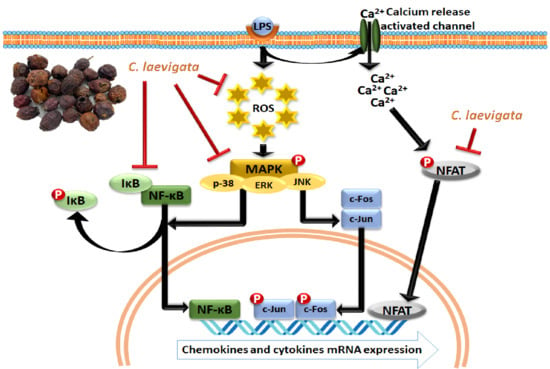
Crataegus laevigata Suppresses LPS-Induced Oxidative Stress during Inflammatory Response in Human Keratinocytes by Regulating the MAPKs/AP-1, NFκB, and NFAT Signaling Pathways
Abstract
1. Introduction
2. Results
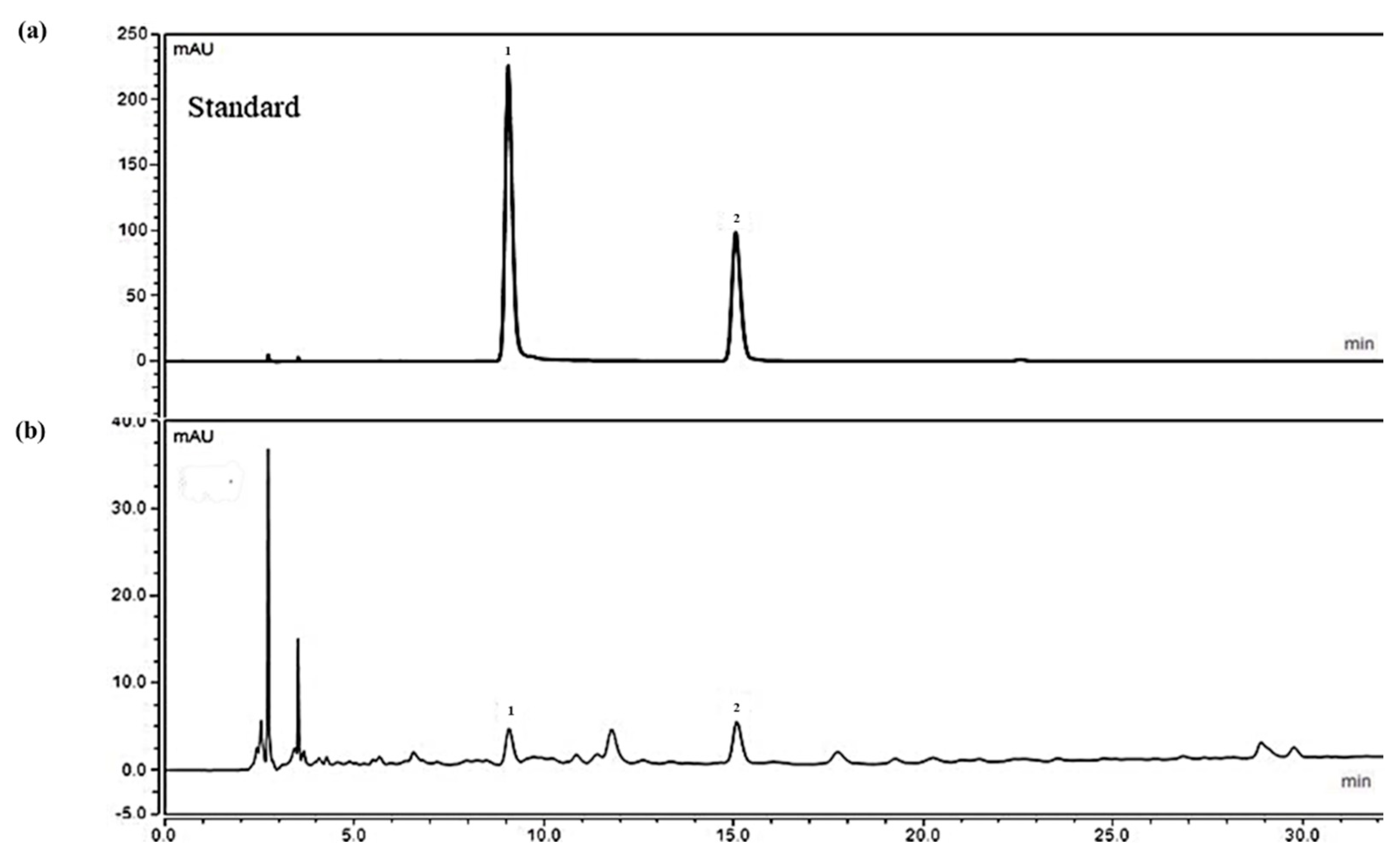
2.1. Analysis of Ethanolic Crataegus Laevigata Berry Extract
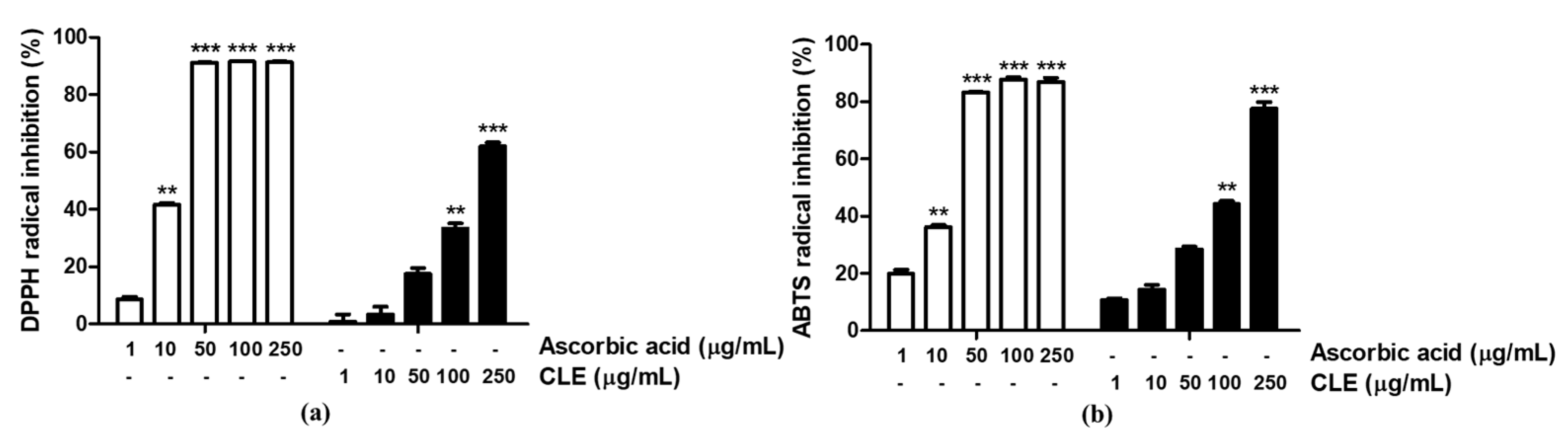
2.2. Antioxidative Activity of CLE
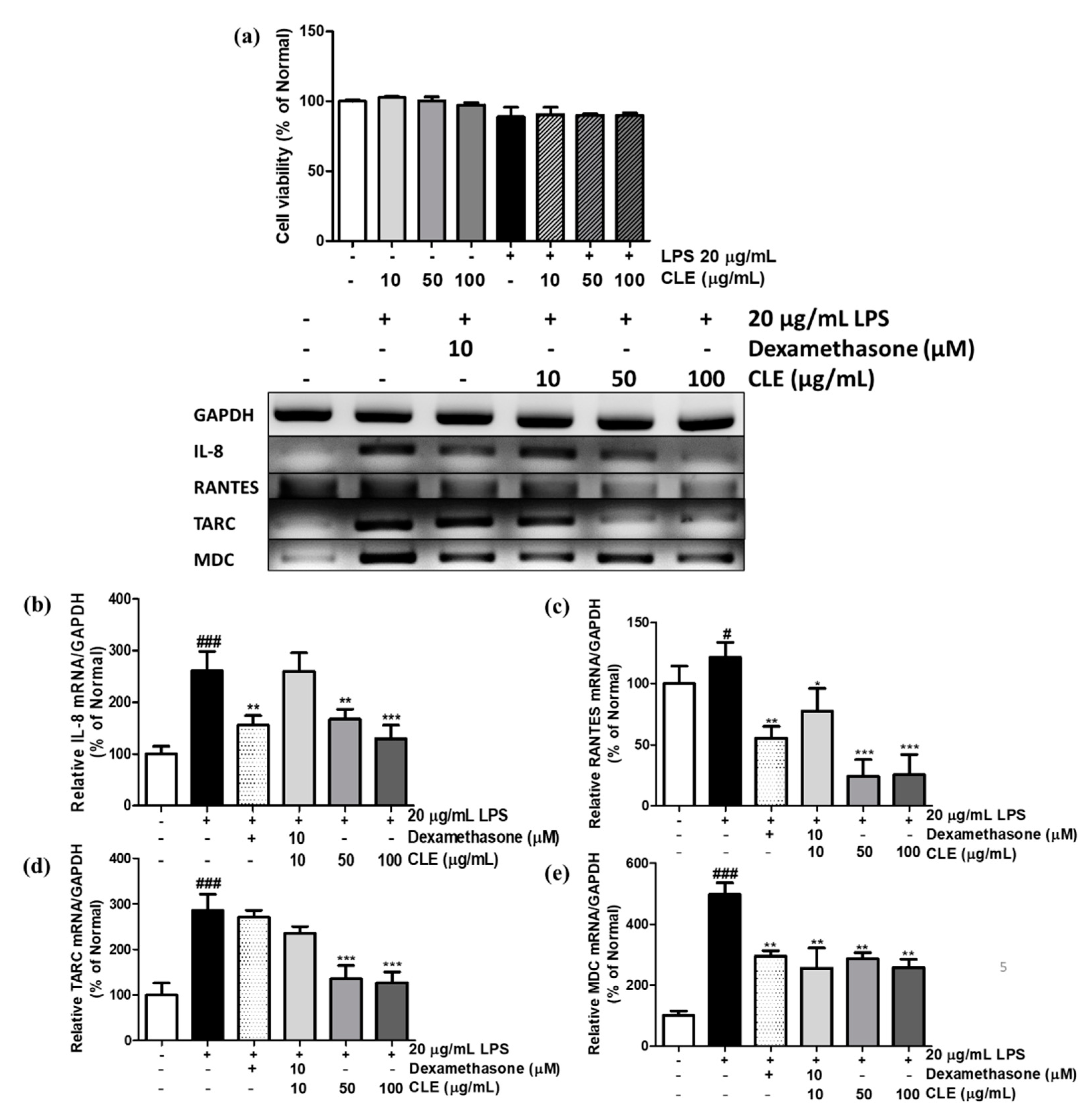
2.3. Cell Viability and Chemokine mRNA Expression Levels in LPS-Treated and CLE-Treated HaCaTs
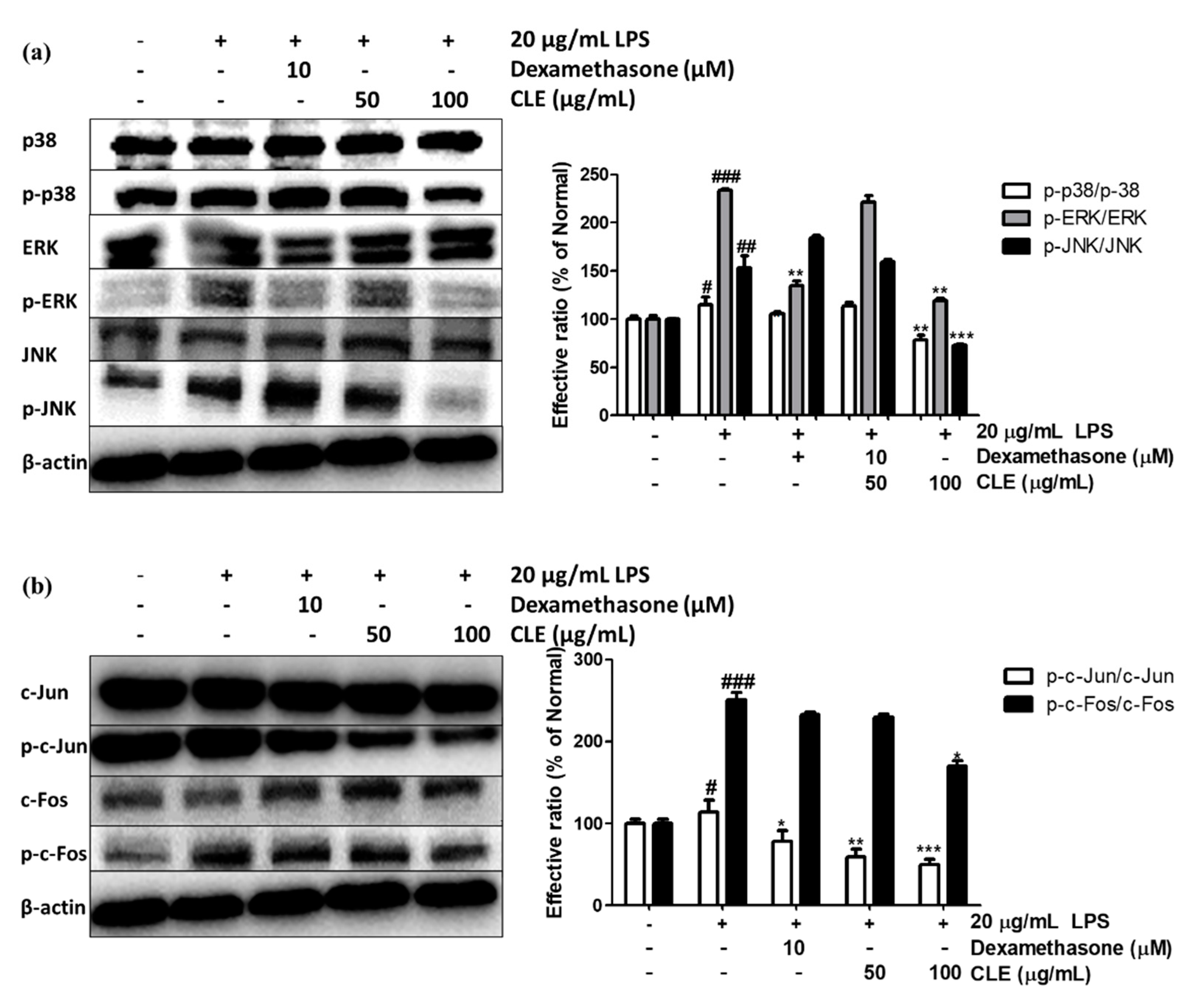
2.4. Regulatory Effect of CLE Treatment on MAPK Signaling Pathway and AP-1 Activation
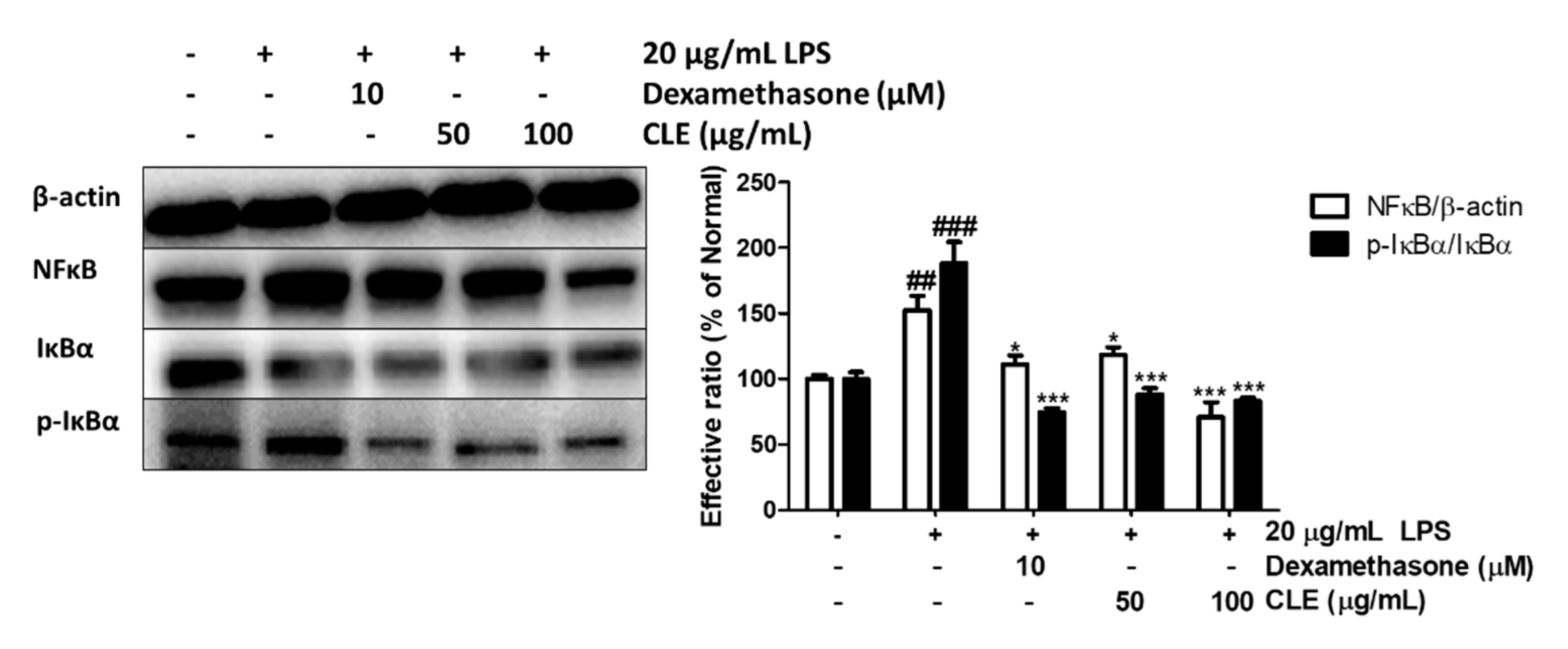
2.5. Regulatory Effect of CLE Treatment on NFκB Signaling Pathways
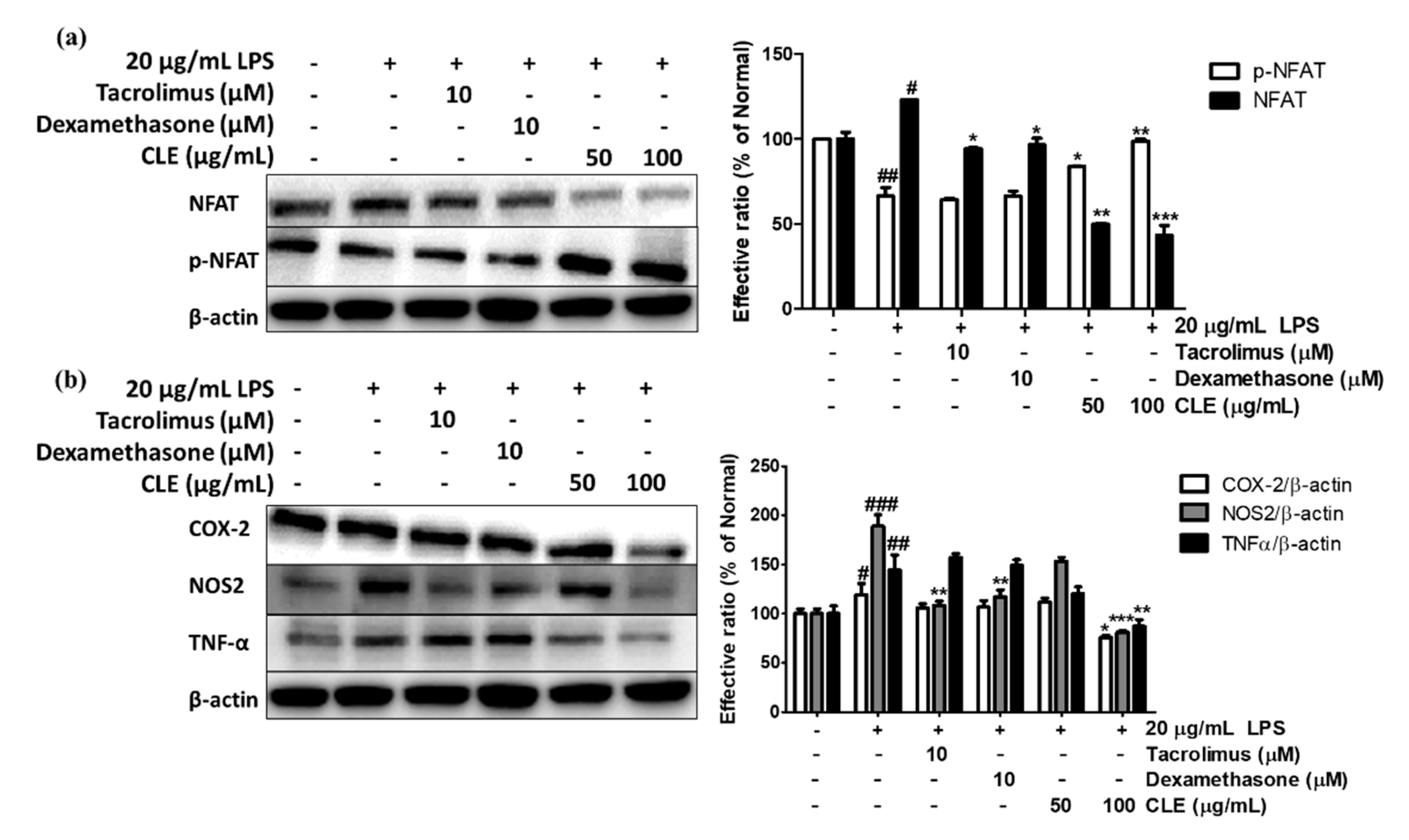
2.6. Regulatory Effect of CLE Treatment on NFAT Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Sample Preparation
4.3. HPLC Analysis
4.4. 1,1-Diphenyl-2-picrythydrazyl Radical Scavenging Activity
4.5. ABTS•+ Radical Scavenging Activity
4.6. Cell Culture
4.7. Cell Viability
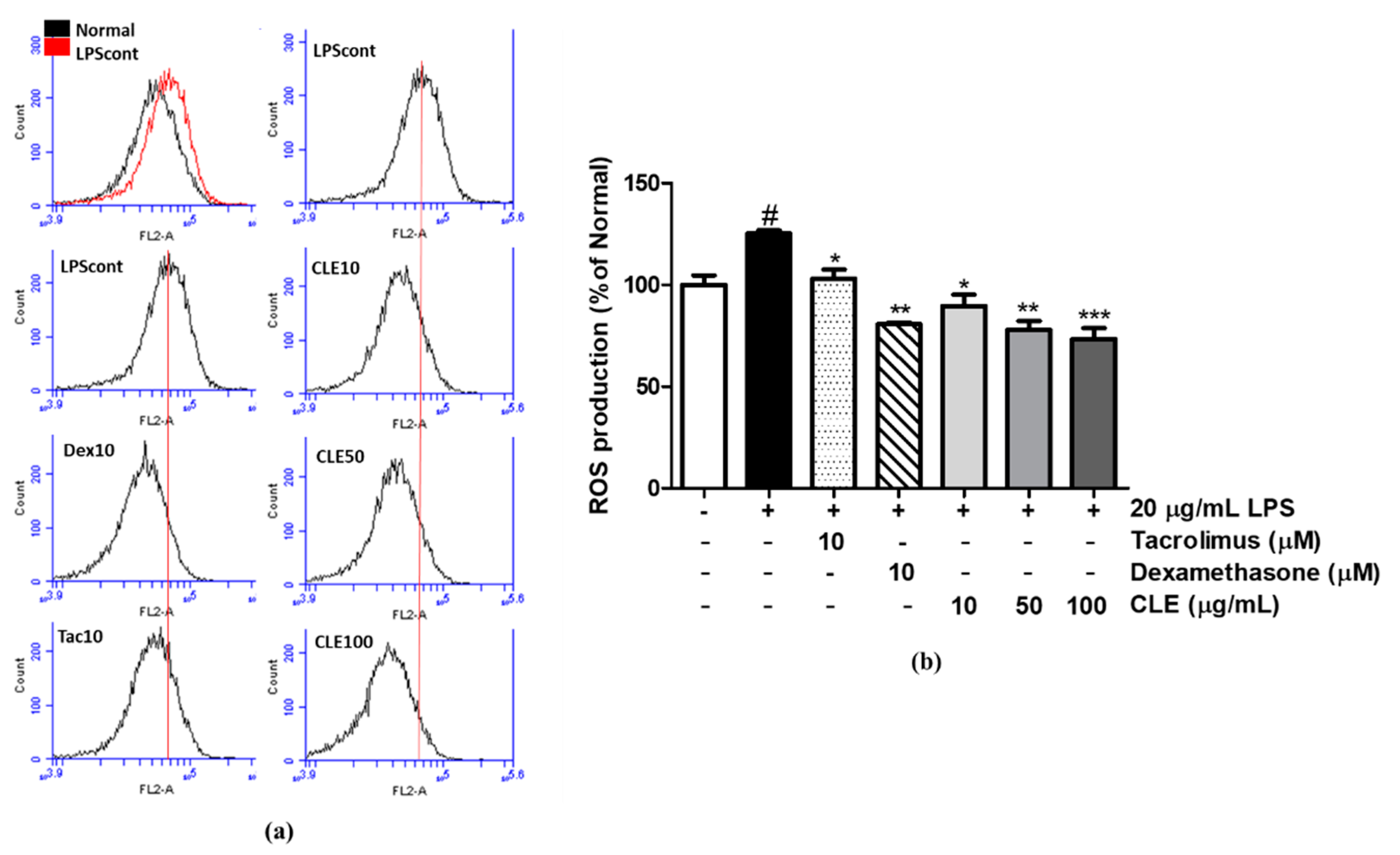
4.8. ROS Measurement
4.9. Reverse-Transcription Polymerase Reaction
4.10. Western Blot Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Available online: https://www.hindawi.com/journals/omcl/2016/5698931/ (accessed on 13 December 2020).
- The Role of Oxidative Stress and Systemic Inflammation in Kidney Disease and Its Associated Cardiovascular Risk|IntechOpen. Available online: https://www.intechopen.com/books/novel-prospects-in-oxidative-and-nitrosative-stress/the-role-of-oxidative-stress-and-systemic-inflammation-in-kidney-disease-and-its-associated-cardiova (accessed on 13 December 2020).
- The Role of Oxidative Stress during Inflammatory Processes—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/24127541/ (accessed on 13 December 2020).
- Oxidative Stress and Inflammation in the Liver—The Liver—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781119436812.ch55 (accessed on 13 December 2020).
- Mechanisms of Disease: Oxidative Stress and Inflammation in the Pathogenesis of Hypertension|Nature Reviews Nephrology. Available online: https://www.nature.com/articles/ncpneph0283 (accessed on 13 December 2020).
- Lauridsen, C. From Oxidative Stress to Inflammation: Redox Balance and Immune System. Poult. Sci. 2019, 98, 4240–4246. [Google Scholar] [CrossRef]
- Role of Reactive Oxygen Species in Inflammation: A Minireview|SpringerLink. Available online: https://link.springer.com/article/10.3103/S009639251804003X (accessed on 13 December 2020).
- Reactive Oxygen Species in Metabolic and Inflammatory Signaling|Circulation Research. Available online: https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.117.311401 (accessed on 13 December 2020).
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2013, 20, 1126–1167. [Google Scholar] [CrossRef]
- Park, J.; Min, J.-S.; Kim, B.; Chae, U.-B.; Yun, J.W.; Choi, M.-S.; Kong, I.-K.; Chang, K.-T.; Lee, D.-S. Mitochondrial ROS Govern the LPS-Induced pro-Inflammatory Response in Microglia Cells by Regulating MAPK and NF-ΚB Pathways. Neurosci. Lett. 2015, 584, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Liu, C.; Tu, Z.; Xu, Q.; Chen, S.; Zhang, Y.; Wang, X.; Zhang, J.; Hu, C.-A.A.; Liu, Y. Activation of the NF-ΚB and MAPK Signaling Pathways Contributes to the Inflammatory Responses, but Not Cell Injury, in IPEC-1 Cells Challenged with Hydrogen Peroxide. Available online: https://www.hindawi.com/journals/omcl/2020/5803639/ (accessed on 13 December 2020).
- Qi, X.-F.; Kim, D.-H.; Yoon, Y.-S.; Li, J.-H.; Song, S.-B.; Jin, D.; Huang, X.-Z.; Teng, Y.-C.; Lee, K.-J. The Adenylyl Cyclase-CAMP System Suppresses TARC/CCL17 and MDC/CCL22 Production through P38 MAPK and NF-KappaB in HaCaT Keratinocytes. Mol. Immunol. 2009, 46, 1925–1934. [Google Scholar] [CrossRef]
- Yano, C.; Saeki, H.; Komine, M.; Kagami, S.; Tsunemi, Y.; Ohtsuki, M.; Nakagawa, H. Mechanism of Macrophage-Derived Chemokine/CCL22 Production by HaCaT Keratinocytes. Ann. Derm. 2015, 27, 152. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.-P.; Liu, C.; Chiang, F.-Y.; Wang, L.-F.; Lee, K.-W.; Chen, W.-T.; Kuo, P.-L.; Liang, C.-H. IL-8 Promotes Inflammatory Mediators and Stimulates Activation of P38 MAPK/ERK-NF-ΚB Pathway and Reduction of JNK in HNSCC. Oncotarget 2017, 8, 56375–56388. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Gutti, U.; Mercado, J.; Moore, C.; Pollard, H.B.; Biswas, R. MAPK Signaling Pathways Regulate IL-8 MRNA Stability and IL-8 Protein Expression in Cystic Fibrosis Lung Epithelial Cell Lines. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 300, L81–L87. [Google Scholar] [CrossRef]
- Involvement of NFkB and STAT6 on Eotaxin-1, IL-8 and RANTES Production in Human Conjunctival Fibroblasts and Epithelial Cells|IOVS|ARVO Journals. Available online: https://iovs.arvojournals.org/article.aspx?articleid=2375839 (accessed on 13 December 2020).
- Cai, T.; Li, X.; Ding, J.; Luo, W.; Li, J.; Huang, C. A Cross-Talk between NFAT and NF-ΚB Pathways Is Crucial for Nickel-Induced COX-2 Expression in Beas-2B Cells. Curr. Cancer Drug Targets 2011, 11, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, I.; Granucci, F. Regulation and Dysregulation of Innate Immunity by NFAT Signaling Downstream of Pattern Recognition Receptors (PRRs). Eur. J. Immunol. 2012, 42, 1924–1931. [Google Scholar] [CrossRef] [PubMed]
- Interaction Between NFκB and NFAT Coordinates Cardiac Hypertrophy and Pathological Remodeling|Circulation Research. Available online: https://www.ahajournals.org/doi/full/10.1161/circresaha.111.260729 (accessed on 13 December 2020).
- Chand, D.H.; Southerland, S.M.; Cunningham, R.J. Tacrolimus: The Good, the Bad, and the Ugly. Pediatr. Transpl. 2001, 5, 32–36. [Google Scholar] [CrossRef]
- Hawthorn: Pharmacology and Therapeutic Uses|American Journal of Health-System Pharmacy|Oxford Academic. Available online: https://academic.oup.com/ajhp/article/59/5/417/5157981 (accessed on 9 December 2020).
- Pittler, M.H.; Schmidt, K.; Ernst, E. Hawthorn Extract for Treating Chronic Heart Failure: Meta-Analysis of Randomized Trials. Am. J. Med. 2003, 114, 665–674. [Google Scholar] [CrossRef]
- Veveris, M.; Koch, E.; Chatterjee, S.S. Crataegus Special Extract WS® 1442 Improves Cardiac Function and Reduces Infarct Size in a Rat Model of Prolonged Coronary Ischemia and Reperfusion. Life Sci. 2004, 74, 1945–1955. [Google Scholar] [CrossRef]
- Kim, S.H.; Kang, K.W.; Kim, K.W.; Kim, N.D. Procyanidins in Crataegus Extract Evoke Endothelium-Dependent Vasorelaxation in Rat Aorta. Life Sci. 2000, 67, 121–131. [Google Scholar] [CrossRef]
- Tassell, M.C.; Kingston, R.; Gilroy, D.; Lehane, M.; Furey, A. Hawthorn (Crataegus Spp.) in the Treatment of Cardiovascular Disease. Pharm. Rev. 2010, 4, 32–41. [Google Scholar] [CrossRef]
- Chai, W.-M.; Chen, C.-M.; Gao, Y.-S.; Feng, H.-L.; Ding, Y.-M.; Shi, Y.; Zhou, H.-T.; Chen, Q.-X. Structural Analysis of Proanthocyanidins Isolated from Fruit Stone of Chinese Hawthorn with Potent Antityrosinase and Antioxidant Activity. J. Agric. Food Chem. 2014, 62, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Alirezalu, A.; Ahmadi, N.; Salehi, P.; Sonboli, A.; Alirezalu, K.; Mousavi Khaneghah, A.; Barba, F.J.; Munekata, P.E.S.; Lorenzo, J.M. Physicochemical Characterization, Antioxidant Activity, and Phenolic Compounds of Hawthorn (Crataegus Spp.) Fruits Species for Potential Use in Food Applications. Foods 2020, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Kirakosyan, A.; Seymour, E.; Kaufman, P.B.; Warber, S.; Bolling, S.; Chang, S.C. Antioxidant Capacity of Polyphenolic Extracts from Leaves of Crataegus Laevigata and Crataegus Monogyna (Hawthorn) Subjected to Drought and Cold Stress. J. Agric. Food Chem. 2003, 51, 3973–3976. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; You, L.; Zhao, Y.; Chang, X. Hawthorn Polyphenol Extract Inhibits UVB-Induced Skin Photoaging by Regulating MMP Expression and Type I Procollagen Production in Mice. J. Agric. Food Chem. 2018, 66, 8537–8546. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, L.; Xing, Y.; Yang, S.; Li, H.; Cao, Y. Roles and Mechanisms of Hawthorn and Its Extracts on Atherosclerosis: A Review. Front. Pharm. 2020, 11. [Google Scholar] [CrossRef]
- Bubik, M.F.; Willer, E.A.; Bihari, P.; Jürgenliemk, G.; Ammer, H.; Krombach, F.; Zahler, S.; Vollmar, A.M.; Fürst, R. A Novel Approach to Prevent Endothelial Hyperpermeability: The Crataegus Extract WS® 1442 Targets the CAMP/Rap1 Pathway. J. Mol. Cell. Cardiol. 2012, 52, 196–205. [Google Scholar] [CrossRef]
- Idris-Khodja, N.; Auger, C.; Koch, E.; Schini-Kerth, V.B. Crataegus Special Extract WS®1442 Prevents Aging-Related Endothelial Dysfunction. Phytomedicine 2012, 19, 699–706. [Google Scholar] [CrossRef]
- Dalli, E.; Milara, J.; Cortijo, J.; Morcillo, E.J.; Cosín-Sales, J.; Sotillo, J.F. Hawthorn Extract Inhibits Human Isolated Neutrophil Functions. Pharmacol. Res. 2008, 57, 445–450. [Google Scholar] [CrossRef]
- Effects of Chlorogenic Acid on Capacity of Free Radicals Scavenging and Proteomic Changes in Postharvest Fruit of Nectarine. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5542658/ (accessed on 17 December 2020).
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Chlorogenic Acid (CGA): A Pharmacological Review and Call for Further Research—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0753332217339963 (accessed on 17 December 2020).
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Hüttemann, M. Molecular Mechanisms and Therapeutic Effects of (−)-Epicatechin and Other Polyphenols in Cancer, Inflammation, Diabetes, and Neurodegeneration. Available online: https://www.hindawi.com/journals/omcl/2015/181260/ (accessed on 17 December 2020).
- Gao, W.; Wang, C.; Yu, L.; Sheng, T.; Wu, Z.; Wang, X.; Zhang, D.; Lin, Y.; Gong, Y. Chlorogenic Acid Attenuates Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice through MAPK/ERK/JNK Pathway. Available online: https://www.hindawi.com/journals/bmri/2019/6769789/ (accessed on 18 January 2021).
- Kwak, S.C.; Lee, C.; Kim, J.-Y.; Oh, H.M.; So, H.-S.; Lee, M.S.; Rho, M.C.; Oh, J. Chlorogenic Acid Inhibits Osteoclast Differentiation and Bone Resorption by Down-Regulation of Receptor Activator of Nuclear Factor Kappa-B Ligand-Induced Nuclear Factor of Activated T Cells C1 Expression. Biol. Pharm. Bull. 2013, 36, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Ngo, H.T.T.; Seo, S.A.; Park, B.; Zhang, M.; Gao, W.; Hoo Yi, T. Urtica Thunbergiana Prevents UVB-Induced Premature Skin Aging by Regulating the Transcription Factor NFATc1: An in Vitro and in Vivo Study. J. Funct. Foods 2017, 36, 162–177. [Google Scholar] [CrossRef]
- Seo, C.-S.; Lim, H.-S.; Ha, H.; Jin, S.E.; Shin, H.-K. Quantitative Analysis and Anti-Inflammatory Effects of Gleditsia Sinensis Thorns in RAW 264.7 Macrophages and HaCaT Keratinocytes. Mol. Med. Rep. 2015, 12, 4773–4781. [Google Scholar] [CrossRef] [PubMed]
- Bourke, C.D.; Prendergast, C.T.; Sanin, D.E.; Oulton, T.E.; Hall, R.J.; Mountford, A.P. Epidermal Keratinocytes Initiate Wound Healing and Pro-Inflammatory Immune Responses Following Percutaneous Schistosome Infection. Int. J. Parasitol. 2015, 45, 215. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.-D.; Kang, T.J.; Lee, C.H.; Lee, A.-Y.; Noh, M. HaCaT Keratinocytes and Primary Epidermal Keratinocytes Have Different Transcriptional Profiles of Cornified Envelope-Associated Genes to T Helper Cell Cytokines. Biomol. Ther. 2012, 20, 171–176. [Google Scholar] [CrossRef]
- Jiang, X.; He, H.; Xie, Z.; Wen, H.; Li, X.; Li, X.; Ma, J.; Umezawa, K.; Zhang, Y. Dehydroxymethylepoxyquinomicin Suppresses Atopic Dermatitis-like Lesions in a Stratum Corneum-Removed Murine Model through NF-ΚB Inhibition. Immunopharmacol Immunotoxicol 2019, 41, 32–39. [Google Scholar] [CrossRef]
- Jin, S.E.; Ha, H.; Shin, H.-K.; Seo, C.-S. Anti-Allergic and Anti-Inflammatory Effects of Kuwanon G and Morusin on MC/9 Mast Cells and HaCaT Keratinocytes. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Henkels, K.M.; Frondorf, K.; Gonzalez-Mejia, M.E.; Doseff, A.L.; Gomez-Cambronero, J. IL-8-INDUCED NEUTROPHIL CHEMOTAXIS IS MEDIATED BY JANUS KINASE 3 (JAK3). FEBS Lett. 2011, 585, 159. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef]
- Huang-Lian-Jie-Du Extract Ameliorates Atopic Dermatitis-like Skin Lesions Induced by 2,4-Dinitrobenzene in Mice via Suppression of MAPKs and NF-ΚB ... -PubMed-NCBI. Available online: https://www.ncbi.nlm.nih.gov/pubmed/31678637 (accessed on 11 January 2020).
- Park, J.-H.; Ahn, E.-K.; Ko, H.-J.; Lee, J.Y.; Hwang, S.-M.; Ko, S.; Oh, J.S. Korean Red Ginseng Water Extract Alleviates Atopic Dermatitis-like Inflammatory Responses by Negative Regulation of Mitogen-Activated Protein Kinase Signaling Pathway in Vivo. Biomed. Pharm. 2019, 117, 109066. [Google Scholar] [CrossRef]
- Lawrence, T. The Nuclear Factor NF-ΚB Pathway in Inflammation. Cold Spring Harb. Perspect Biol. 2009, 1. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, S.; Novak, N. Atopic Dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Sig. Transduct. Target Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fric, J.; Zelante, T.; Wong, A.Y.W.; Mertes, A.; Yu, H.-B.; Ricciardi-Castagnoli, P. NFAT Control of Innate Immunity. Blood 2012, 120, 1380–1389. [Google Scholar] [CrossRef]
- Yan, Y.; Li, J.; Ouyang, W.; Ma, Q.; Hu, Y.; Zhang, D.; Ding, J.; Qu, Q.; Subbaramaiah, K.; Huang, C. NFAT3 Is Specifically Required for TNF-α-Induced Cyclooxygenase-2 (COX-2) Expression and Transformation of Cl41 Cells. J. Cell Sci. 2006, 119, 2985–2994. [Google Scholar] [CrossRef]
- Wierenga, E.A.; Walchner, M.; Kick, G.; Kapsenberg, M.L.; Weiss, E.H.; Messer, G. Evidence for Suppressed Activity of the Transcription Factor NFAT1 at Its Proximal Binding Element P0 in the IL-4 Promoter Associated with Enhanced IL-4 Gene Transcription in T Cells of Atopic Patients. Int. Immunol. 1999, 11, 297–306. [Google Scholar] [CrossRef][Green Version]
- Research, C. for D.E. and Q2 (R1) Validation of Analytical Procedures: Text and Methodology. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q2-r1-validation-analytical-procedures-text-and-methodology (accessed on 2 February 2021).
| Gene | Sense | Antisense |
|---|---|---|
| TARC/CCL17 | ATGGCCCCACTGAAGATGCT | TGAACACCAACGGTGGAGGT |
| MDC/CCL22 | AGGACAGAGCATGGCTCGCCTACAGA | TAATGGCAGGGAGGTAGGGCTCCTGA |
| RANTES/CCL5 | CCCCGTGCCCACATCAAGGAGTATTT | CGTCCAGCCTGGGGAAGGTTTTTGTA |
| IL-8 | TCAGTGCATAAAGACATACTCC | TGGCATCTTCACTGATTCTTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, Q.T.N.; Fang, M.; Zhang, M.; Do, N.Q.; Kim, M.; Zheng, S.D.; Hwang, E.; Yi, T.H. Crataegus laevigata Suppresses LPS-Induced Oxidative Stress during Inflammatory Response in Human Keratinocytes by Regulating the MAPKs/AP-1, NFκB, and NFAT Signaling Pathways. Molecules 2021, 26, 869. https://doi.org/10.3390/molecules26040869
Nguyen QTN, Fang M, Zhang M, Do NQ, Kim M, Zheng SD, Hwang E, Yi TH. Crataegus laevigata Suppresses LPS-Induced Oxidative Stress during Inflammatory Response in Human Keratinocytes by Regulating the MAPKs/AP-1, NFκB, and NFAT Signaling Pathways. Molecules. 2021; 26(4):869. https://doi.org/10.3390/molecules26040869
Chicago/Turabian StyleNguyen, Quynh T. N., Minzhe Fang, Mengyang Zhang, Nhung Quynh Do, Minseon Kim, Sheng Dao Zheng, Eunson Hwang, and Tae Hoo Yi. 2021. "Crataegus laevigata Suppresses LPS-Induced Oxidative Stress during Inflammatory Response in Human Keratinocytes by Regulating the MAPKs/AP-1, NFκB, and NFAT Signaling Pathways" Molecules 26, no. 4: 869. https://doi.org/10.3390/molecules26040869
APA StyleNguyen, Q. T. N., Fang, M., Zhang, M., Do, N. Q., Kim, M., Zheng, S. D., Hwang, E., & Yi, T. H. (2021). Crataegus laevigata Suppresses LPS-Induced Oxidative Stress during Inflammatory Response in Human Keratinocytes by Regulating the MAPKs/AP-1, NFκB, and NFAT Signaling Pathways. Molecules, 26(4), 869. https://doi.org/10.3390/molecules26040869