Molecular and Cellular Effects of Chemical Chaperone—TUDCA on ER-Stressed NHAC-kn Human Articular Chondrocytes Cultured in Normoxic and Hypoxic Conditions
Abstract
1. Introduction
2. Results
2.1. Analysis of Cell Viability
2.2. Induction of Hypoxia, Cell Morphology, and Apoptosis
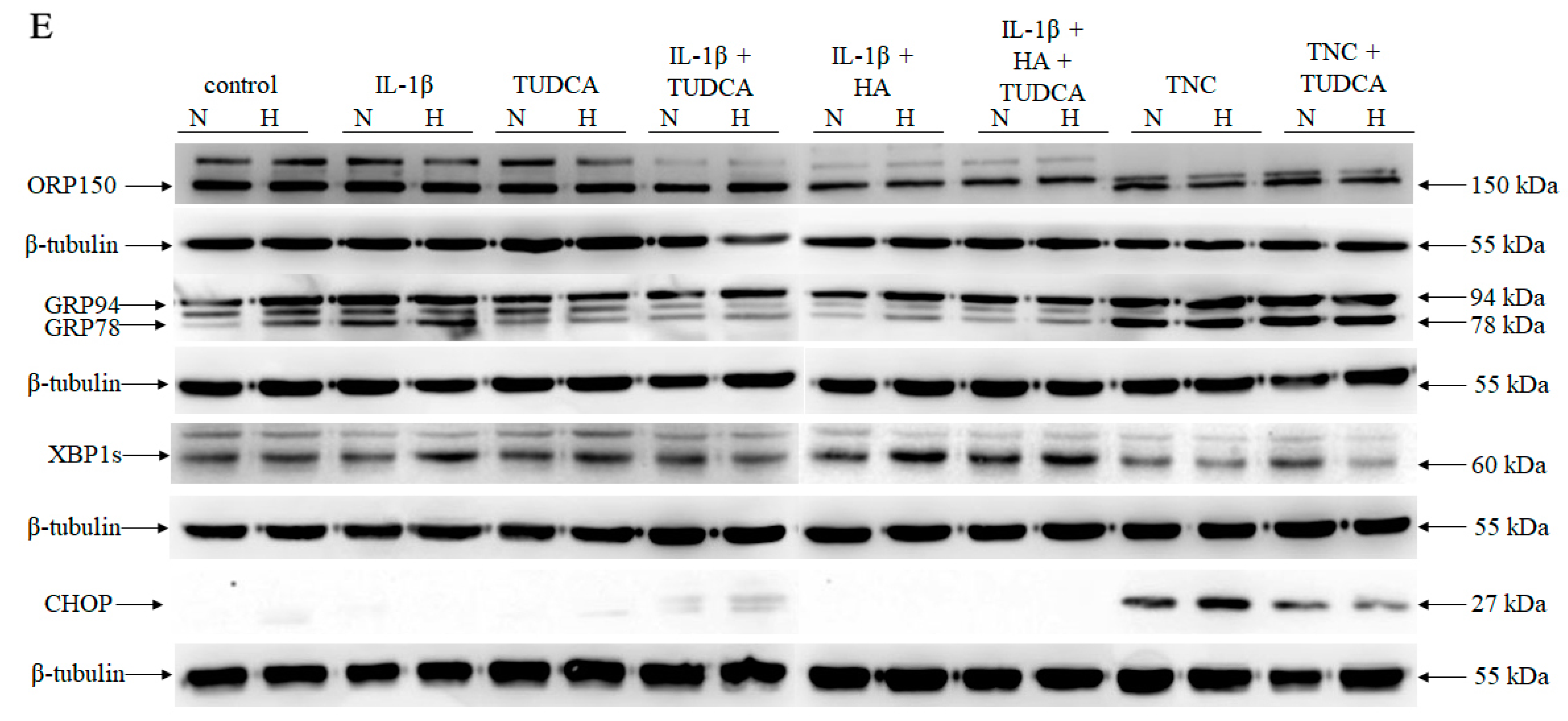
2.3. Analysis of ER Stress
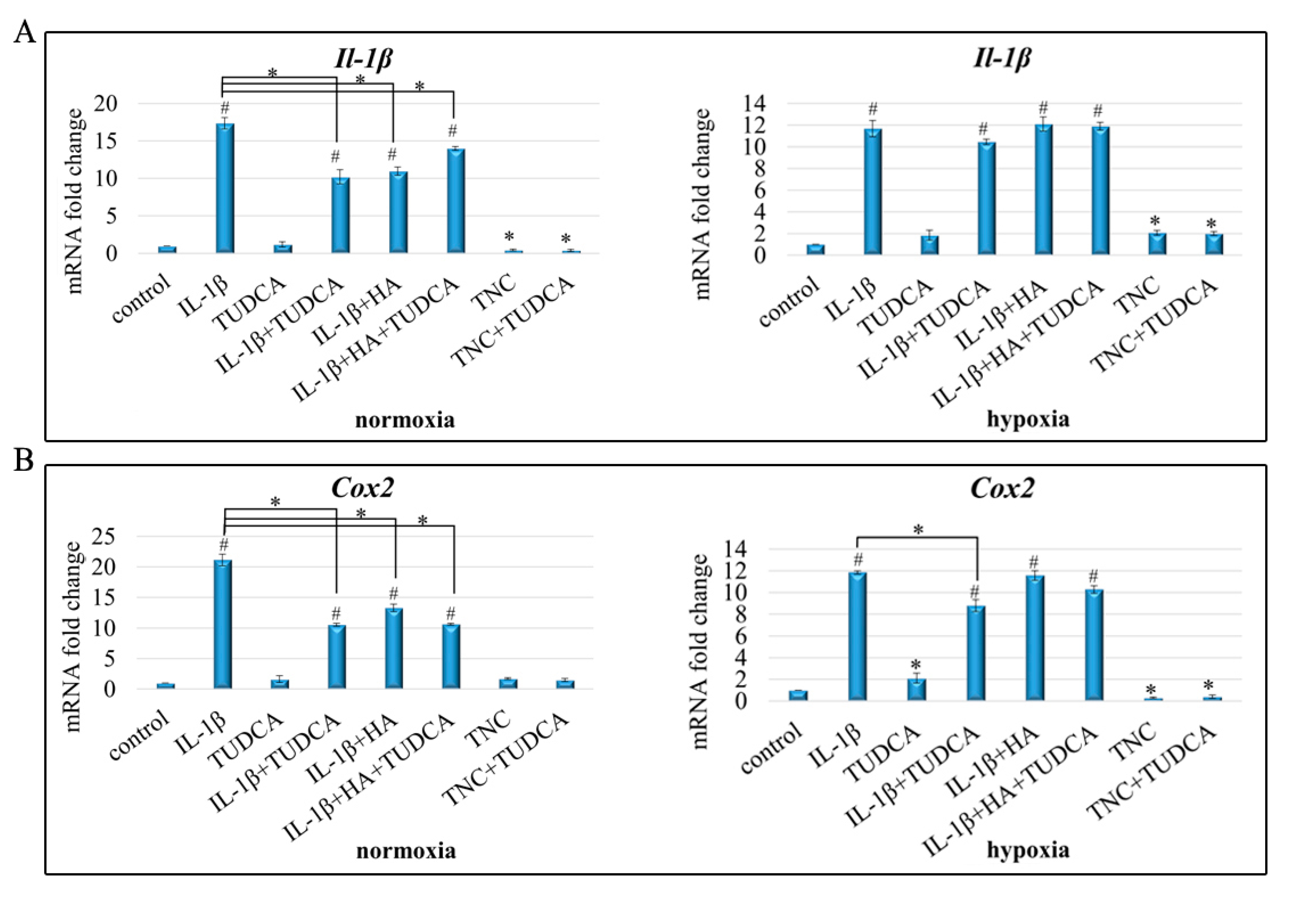
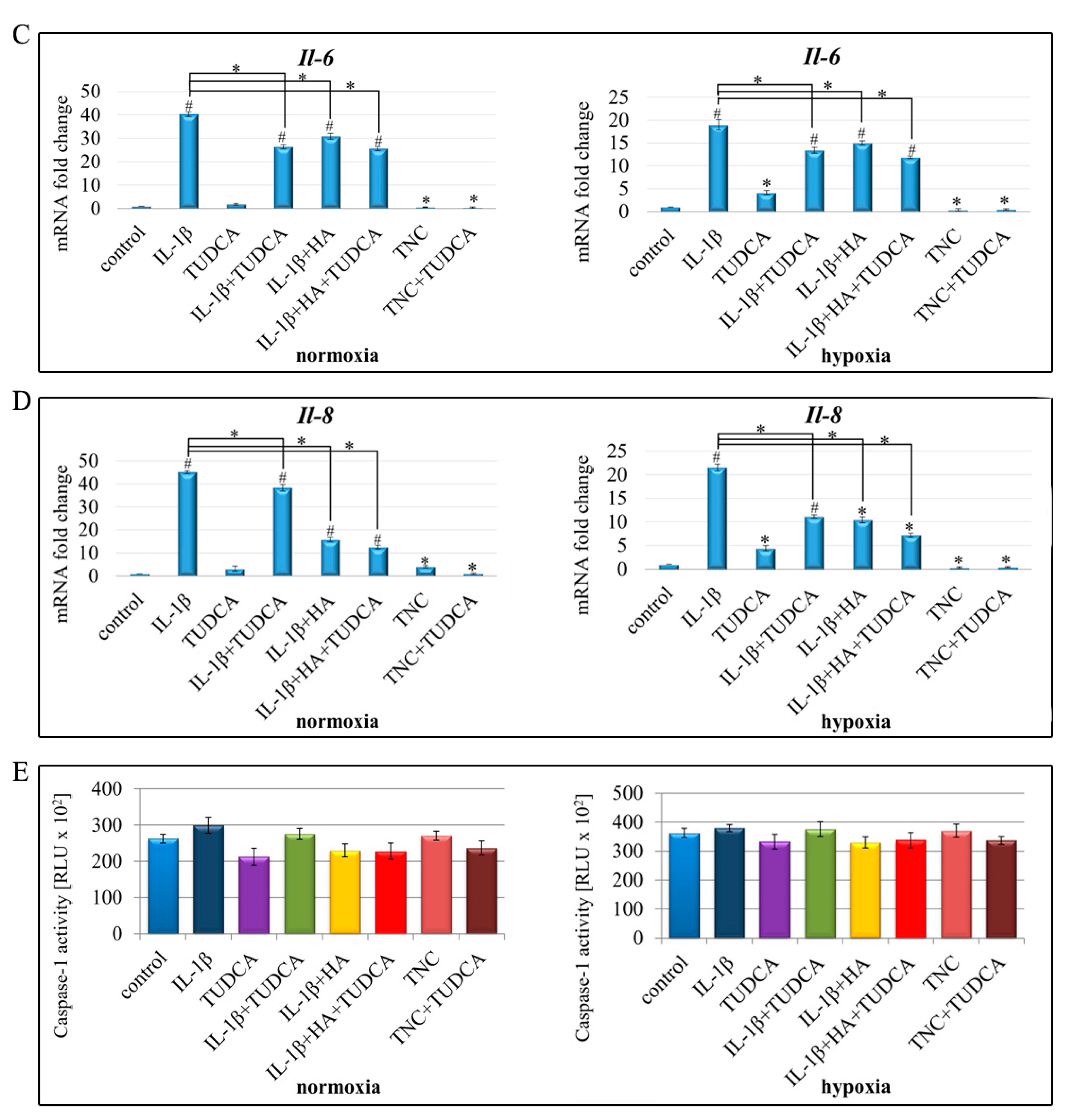
2.4. Analysis of Inflammation
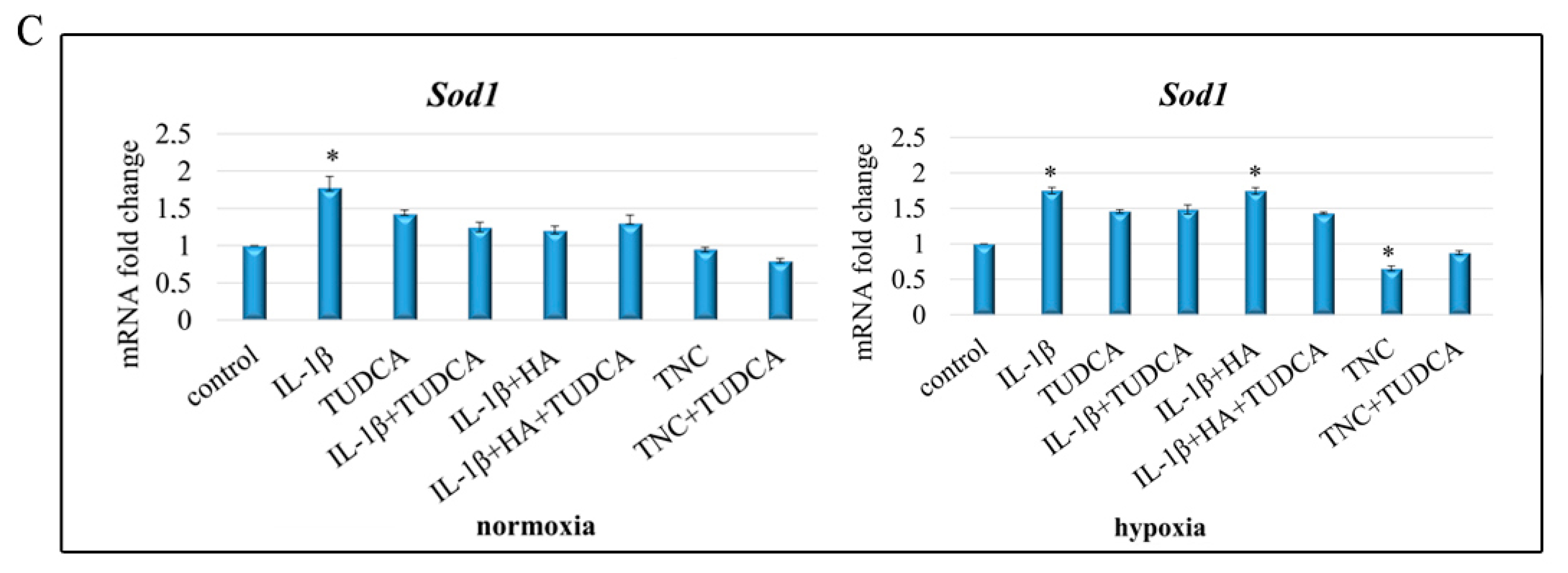
2.5. Analysis of ROS Production
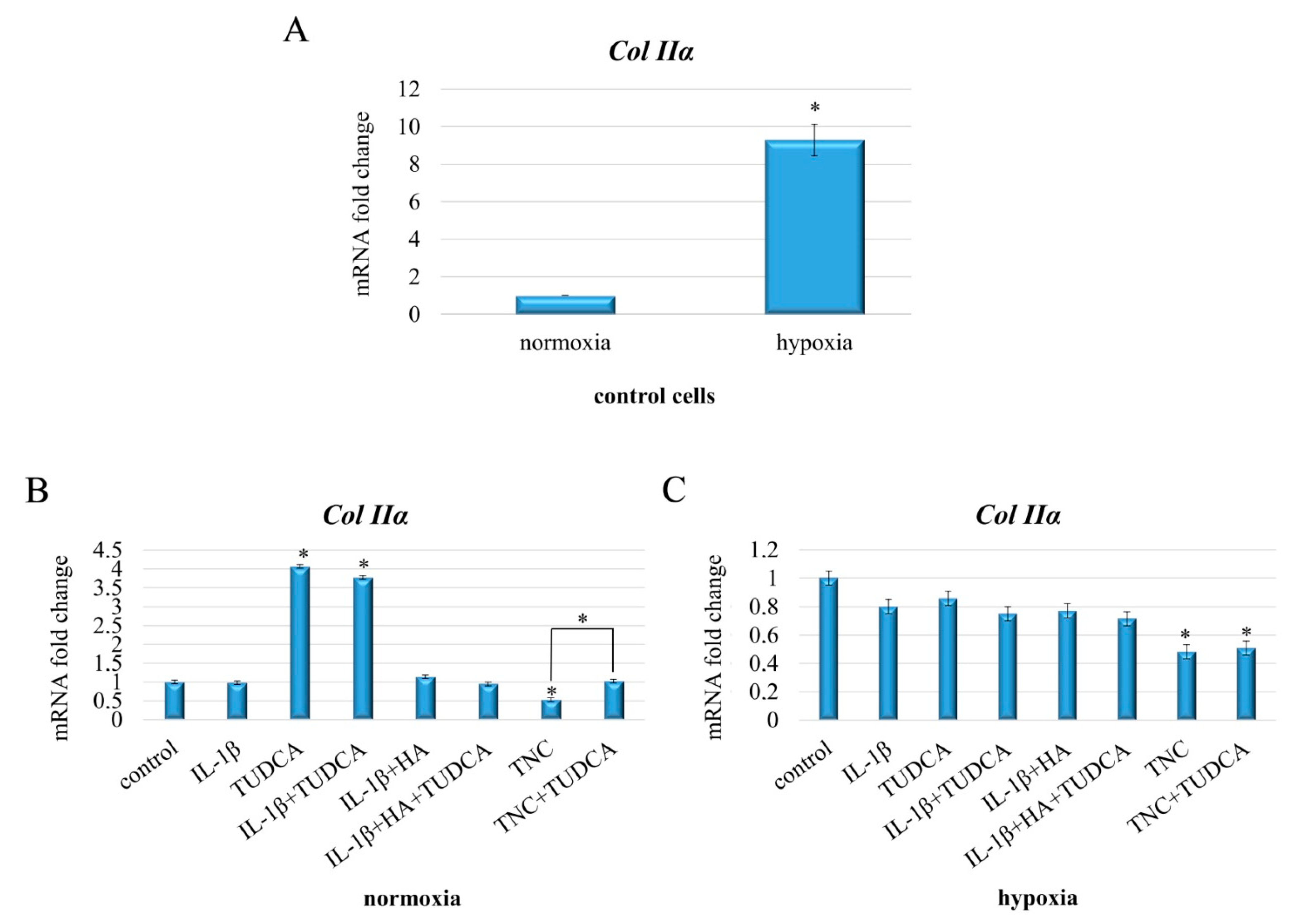
2.6. Analysis of Collagen Production
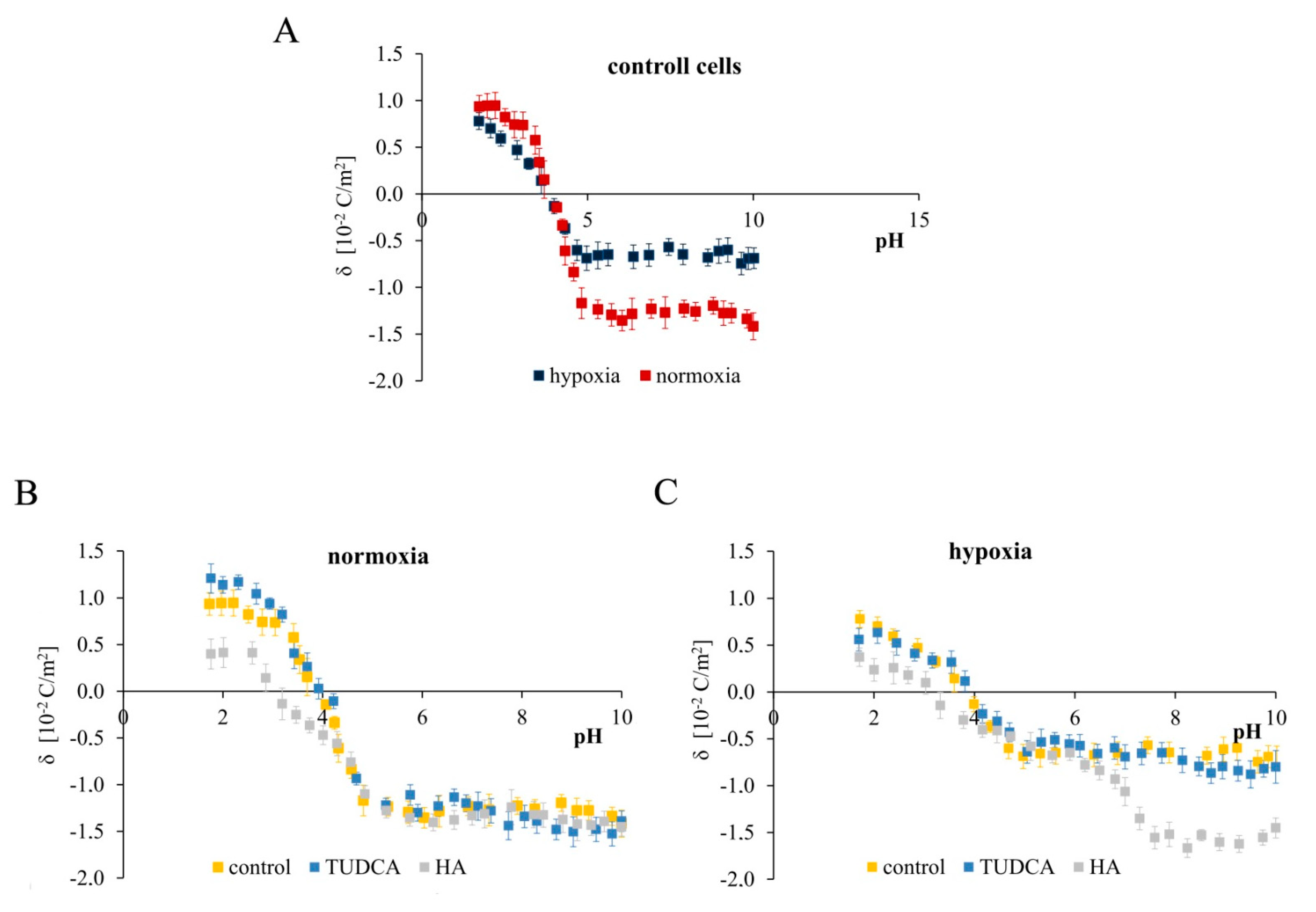
2.7. Analysis of Membrane Surface Charge Density
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Induction of Hypoxia
4.3. Cell Viability
4.4. Cell Morphological Analysis
4.5. Detection of Apoptosis
4.6. RNA Isolation and Gene Expression Analysis
4.7. Protein Assay
4.8. Sodium Dodecyl Sulfate–Polyacrylamide-Gel Electrophoresis
4.9. Immunoblotting
4.10. Reactive Oxygen Species Generation
4.11. Caspase 1 Activity Assessment
4.12. Zeta Potential Measurements
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- D’Adamo, S.; Cetrullo, S.; Panichi, V.; Mariani, E.; Flamigni, F.; Borzì, R.M. Nutraceutical Activity in Osteoarthritis Biology: A Focus on the Nutrigenomic Role. Cells 2020, 9, 1232. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Huang, Z.; Kraus, V.B. Does lipopolysaccharide-mediated inflammation have a role in OA? Nat. Rev. Rheumatol. 2016, 12, 123–129. [Google Scholar] [CrossRef]
- Jiang, M.; He, J.; Gu, H.; Yang, Y.; Huang, Y.; Xu, X.; Liu, L. Protective effect of resveratrol on obesity-related osteoarthritis via alleviating JAK2/STAT3 signaling pathway is independent of SOCS3. Toxicol. Appl. Pharmcol. 2020, 388, 114871. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, M.; Li, W.; Zhu, S.; Zhang, D. Stachydrine attenuates IL-1β-induced inflammatory response in osteoarthritis chondrocytes through the NF-κB signaling pathway. Chem. Biol. Interact. 2020, 326, 10913. [Google Scholar] [CrossRef]
- Liu, C.; Cao, Y.; Yang, X.; Shan, P.; Liu, H. Tauroursodeoxycholic acid suppresses endoplasmic reticulum stress in the chondrocytes of patients with osteoarthritis. Int. J. Mol. Med. 2015, 36, 1081–1087. [Google Scholar] [CrossRef]
- Vonk, L.A.; Zandieh-Doulabi, B.; Huang, C.-L.; Helder, M.N.; Everts, V.; Bank, R.A. Endoplasmic reticulum stress inhibits collagen synthesis independent of collagen-modifying enzymes in different chondrocyte populations and dermal fibroblasts. Biochem. Cell Biol. 2010, 88, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.L.; Li, J.L.; Xue, E.X.; Dou, H.C.; Lin, J.T.; Chen, K.; Wu, H.Q.; Wu, L.; Xuan, J.; Huang, Q.S. Vitexin alleviates ER-stress-activated apoptosis and the related inflammation in chondrocytes and inhibits the degeneration of cartilage in rats. Food Funct. 2018, 9, 5740–5749. [Google Scholar] [CrossRef] [PubMed]
- Boyce, M.; Yuan, J. Cellular response to endoplasmic reticulum stress: A matter of life or death. Cell Death Differ. 2006, 13, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Brostrom, M.A.; Brostrom, C.O. Calcium dynamics and endoplasmic reticular function in the regulation of protein synthesis: Implications for cell growth and adaptability. Cell Calcium 2003, 34, 345–363. [Google Scholar] [CrossRef]
- Yang, L.; Carlson, S.G.; McBurney, D.; Horton, W.E. Multiple Signals Induce Endoplasmic Reticulum Stress in Both Primary and Immortalized Chondrocytes Resulting in Loss of Differentiation, Impaired Cell Growth, and Apoptosis. J. Biol. Chem. 2005, 280, 31156–31165. [Google Scholar] [CrossRef]
- Ruiz-Romero, C.; Carreira, V.; Rego-Pérez, I.; Remeseiro, S.; López-Armada, M.J.; Blanco, F.J. Proteomic analysis of human osteoarthritic chondrocytes reveals protein changes in stress and glycolysis. Proteomics 2008, 8, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Nugent, A.E.; Speicher, D.M.; Gradisar, I.; McBurney, D.L.; Baraga, A.; Doane, K.J.; Horton, W.E. Advanced Osteoarthritis in Humans Is Associated With Altered Collagen VI Expression and Upregulation of ER-stress Markers Grp78 and Bag-1. J. Histochem. Cytochem. 2009, 57, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.; Oxford, A.E.; Tawara, K.; Jorcyk, C.L.; Oxford, J.T. Endoplasmic reticulum stress and unfolded protein response in cartilage pathophysiology; contributing factors to apoptosis and osteoarthritis. Int. J. Mol. Sci. 2017, 18, 665. [Google Scholar] [CrossRef] [PubMed]
- Uehara, Y.; Hirose, J.; Yamabe, S.; Okamoto, N.; Okada, T.; Oyadomari, S.; Mizuta, H. Endoplasmic reticulum stress-induced apoptosis contributes to articular cartilage degeneration via C/EBP homologous protein. Osteoarthr. Cartil. 2014, 22, 1007–1017. [Google Scholar] [CrossRef]
- Bian, Y.; Wang, H.; Sun, S. Taurine alleviates endoplasmic reticulum stress in the chondrocytes from patients with osteoarthritis. Redox Rep. 2018, 23, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-H.; Tardif, G.; Hum, D.; Kapoor, M.; Fahmi, H.; Pelletier, J.-P.; Martel-Pelletier, J. The unfolded protein response genes in human osteoarthritic chondrocytes: PERK emerges as a potential therapeutic target. Arthritis Res. 2016, 18, 1–12. [Google Scholar] [CrossRef]
- Takada, K.; Hirose, J.; Senba, K.; Yamabe, S.; Oike, Y.; Gotoh, T.; Mizuta, H. Enhanced apoptotic and reduced protective response in chondrocytes following endoplasmic reticulum stress in osteoarthritic cartilage. Int. J. Exp. Pathol. 2011, 92, 232–242. [Google Scholar] [CrossRef]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Shakibaei, M.; Schulze-Tanzil, G.; John, T.; Mobasheri, A. Curcumin protects human chondrocytes from IL-l1beta-induced inhibition of collagen type II and beta1-integrin expression and activation of caspase-3: An immunomorphological study. Ann. Anat. 2005, 187, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, H.; Yan, X.; Gu, H.; Gu, Z.; Liu, F. Endoplasmic reticulum stress participates in the progress of senescence and apoptosis of osteoarthritis chondrocytes. Biochem. Biophys. Res. Commun. 2017, 491, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Kusaczuk, M.; Bartoszewicz, M.; Cechowska-Pasko, M. Phenylbutyric Acid: Simple structure—Multiple effects. Curr. Pharm. Des. 2015, 21, 2147–2166. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Endoplasmic Reticulum Stress and the Inflammatory Basis of Metabolic Disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Otero, M.; Tsuchimochi, K.; Ijiri, K.; Li, Y. Defining the roles of inflammatory and anabolic cytokines in cartilage metabolism. Ann. Rheum. Dis. 2008, 67, iii75–iii82. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.I.; Argyle, D.J.; Clements, D.N. In vitro models for the study of osteoarthritis. Vet. J. 2016, 209, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Husa, M.; Petursson, F.; Lotz, M.K.; Terkeltaub, R.; Liu-Bryan, R. C/EBP homologous protein drives pro-catabolic responses in chondrocytes. Arthritis Res. Ther. 2013, 15, R218. [Google Scholar] [CrossRef] [PubMed]
- Briggs, M.D.; Dennis, E.P.; Dietmar, H.; Piróg, K.A. New developments in chondrocyte ER-stress and related diseases. F1000Research 2020, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Beaumont, G.; Ivorra, J.A.R.; del Carmen Trabado, M.; Blanco, F.J.; Benito, P.; Martín-Mola, E.; Paulino, J.; Marenco, J.L.; Porto, A.; Laffon, A.; et al. Glucosamine sulfate in the treatment of knee osteoarthritis symptoms: A randomized, double-blind, placebo-controlled study using acetaminophen as a side comparator. Arthritis Rheum. 2007, 56, 555–567. [Google Scholar] [CrossRef]
- Mazières, B.; Hucher, M.; Zaïm, M.; Garnero, P. Effect of chondroitin sulphate in symptomatic knee osteoarthritis: A multicentre, randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 2007, 66, 639–645. [Google Scholar] [CrossRef]
- Tashiro, T.; Seino, S.; Sato, T.; Matsuoka, R.; Masuda, Y.; Fukui, N. Oral administration of polymer hyaluronic acid alleviates symptoms of knee osteoarthritis: A double-blind, placebo-controlled study over a 12-month period. Sci. World J. 2012, 2012, 167928. [Google Scholar] [CrossRef] [PubMed]
- Kusaczuk, M. Tauroursodeoxycholate—Bile Acid with Chaperoning Activity: Molecular and Cellular Effects and Therapeutic Perspectives. Cells 2019, 8, 1471. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Choi, B.; Kim, B.J.; Rim, W.; Park, S.; Park, H.; Ahn, J.; Park, H. Tauroursodeoxycholic acid (TUDCA) counters osteoarthritis by regulating intracellular cholesterol levels and membrane fluidity of degenerated chondrocytes. Biomater. Sci. 2019, 7, 3178–3189. [Google Scholar] [CrossRef] [PubMed]
- Surażyński, A.; Miltyk, W.; Czarnomysy, R.; Grabowska, J.; Pałka, J. Hyaluronic acid abrogates nitric oxide-dependent stimulation of collagen degradation in cultured human chondrocytes. Pharmcol. Res. 2009, 60, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Karna, E.; Miltyk, W.; Pałka, J.A.; Jarząbek, K.; Wołczyński, S. Hyaluronic acid counteracts interleukin-1-induced inhibition of collagen biosynthesis in cultured human chondrocytes. Pharmacol. Res. 2006, 54, 275–281. [Google Scholar] [CrossRef]
- Chen, J.; Gu, Y.-T.; Xie, J.-J.; Wu, C.-C.; Xuan, J.; Guo, W.-J.; Xiang-Yang, W.; Chen, L.; Wu, Y.-S.; Zhang, X.-L.; et al. Gastrodin reduces IL-1β-induced apoptosis, inflammation, and matrix catabolism in osteoarthritis chondrocytes and attenuates rat cartilage degeneration in vivo. Biomed. Pharmcol. 2018, 97, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Fan, L.; Tian, S.; Ding, S.; Luo, J.; Zheng, J. Polydatin reduces IL-1 β-induced chondrocytes apoptosis and inflammatory response via p 38 MAPK signaling pathway in a rat model of osteoarthritis. Int. J. Clin. Exp. Med. 2017, 10, 2263–2273. [Google Scholar]
- Yu, C.-J.; Ko, C.; Hsieh, C.-H.; Chien, C.-T.; Huang, L.-H.; Lee, C.-W.; Jiang, C.-C. Proteomic analysis of osteoarthritic chondrocyte reveals the hyaluronic acid-regulated proteins involved in chondroprotective effect under oxidative stress. J. Proteom. 2014, 99, 40–53. [Google Scholar] [CrossRef]
- Qing, L.; Lei, P.; Liu, H.; Xie, J.; Wang, L.; Wen, T.; Hu, Y. Expression of hypoxia-inducible factor-1α in synovial fluid and articular cartilage is associated with disease severity in knee osteoarthritis. Exp. Ther. Med. 2017, 13, 63–68. [Google Scholar] [CrossRef]
- Sieber, S.; Michaelis, M.; Gühring, H.; Lindemann, S.; Gigout, A. Importance of osmolarity and oxygen tension for cartilage tissue engineering. BioRes. Open Access. 2020, 9, 106–115. [Google Scholar] [CrossRef]
- Bentovim, L.; Amarilio, R.; Zelzer, E. HIF1α is a central regulator of collagen hydroxylation and secretion under hypoxia during bone development. Development 2012, 139, 4473–4483. [Google Scholar] [CrossRef]
- Wenger, R.H.; Kurtcuoglu, V.; Scholz, C.C.; Marti, H.H.; Hoogewijs, D. Frequently asked questions in hypoxia research. Hypoxia 2015, 3, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Koay, E.; Athanasiou, K. Hypoxic chondrogenic differentiation of human embryonic stem cells enhances cartilage protein synthesis and biomechanical functionality. Osteoarthr. Cartil. 2008, 16, 1450–1456. [Google Scholar] [CrossRef]
- Thoms, B.L.; Dudek, K.A.; Lafont, J.E.; Murphy, C.L. Chondrocyte biology hypoxia promotes the production and inhibits the destruction of human articular cartilage. Arthritis Rheum. 2013, 65, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Kusaczuk, M.; Krętowski, R.; Naumowicz, M.; Stypułkowska, A.; Cechowska-Pasko, M. Silica nanoparticle-induced oxidative stress and mitochondrial damage is followed by activation of intrinsic apoptosis pathway in glioblastoma cells. Int. J. Nanomed. 2018, 13, 2279–2294. [Google Scholar] [CrossRef] [PubMed]
- Coyle, C.H.; Izzo, N.J.; Chu, C.R. Sustained hypoxia enhances chondrocyte matrix synthesis. J. Orthop. Res. 2009, 27, 793–799. [Google Scholar] [CrossRef]
- Mennan, C.; García, J.; McCarthy, H.; Owen, S.; Perry, J.; Wright, K.; Banerjee, R.; Richardson, J.B.; Roberts, S. Human Articular Chondrocytes Retain Their Phenotype in Sustained Hypoxia While Normoxia Promotes Their Immunomodulatory Potential. Cartilage 2018, 10, 467–479. [Google Scholar] [CrossRef]
- Aoe, T. Pathological Aspects of COVID-19 as a Conformational Disease and the Use of Pharmacological Chaperones as a Potential Therapeutic Strategy. Front. Pharmcol. 2020, 11, 1095. [Google Scholar] [CrossRef]
- Ito, H.; Yamashita, Y.; Tanaka, T.; Takaki, M.; Le, M.N.; Yoshida, L.-M.; Morimoto, K. Cigarette smoke induces endoplasmic reticulum stress and suppresses efferocytosis through the activation of RhoA. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Paganoni, S.; Macklin, E.A.; Hendrix, S.; Berry, J.D.; Elliott, M.A.; Maiser, S.; Karam, C.; Caress, J.B.; Owegi, M.A.; Quick, A.; et al. Trial of Sodium Phenylbutyrate–Taurursodiol for Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2020, 383, 919–930. [Google Scholar] [CrossRef]
- Liu, F.; Weng, X.; Lin, P.; Zheng, C.; Xu, H.; Liu, X.-X.; Ye, H.; Li, X. Duhuo Jisheng decoction inhibits endoplasmic reticulum stress in chondrocytes induced by tunicamycin through the downregulation of miR-34a. Int. J. Mol. Med. 2015, 36, 1311–1318. [Google Scholar] [CrossRef]
- Wu, H.; Meng, Z.; Jiao, Y.; Ren, Y.; Yang, X.; Liu, H.; Wang, R.; Cui, Y.; Pan, L.; Cao, Y. The endoplasmic reticulum stress induced by tunicamycin affects the viability and autophagy activity of chondrocytes. J. Clin. Lab. Anal. 2020, 34, e23437. [Google Scholar] [CrossRef] [PubMed]
- Chai, B.; Zheng, Z.-H.; Liao, X.; Li, K.-Y.; Liang, J.-S.; Huang, Y.-X.; Tong, C.-J.; Ou, D.-J.; Lu, J. The protective role of omentin-1 in IL-1β-induced chondrocyte senescence. Artif. Cells Nanomed. Biotechnol. 2019, 48, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Wang, L.; Wang, B.; Wang, T.; Yang, G.; Shen, L.; Wang, T.; Guo, X.; Liu, Y.; Xia, Y.; et al. Activation of volume-sensitive outwardly rectifying chloride channel by ROS contributes to ER stress and cardiac contractile dysfunction: Involvement of CHOP through Wnt. Cell Death Dis. 2014, 5, e1528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, H.; Wang, S.; Liu, M.; Feng, Y.; Wang, X. Icariin Protects Rat Cardiac H9c2 Cells from Apoptosis by Inhibiting Endoplasmic Reticulum Stress. Int. J. Mol. Sci. 2013, 14, 17845–17860. [Google Scholar] [CrossRef]
- Ansari, M.; Khan, N.; Ahmad, I.; Haqqi, T. Parkin clearance of dysfunctional mitochondria regulates ROS levels and increases survival of human chondrocytes. Osteoarthr. Cartil. 2018, 26, 1087–1097. [Google Scholar] [CrossRef]
- Khan, N.M.; Ahmad, I.; Haqqi, T.M. Nrf2/ARE pathway attenuates oxidative and apoptotic response in human osteoarthritis chondrocytes by activating ERK1/2/ELK1-P70S6K-P90RSK signaling axis. Free Radic. Biol. Med. 2018, 116, 159–171. [Google Scholar] [CrossRef]
- Jia, Y.; Pang, C.; Zhao, K.; Jiang, J.; Zhang, T.; Peng, J.; Sun, P.; Qian, Y. Garcinol Suppresses IL-1β-Induced Chondrocyte Inflammation and Osteoarthritis via Inhibition of the NF-κB Signaling Pathway. Inflammation 2019, 42, 1754–1766. [Google Scholar] [CrossRef]
- Chernos, M.; Grecov, D.; Kwok, E.; Bebe, S.; Babsola, O.; Anastassiades, T. Rheological study of hyaluronic acid derivatives. Biomed. Eng. Lett. 2017, 7, 17–24. [Google Scholar] [CrossRef]
- Migliore, A.; Giovannangeli, F.; Granata, M.; Laganà, B. Hylan G-F 20: Review of its Safety and Efficacy in the Management of Joint Pain in Osteoarthritis. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2010, 3, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Masuko, K.; Murata, M.; Yudoh, K.; Kato, T.; Nakamura, H. Anti-inflammatory effects of hyaluronan in arthritis therapy: Not just for viscosity. Int. J. Gen. Med. 2009, 2, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Campo, G.M.; Avenoso, A.; Campo, S.; Ferlazzo, A.M.; Altavilla, D.; Calatroni, A. Efficacy of treatment with glycosaminoglycans on experimental collagen-induced arthritis in rats. Arthritis Res. Ther. 2003, 5, 1–10. [Google Scholar] [CrossRef]
- Grishko, V.; Xu, M.; Ho, R.; Mates, A.; Watson, S.; Kim, J.T.; Wilson, G.L.; Pearsall, A.W. Effects of Hyaluronic Acid on Mitochondrial Function and Mitochondria-driven Apoptosis following Oxidative Stress in Human Chondrocytes. J. Biol. Chem. 2009, 284, 9132–9139. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef]
- Sasaki, A.; Sasaki, K.; Konttinen, Y.T.; Santavirta, S.; Takahara, M.; Takei, H.; Ogino, T.; Takagi, M. Hayluronan inhibits the interleukin-1beta-induced expression of matrix metalloproteinase. (MMP)-1 and MMP-3 in human synovial cells. Tohoku J. Exp. Med. 2004, 204, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.-H.; Chae, C.-S.; Kwak, J.-S.; Oh, H.; Shin, Y.; Huh, Y.H.; Lee, C.-G.; Park, Y.-W.; Chun, C.-H.; Kim, Y.-M.; et al. Hypoxia-Inducible Factor-2α Is an Essential Catabolic Regulator of Inflammatory Rheumatoid Arthritis. PLoS Biol. 2014, 12, e1001881. [Google Scholar] [CrossRef]
- Zhang, W.; Petrovic, J.M.; Callaghan, D.; Jones, A.; Cui, H.; Howlett, C.; Stanimirovic, D. Evidence that hypoxia-inducible factor-1 (HIF-1) mediates transcriptional activation of interleukin-1beta (IL-1beta) in astrocyte cultures. J. Neuroimmunol. 2006, 174, 63–73. [Google Scholar] [CrossRef]
- Lafont, J.E. Lack of oxygen in articular cartilage: Consequences for chondrocyte biology. Int. J. Exp. Pathol. 2010, 91, 99–106. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Jing, X.; Li, M.; Ren, Y.; Chen, J.; Yang, C.; Wu, H.; Guo, F. Hypoxia promotes maintenance of the chondrogenic phenotype in rat growth plate chondrocytes through the HIF-1α/YAP signaling pathway. Int. J. Mol. Med. 2018, 42, 3181–3192. [Google Scholar] [CrossRef]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar]
- Bandarra, D.; Biddlestone, J.; Mudie, S.; Müller, H.-A.J.; Rocha, S. HIF-1α restricts NF-κB-dependent gene expression to control innate immunity signals. Dis. Models Mech. 2015, 8, 169–181. [Google Scholar] [CrossRef]
- Zhou, Y.; Liao, Q.; Li, X.; Wang, H.; Wei, F.; Chen, J.; Yang, J.; Zeng, Z.; Guo, X.; Chen, P.; et al. HYOU1, Regulated by LPLUNC1, Is Up-Regulated in Nasopharyngeal Carcinoma and Associated with Poor Prognosis. J. Cancer 2016, 7, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Krohn, R.; Hermanson, G.; Mallia, A.; Gartner, F.; Provenzano, M.; Fujimoto, E.; Goeke, N.; Olson, B.; Klenk, D. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]




| Agent | Symbol | Reported Effect | References |
|---|---|---|---|
| Tauroursodeoxycholic acid | TUDCA | Alleviation of ER stress | [6] |
| Alleviation of inflammation | [32] 1 | ||
| Alleviation of oxidative stress | [32] 1 | ||
| Restoration of collagen expression | [6,33] | ||
| Hyaluronic acid | HA | Alleviation of oxidative stress | [34,35,36] |
| Alleviation of inflammation | [37,38,39] | ||
| Restoration of collagen expression | [40,41] | ||
| Interleukin-1β | IL-1β | Induction of inflammation | [5,42,43,44] |
| Apoptosis | [21,22,27,42,43,45] | ||
| Induction of ER stress | [8,17,22,27] | ||
| Induction of oxidative stress | [27,45,46] | ||
| Reduction of collagen expression | [21,22,40,41] | ||
| Tunicamycin | TNC | Induction of ER stress | [6,11,18,27,47] |
| Apoptosis | [6,11,18,47] | ||
| Induction of oxidative stress | [48,49] 1 | ||
| Reduction of collagen expression | [6,18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusaczuk, M.; Naumowicz, M.; Krętowski, R.; Cukierman, B.; Cechowska-Pasko, M. Molecular and Cellular Effects of Chemical Chaperone—TUDCA on ER-Stressed NHAC-kn Human Articular Chondrocytes Cultured in Normoxic and Hypoxic Conditions. Molecules 2021, 26, 878. https://doi.org/10.3390/molecules26040878
Kusaczuk M, Naumowicz M, Krętowski R, Cukierman B, Cechowska-Pasko M. Molecular and Cellular Effects of Chemical Chaperone—TUDCA on ER-Stressed NHAC-kn Human Articular Chondrocytes Cultured in Normoxic and Hypoxic Conditions. Molecules. 2021; 26(4):878. https://doi.org/10.3390/molecules26040878
Chicago/Turabian StyleKusaczuk, Magdalena, Monika Naumowicz, Rafał Krętowski, Bartosz Cukierman, and Marzanna Cechowska-Pasko. 2021. "Molecular and Cellular Effects of Chemical Chaperone—TUDCA on ER-Stressed NHAC-kn Human Articular Chondrocytes Cultured in Normoxic and Hypoxic Conditions" Molecules 26, no. 4: 878. https://doi.org/10.3390/molecules26040878
APA StyleKusaczuk, M., Naumowicz, M., Krętowski, R., Cukierman, B., & Cechowska-Pasko, M. (2021). Molecular and Cellular Effects of Chemical Chaperone—TUDCA on ER-Stressed NHAC-kn Human Articular Chondrocytes Cultured in Normoxic and Hypoxic Conditions. Molecules, 26(4), 878. https://doi.org/10.3390/molecules26040878







