Synthesis of Four Pentacyclic Triterpene–Sialylglycopeptide Conjugates and Their Affinity Assays with Hemagglutinin
Abstract
1. Introduction
2. Results and Discussion
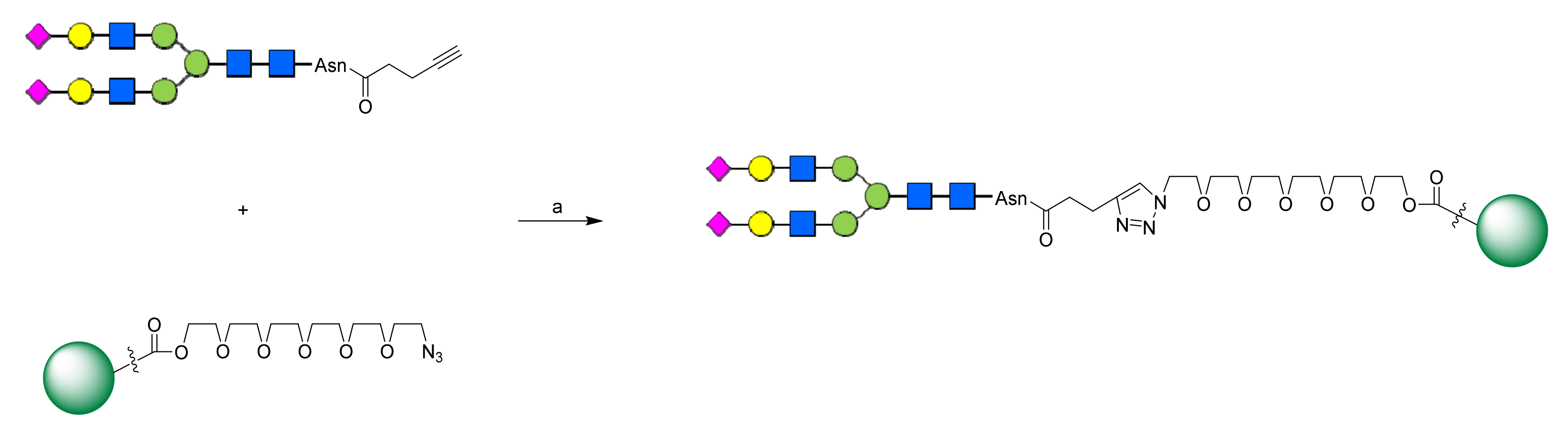
2.1. Chemistry
2.2. SPR Assay
3. Materials and Methods
3.1. Chemistry
3.1.1. Preparation of SGP
3.1.2. Preparation of SCT-Asn
3.1.3. Preparation of SCT-Asn-Alkyne
3.1.4. General Procedure for Preparing Pentacyclic Triterpenes with the Azide Group
UA-N3
OA-N3
BA-N3
GA-N3
3.1.5. General Procedure for Preparing SCT-Asn-Pentacyclic Triterpene Conjugates
SCT-Asn-UA
SCT-Asn-OA
SCT-Asn-BA
SCT-Asn-GA
3.2. SPR Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 1 January 2021).
- Johnson, N.; Mueller, J. Updating the accounts: Global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull. Hist. Med. 2002, 76, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Carrat, F.; Flahault, A. Influenza vaccine: The challenge of antigenic drift. Vaccine 2007, 25, 6852–6862. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, N.M.; Palese, P. The biology of influenza viruses. Vaccine 2008, 26, D49–D53. [Google Scholar] [CrossRef]
- Gong, J.; Fang, H.; Li, M.; Liu, Y.; Yang, K.; Liu, Y.; Xu, W. Potential targets and their relevant inhibitors in anti-influenza fields. Curr. Med. Chem. 2009, 16, 3716–3739. [Google Scholar] [CrossRef]
- Takashita, E.; Meijer, A.; Lackenby, A.; Gubareva, L.; Rebelo-de-Andrade, H.; Besselaar, T.; Fry, A.; Gregory, V.; Leang, S.-K.; Huang, W.; et al. Global update on the susceptibility of human influenza viruses to neuraminidase inhibitors, 2013–2014. Antiviral Res. 2015, 117, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Y.; Ma, C.; Fiorin, G.; Wang, J.; Pinto, L.H.; Lamb, R.A.; Klein, M.L.; DeGrado, W.F. Structure and inhibition of the drug-resistant S31N mutant of the M2 ion channel of influenza A virus. Proc. Natl. Acad. Sci. USA 2013, 110, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Copeland, C.S.; Doms, R.W.; Bolzau, E.M.; Webster, R.G.; Helenius, A. Assembly of influenza hemagglutinin trimers and its role in intracellular transport. J. Cell Biol. 1986, 103, 1179–1191. [Google Scholar] [CrossRef]
- Boonstra, S.; Blijleven, J.S.; Roos, W.H.; Onck, P.R.; van der Giessen, E.; van Oijen, A.M. Hemagglutinin-mediated membrane fusion: A biophysical perspective. Ann. Rev. Biophys. 2018, 47, 153–173. [Google Scholar] [CrossRef]
- Caton, A.J.; Brownlee, G.G.; Yewdell, J.W.; Gerhard, W. The antigenic structure of the influenza-virus A/PR/8/34 hemagglutinin (H1 subtype). Cell 1982, 31, 417–427. [Google Scholar] [CrossRef]
- Seko, A.; Koketsu, M.; Nishizono, M.; Enoki, Y.; Ibrahim, H.R.; Juneja, L.R.; Kim, M.; Yamamoto, T. Occurrence of a sialylglycopeptide and free sialylglycans in hen’s egg yolk. Biochim. Biophys. Acta Gen. Subj. 1997, 1335, 23–32. [Google Scholar] [CrossRef]
- Liu, L.; Prudden, A.R.; Bosman, G.P.; Boons, G.-J. Improved isolation and characterization procedure of sialylglycopeptide from egg yolk powder. Carbohydr. Res. 2017, 452, 122–128. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nagao, Y.; Kato, H.; Suzuki, T.; Matsumoto, M.; Murayama, J.-I. The hemagglutinins of the human influenza viruses A and B recognize different receptor microdomains. Biochim. Biophys. Acta Biomembr. 1987, 903, 417–424. [Google Scholar] [CrossRef]
- Gambaryan, A.; Tuzikov, A.; Chinarev, A.; Juneja, L.; Bovin, N.; Matrosovich, M. Polymeric inhibitor of influenza virus attachment protects mice from experimental influenza infection. Antiviral Res. 2002, 55, 201–205. [Google Scholar] [CrossRef]
- Makimura, Y.; Watanabe, S.; Suzuki, T.; Suzuki, Y.; Ishida, H.; Kiso, M.; Katayama, T.; Kumagai, H.; Yamamoto, K. Chemoenzymatic synthesis and application of a sialoglycopolymer with a chitosan backbone as a potent inhibitor of human influenza virus hemagglutination. Carbohydr. Res. 2006, 341, 1803–1808. [Google Scholar] [CrossRef]
- Wang, H.; Huang, W.; Orwenyo, J.; Banerjee, A.; Vasta, G.R.; Wang, L.X. Design and synthesis of glycoprotein-based multivalent glyco-ligands for influenza hemagglutinin and human galectin-3. Bioorg. Med. Chem. 2013, 21, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Ishitani, H.; Miura, Y.; Oishi, K.; Takahashi, T.; Suzuki, T.; Shoda, S.-I.; Kimura, Y. Protecting-group-free synthesis of glycopolymers bearing sialyloligosaccharide and their high binding with the influenza virus. ACS Macro Lett. 2014, 3, 1074–1078. [Google Scholar] [CrossRef]
- Murakami, M.; Okamoto, R.; Izumi, M.; Kajihara, Y. Chemical synthesis of an erythropoietin glycoform containing a complex-type disialyloligosaccharide. Angew. Chem. Int. Ed. Engl. 2012, 51, 3567–3572. [Google Scholar] [CrossRef]
- Huang, W.; Li, C.; Li, B.; Umekawa, M.; Yamamoto, K.; Zhang, X.; Wang, L.-X. Glycosynthases enable a highly efficient chemoenzymatic synthesis of N-glycoproteins carrying intact natural N-glycans. J. Am. Chem. Soc. 2009, 131, 2214–2223. [Google Scholar] [CrossRef]
- Huang, W.; Wang, D.N.; Yamada, M.; Wang, L.X. Chemoenzymatic synthesis and lectin array characterization of a class of N-glycan clusters. J. Am. Chem. Soc. 2009, 131, 17963–17971. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Yang, Y.; Tang, Y.; Tang, S.; Yang, L.; Sun, B.; Jiang, B.; Dong, J.; Liu, H.; Huang, M.; et al. One-pot N-glycosylation remodeling of IgG with non-natural sialylglycopeptides enables glycosite-specific and dual-payload antibody–drug conjugates. Org. Biomol. Chem. 2016, 14, 9501–9518. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-X. Chemoenzymatic synthesis of glycopeptides and glycoproteins through endoglycosidase-catalyzed transglycosylation. Carbohydr. Res. 2008, 343, 1509–1522. [Google Scholar] [CrossRef]
- Yamamoto, N.; Takayanagi, A.; Yoshino, A.; Sakakibara, T.; Kajihara, Y. An approach for a synthesis of asparagine-linked sialylglycopeptides having intact and homogeneous complex-type undecadisialyloligosaccharides. Chemistry 2007, 13, 613–625. [Google Scholar] [CrossRef]
- Huang, W.; Ochiai, H.; Zhang, X.Y.; Wang, L.X. Introducing N-glycans into natural products through a chemoenzymatic approach. Carbohydr. Res. 2008, 343, 2903–2913. [Google Scholar] [CrossRef] [PubMed]
- Ogata, M.; Murata, T.; Murakami, K.; Suzuki, T.; Hidari, K.; Suzuki, Y.; Usui, T. Chemoenzymatic synthesis of artificial glycopolypeptides containing multivalent sialyloligosaccharides with a gamma-polyglutamic acid backbone and their effect on inhibition of infection by influenza viruses. Biorg. Med. Chem. 2007, 15, 1383–1393. [Google Scholar] [CrossRef]
- Narla, S.N.; Sun, X.-L. Immobilized sialyloligo-macroligand and its protein binding specificity. Biomacromolecules 2012, 13, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Shi, Y.; Si, L.; Fan, Z.; Wang, H.; Xu, R.; Jiao, P.; Meng, K.; Tian, Z.; Zhou, X. Design, synthesis and biological activity evaluation of novel conjugated sialic acid and pentacyclic triterpene derivatives as anti-influenza entry inhibitors. MedChemComm 2016, 7, 1932–1945. [Google Scholar] [CrossRef]
- Shi, Y.; Si, L.; Han, X.; Fan, Z.; Wang, S.; Li, M.; Sun, J.; Zhang, Y.; Zhou, D.; Xiao, S. Synthesis of novel pentacyclic triterpene–Neu5Ac2en derivatives and investigation of their in vitro anti-influenza entry activity. MedChemComm 2017, 8, 1531–1541. [Google Scholar] [CrossRef]
- Han, X.; Si, L.L.; Shi, Y.Y.; Fan, Z.B.; Wang, S.X.; Tian, Z.Y.; Li, M.; Sun, J.Q.; Jiao, P.X.; Ran, F.X.; et al. Synthesis and in vitro anti-influenza virus evaluation of novel sialic acid (C-5 and C-9)-pentacyclic triterpene derivatives. Molecules 2017, 22, 1018. [Google Scholar] [CrossRef] [PubMed]
- Lacaille-Dubois, M.-A. Newest results on the chemistry and pharmacology of TCM drugs containing triterpene and steroid saponins. In Evidence and Rational Based Research on Chinese Drugs; Springer: Berlin/Heidelberg, Germany, 2013; pp. 87–135. [Google Scholar]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants–rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [PubMed]
- Tung, N.H.; Kwon, H.-J.; Kim, J.-H.; Ra, J.C.; Kim, J.A.; Kim, Y.H. An anti-influenza component of the bark of Alnus japonica. Arch. Pharmacal Res. 2010, 33, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Yang, S.; Zhang, W.; Cao, Y.; Wang, P.; Ding, N.; Zhang, Z.; Guo, Y.; Li, Y. Discovery of the first series of small molecule H5N1 entry inhibitors. J. Med. Chem. 2009, 52, 7368–7371. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, R.; Ooi, L.S.; But, P.P.; Ooi, V.E. Antiviral triterpenoids from the medicinal plant Schefflera heptaphylla. Phytother. Res. 2007, 21, 466–470. [Google Scholar] [CrossRef]
- Yu, M.; Si, L.; Wang, Y.; Wu, Y.; Yu, F.; Jiao, P.; Shi, Y.; Wang, H.; Xiao, S.; Fu, G. Discovery of pentacyclic triterpenoids as potential entry inhibitors of influenza viruses. J. Med. Chem. 2014, 57, 10058–10071. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Sriwilaijaroen, N.; Inoue, H.; Miki, K.; Kinoshita, K.; Koyama, K.; Furuhata, K.; Suzuki, Y.; Takahashi, K. Synthesis and anti-influenza virus evaluation of triterpene-sialic acid conjugates. Biorg. Med. Chem. 2018, 26, 17–24. [Google Scholar] [CrossRef]
- Wang, H.; Xu, R.; Shi, Y.; Si, L.; Jiao, P.; Fan, Z.; Han, X.; Wu, X.; Zhou, X.; Yu, F. Design, synthesis and biological evaluation of novel L-ascorbic acid-conjugated pentacyclic triterpene derivatives as potential influenza virus entry inhibitors. Eur. J. Med. Chem. 2016, 110, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Si, L.; Tian, Z.; Jiao, P.; Fan, Z.; Meng, K.; Zhou, X.; Wang, H.; Xu, R.; Han, X. Pentacyclic triterpenes grafted on CD cores to interfere with influenza virus entry: A dramatic multivalent effect. Biomaterials 2016, 78, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Si, L.; Meng, K.; Zhou, X.; Zhang, Y.; Zhou, D.; Xiao, S. Inhibition of influenza virus infection by multivalent pentacyclic triterpene-functionalized per-O-methylated cyclodextrin conjugates. Eur. J. Med. Chem. 2017, 134, 133–139. [Google Scholar] [CrossRef]
- Yang, Y.; He, H.-J.; Chang, H.; Yu, Y.; Yang, M.-B.; He, Y.; Fan, Z.-C.; Iyer, S.S.; Yu, P. Multivalent oleanolic acid human serum albumin conjugate as nonglycosylated neomucin for influenza virus capture and entry inhibition. Eur. J. Med. Chem. 2018, 143, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Mangasuli, S.N. Microwave assisted synthesis and biological activity of a novel triazino indole-coumarin hybrid: Crystal structure, hirshfeld surface analysis and DFT calculations. Chem. Data Collect. 2020, 29, 100503. [Google Scholar] [CrossRef]
- Mahmoud, N.F.H.; El-Sewedy, A. Facile synthesis of novel heterocyclic compounds based on pyridine moiety with pharmaceutical activities. J. Heterocycl. Chem. 2020, 57, 1559–1572. [Google Scholar] [CrossRef]
- Durmaz, M.; Halay, E.; Bozkurt, S. Recent applications of chiral calixarenes in asymmetric catalysis. Beilstein J. Org. Chem. 2018, 14, 1389–1412. [Google Scholar] [CrossRef]
- Franz, M.H.; Birzoi, R.; Maftei, C.V.; Maftei, E.; Kelter, G.; Fiebig, H.H.; Neda, I. Studies on the constituents of helleborus purpurascens: Analysis and biological activity of the aqueous and organic extracts. Amino Acids 2018, 50, 163–188. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.H.; Iorga, M.; Maftei, C.V.; Maftei, E.; Neda, I. Studies on the constituents of helleborus purpurascens: Use of derivatives from calix[6]arene, homooxacalix[3]arene and homoazacalix[3]arene as extractant agents for amino acids from the aqueous extract. Amino Acids 2020, 52, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Horatscheck, A.; Wagner, S.; Ortwein, J.; Kim, B.G.; Lisurek, M.; Beligny, S.; Schütz, A.; Rademann, J. Benzoylphosphonate-based photoactive phosphopeptide mimetics for modulation of protein tyrosine phosphatases and highly specific labeling of SH2 domains. Angew. Chem. Int. Ed. 2012, 51, 9441–9447. [Google Scholar] [CrossRef]
- Zhu, T.; Xu, S.; Rahman, A.; Dogdibegovic, E.; Yang, P.; Pageni, P.; Kabir, M.P.; Zhou, X.D.; Tang, C. Cationic metallo-polyelectrolytes for robust alkaline anion-exchange membranes. Angew. Chem. Int. Ed. Engl. 2018, 57, 2388–2392. [Google Scholar] [CrossRef] [PubMed]
- Soriano del Amo, D.; Wang, W.; Jiang, H.; Besanceney, C.; Yan, A.C.; Levy, M.; Liu, Y.; Marlow, F.L.; Wu, P. Biocompatible copper(I) catalysts for in vivo imaging of glycans. J. Am. Chem. Soc. 2010, 132, 16893–16899. [Google Scholar] [CrossRef] [PubMed]
- Sauter, N.K.; Bednarski, M.D.; Wurzburg, B.A.; Hanson, J.E.; Whitesides, G.M.; Skehel, J.J.; Wiley, D.C. Hemagglutinins from two influenza virus variants bind to sialic acid derivatives with millimolar dissociation constants: A 500-MHz proton nuclear magnetic resonance study. Biochemistry 1989, 28, 8388–8396. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, C.; Wang, R.; Xu, J.; Chi, Y.; Qin, J.; Liu, Q. The ω-carboxyl group of 7-ketocholesteryl-9-carboxynonanoate mediates the binding of oxLDL to CD36 receptor and enhances caveolin-1 expression in macrophages. Int. J. Biochem. Cell Biol. 2017, 90, 121–135. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| Compounds | H1N1(A/WSN/1933) | H5N1(A/Hong Kong/483/97) |
|---|---|---|
| SGP | ND | ND |
| SCT-Asn | 29.04 | 75.46 |
| UA | 136.70 | ND |
| BA | ND | ND |
| OA | 31.14 | 47.78 |
| GA | 584.00 | >1000 |
| SCT-Asn-UA | 289.40 | 852.70 |
| SCT-Asn-BA | 6.89 | 251.60 |
| SCT-Asn-OA | 11.24 | 9.10 |
| SCT-Asn-GA | 16.35 | 13.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, M.; Wu, X.; Li, Y.; Guo, F. Synthesis of Four Pentacyclic Triterpene–Sialylglycopeptide Conjugates and Their Affinity Assays with Hemagglutinin. Molecules 2021, 26, 895. https://doi.org/10.3390/molecules26040895
Luo M, Wu X, Li Y, Guo F. Synthesis of Four Pentacyclic Triterpene–Sialylglycopeptide Conjugates and Their Affinity Assays with Hemagglutinin. Molecules. 2021; 26(4):895. https://doi.org/10.3390/molecules26040895
Chicago/Turabian StyleLuo, Mei, Ximin Wu, Yiming Li, and Fujiang Guo. 2021. "Synthesis of Four Pentacyclic Triterpene–Sialylglycopeptide Conjugates and Their Affinity Assays with Hemagglutinin" Molecules 26, no. 4: 895. https://doi.org/10.3390/molecules26040895
APA StyleLuo, M., Wu, X., Li, Y., & Guo, F. (2021). Synthesis of Four Pentacyclic Triterpene–Sialylglycopeptide Conjugates and Their Affinity Assays with Hemagglutinin. Molecules, 26(4), 895. https://doi.org/10.3390/molecules26040895






