. RSC Adv. 2020, 10, 9079–9087. [Google Scholar] [CrossRef]
- Jeon, Y.; Kim, T.-M.; Kim, J.-J.; Hong, J.-I. Vacuum-depositable thiophene- and benzothiadiazole-based donor materials for organic solar cells. N.J.Chem. 2015, 39, 9591–9595. [Google Scholar] [CrossRef]
- Coughlin, J.E.; Henson, Z.B.; Welch, G.C.; Bazan, G.C. Design and Synthesis of Molecular Donors for Solution-Processed High-Efficiency Organic Solar Cells. Acc. Chem. Res. 2014, 47, 257–270. [Google Scholar] [CrossRef]
- Louis, E.; San-Fabián, E.; Díaz-García, M.A.; Chiappe, G.; Vergés, J.A. Are Electron Affinity and Ionization Potential Intrinsic Parameters to Predict the Electron or Hole Acceptor Character of Amorphous Molecular Materials? J. Phys. Chem. Lett. 2017, 8, 2445–2449. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Yeh, S.-C.; Chen, C.-T.; Chen, C.-T. Comparison of Thiophene- and Selenophene-Bridged Donor–Acceptor Low Band-Gap Copolymers Used in Bulk-Heterojunction Organic Photovoltaics. J. Mater. Chem. 2012, 22, 21549. [Google Scholar] [CrossRef]
- Alghamdi, A.A.B.; Watters, D.C.; Yi, H.; Al-Faifi, S.; Almeataq, M.S.; Coles, D.; Kingsley, J.; Lidzey, D.G.; Iraqi, A. Selenophene vs. Thiophene in Benzothiadiazole-Based Low Energy Gap Donor–Acceptor Polymers for Photovoltaic Applications. J. Mater. Chem. A 2013, 1, 5165. [Google Scholar] [CrossRef]
- McCormick, T.M.; Bridges, C.R.; Carrera, E.I.; DiCarmine, P.M.; Gibson, G.L.; Hollinger, J.; Kozycz, L.M.; Seferos, D.S. Conjugated Polymers: Evaluating DFT Methods for More Accurate Orbital Energy Modeling. Macromolecules 2013, 46, 3879–3886. [Google Scholar] [CrossRef]
- Ledwon, P.; Thomson, N.; Angioni, E.; Findlay, N.J.; Skabara, P.J.; Domagala, W. The role of structural and electronic factors in shaping the ambipolar properties of donor–acceptor polymers of thiophene and benzothiadiazole. RSC Adv. 2015, 5, 77303–77315. [Google Scholar] [CrossRef]
- Meloni, F.; Spychalska, K.; Zając, D.; Pilo, M.I.; Zucca, A.; Cabaj, J. Application of a Thiadiazole-derivative in a Tyrosinase-based Amperometric Biosensor for Epinephrine Detection. Electroanalysis, under review.
- Parr, R.G.; Yang, W. Density Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
- Becke, A.D. Density functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate Spin-Dependent Electron Liquid Correlation Energies for Local Spin Density Calculations: A Critical Analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Dunning, T., Jr. H. Gaussian Basis Sets for Use in Correlated Molecular Calculations. I. The Atoms Boron through Neon and Hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Zając, D.; Sołoducho, J.; Jarosz, T.; Łapkowski, M.; Roszak, S. Conjugated silane-based arylenes as luminescent materials. Electrochim. Acta 2015, 173, 105–116. [Google Scholar] [CrossRef]
- Zając, D.; Sołoducho, J.; Jarosz, T.; Łapkowski, M.; Roszak, S. Efficient Synthesis and Physicochemical Characteristic of Novel Anthracene-based Building Blocks for Optical Materials. Indian J. Appl. Res. 2016, 6, 395–403. [Google Scholar]
- Zając, D.; Sołoducho, J.; Jarosz, T.; Łapkowski, M.; Roszak, S. Push-pull structures of symmetric silane derivatives as a novel hosting materials. Indian J. Appl. Res. 2017, 7, 58–66. [Google Scholar]
- Runge, E.; Gross, E.K.U. Density-Functional Theory for Time-Dependent Systems. Phys. Rev. Lett. 1984, 52, 997–1000. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Dennington, R.; Keith, T.A.; Millam, M. GaussView, Version 6.1; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
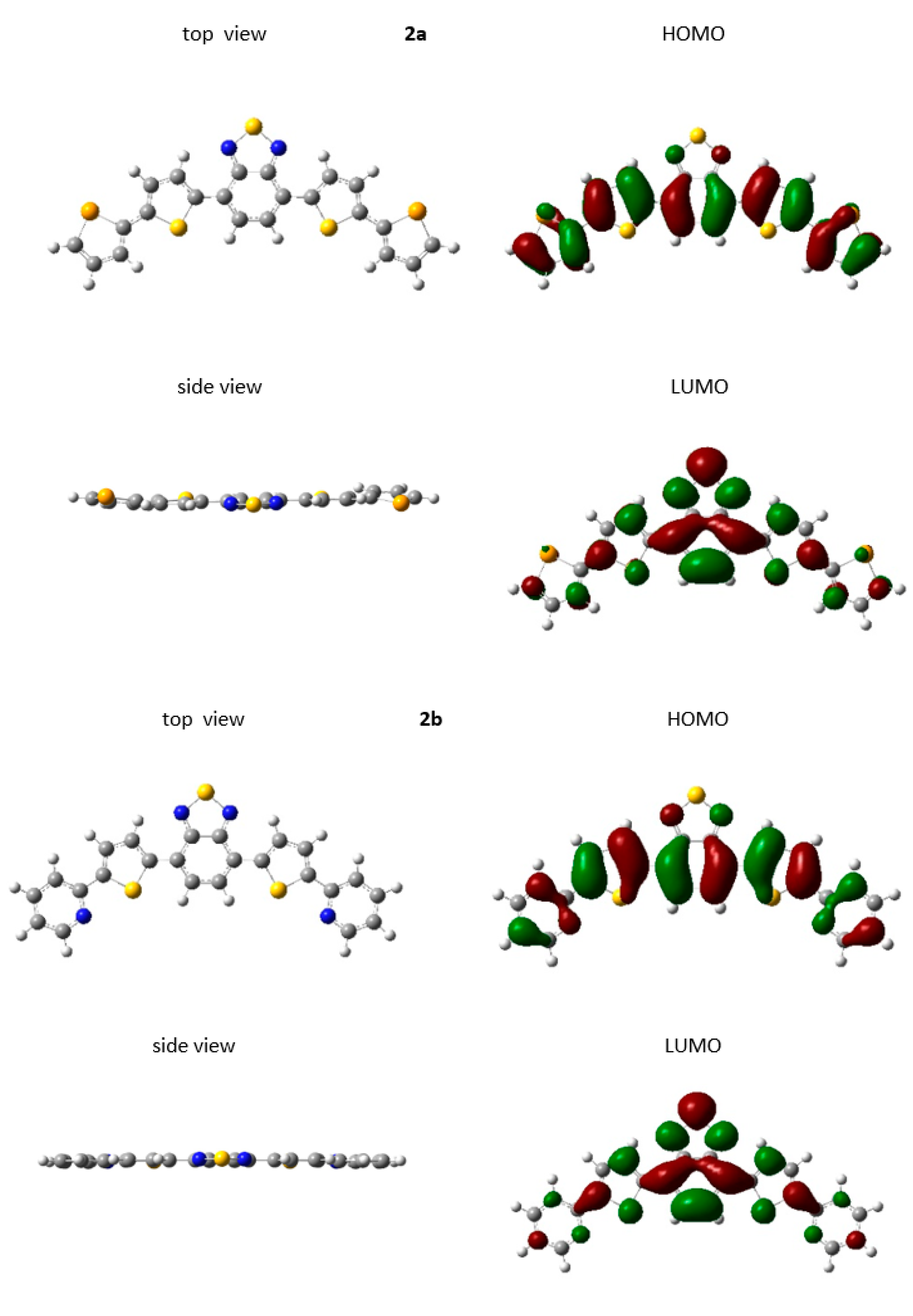
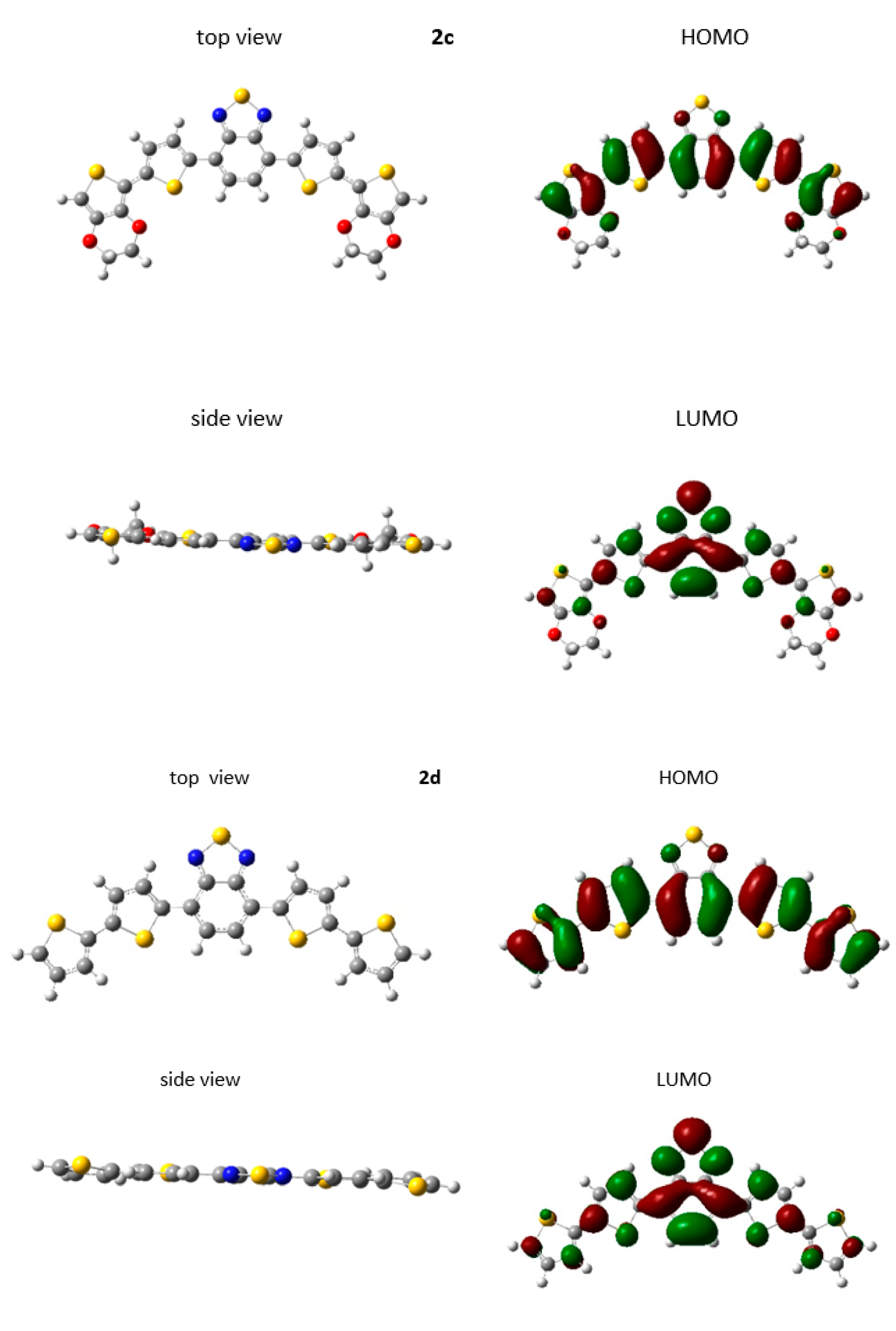
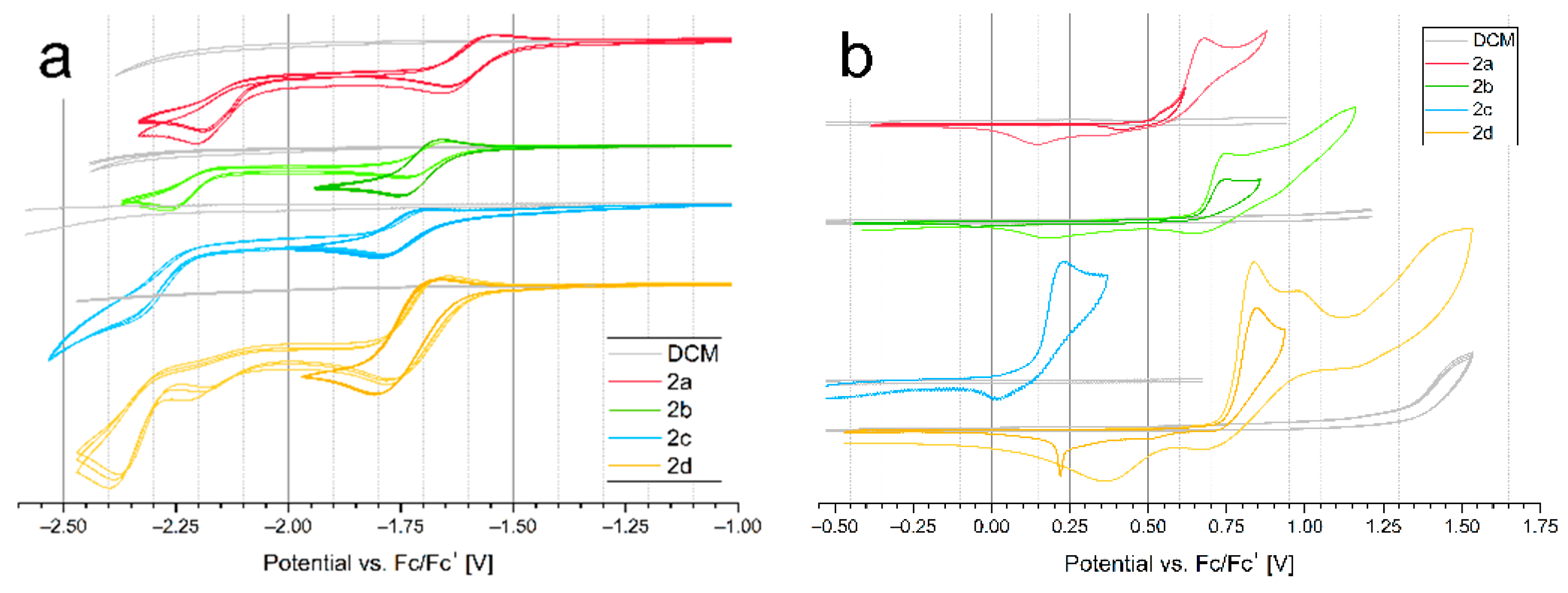
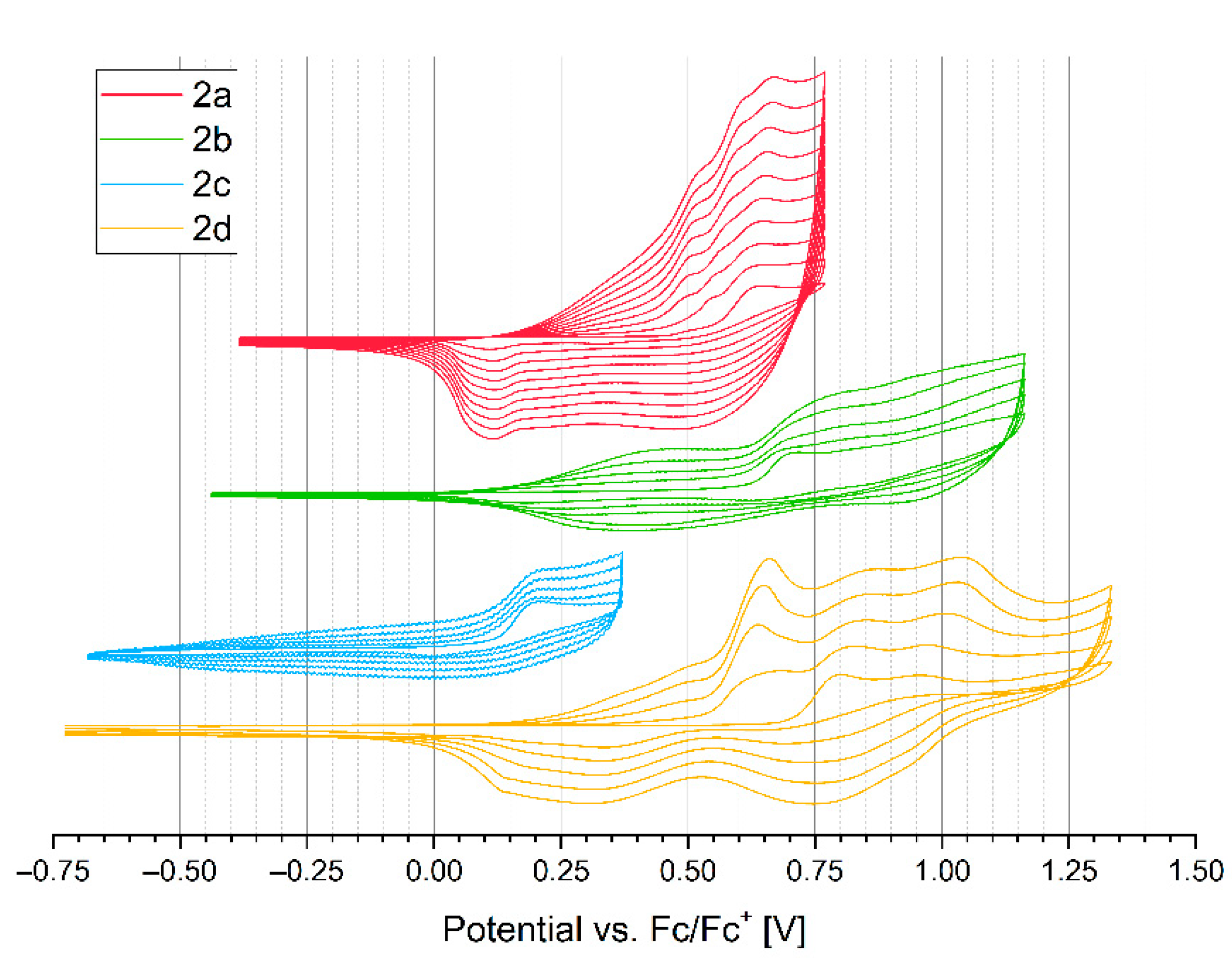
| Compound | Eoxonset (V) | Eredonset (V) | IP (eV) | IPvercal (eV) | IPadcal (eV) | EA (eV) | HOMOcal (eV) | LUMOcal (eV) | Egcal (eV) | ΔEgel (eV) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2a | 0.50 | −1.51 | 5.60 | 6.21 | 6.07 | 3.59 | −5.12 | −2.84 | 2.27 | 2.01 |
| 2b | 0.66 | −1.62 | 5.76 | 6.37 | 6.25 | 3.48 | −5.24 | −2.78 | 2.46 | 2.28 |
| 2c | 0.14 | −1.61 | 5.24 | 5.84 | 5.69 | 3.49 | −4.78 | −2.59 | 2.19 | 1.75 |
| 2d | 0.76 | −1.62 | 5.86 | 6.22 | 6.08 | 3.48 | −5.12 | −2.83 | 2.29 | 2.38 |
| Compound | λmax ex (nm) | λmax cal (nm) | λemmax (nm) | f |
|---|---|---|---|---|
| 2a | 355 | 413 (H-L+1) | 609 | 0.88 |
| 483 | 623 (H-L) | 0.73 | ||
| 2b | 332 | 393 (H-L+1) | 592 | 0.86 |
| 468 | 578 (H-L) | 0.69 | ||
| 2c | 370 | 418 (H-L+1) | 667 | 0.91 |
| 526 | 652 (H-L) | 0.68 | ||
| 2d | 311 | 406 (H-L+1) | 563 | 0.9 |
| 452 | 619 (H-L) | 0.69 |
| Compound | Eoxonset (V) | Eredonset (V) | IP (eV) | EA (eV) | ΔEgel (eV) |
|---|---|---|---|---|---|
| p2a | 0.17 | −1.47 | 5.27 | 3.63 | 1.64 |
| p2b | 0.01 | −1.63 | 5.11 | 3.47 | 1.64 |
| p2c | −0.69 | −1.41 | 4.41 | 3.69 | 0.72 |
| p2d | 0.09 | −1.28 | 5.19 | 3.82 | 1.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zając, D.; Honisz, D.; Łapkowski, M.; Sołoducho, J. 2,1,3-Benzothiadiazole Small Donor Molecules: A DFT Study, Synthesis, and Optoelectronic Properties. Molecules 2021, 26, 1216. https://doi.org/10.3390/molecules26051216
Zając D, Honisz D, Łapkowski M, Sołoducho J. 2,1,3-Benzothiadiazole Small Donor Molecules: A DFT Study, Synthesis, and Optoelectronic Properties. Molecules. 2021; 26(5):1216. https://doi.org/10.3390/molecules26051216
Chicago/Turabian StyleZając, Dorota, Damian Honisz, Mieczysław Łapkowski, and Jadwiga Sołoducho. 2021. "2,1,3-Benzothiadiazole Small Donor Molecules: A DFT Study, Synthesis, and Optoelectronic Properties" Molecules 26, no. 5: 1216. https://doi.org/10.3390/molecules26051216
APA StyleZając, D., Honisz, D., Łapkowski, M., & Sołoducho, J. (2021). 2,1,3-Benzothiadiazole Small Donor Molecules: A DFT Study, Synthesis, and Optoelectronic Properties. Molecules, 26(5), 1216. https://doi.org/10.3390/molecules26051216







