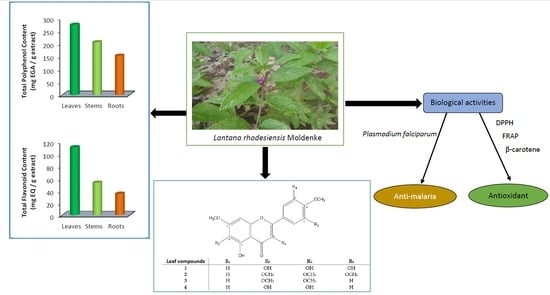
Phytochemical Investigation and Biological Activities of Lantana rhodesiensis
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Screening
2.1.1. Determination of Phytochemical Classes
2.1.2. Polyphenolic Compound Quantification
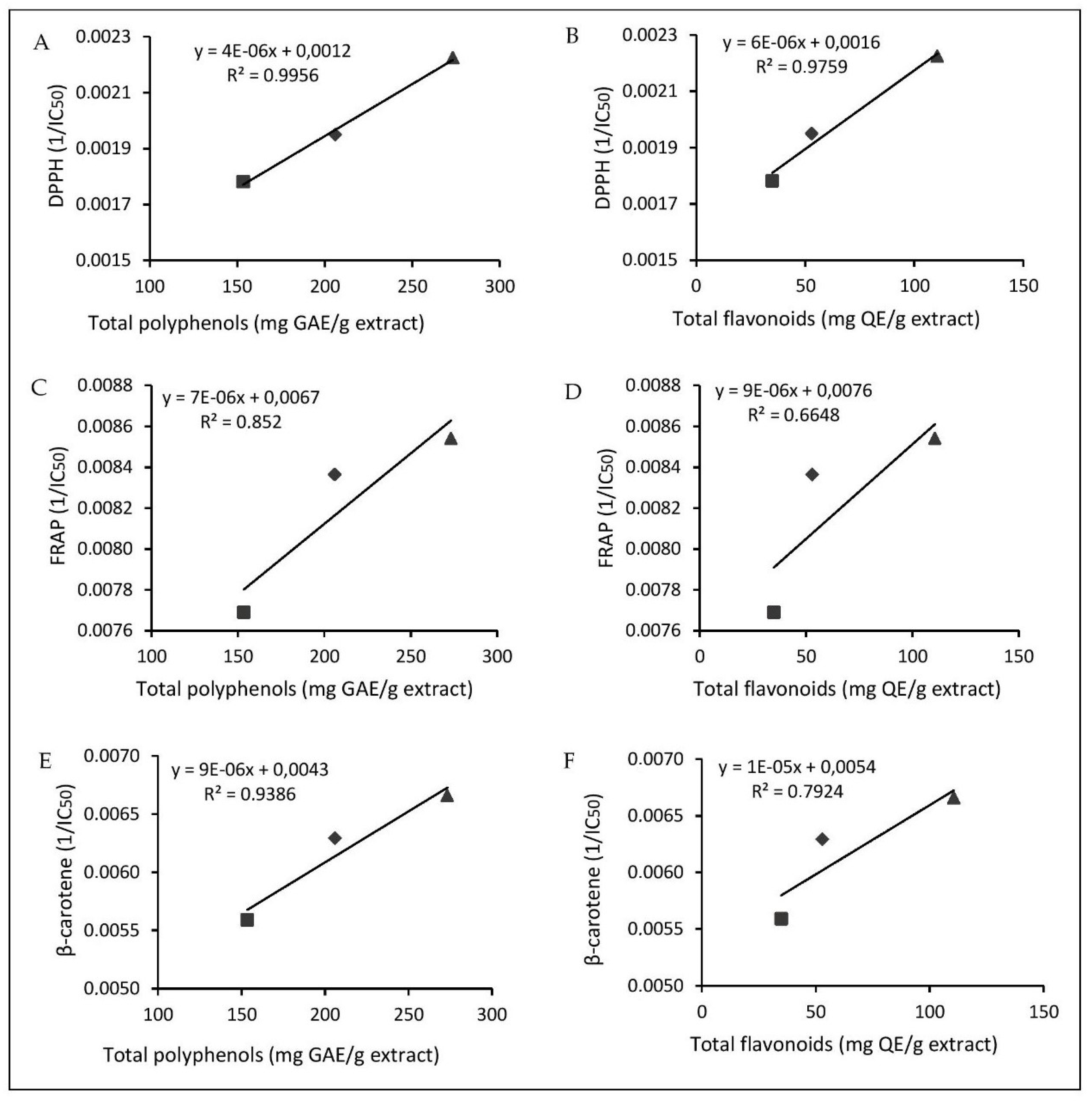
2.2. Antioxidant Activity
2.3. Anti-Malarial Activity
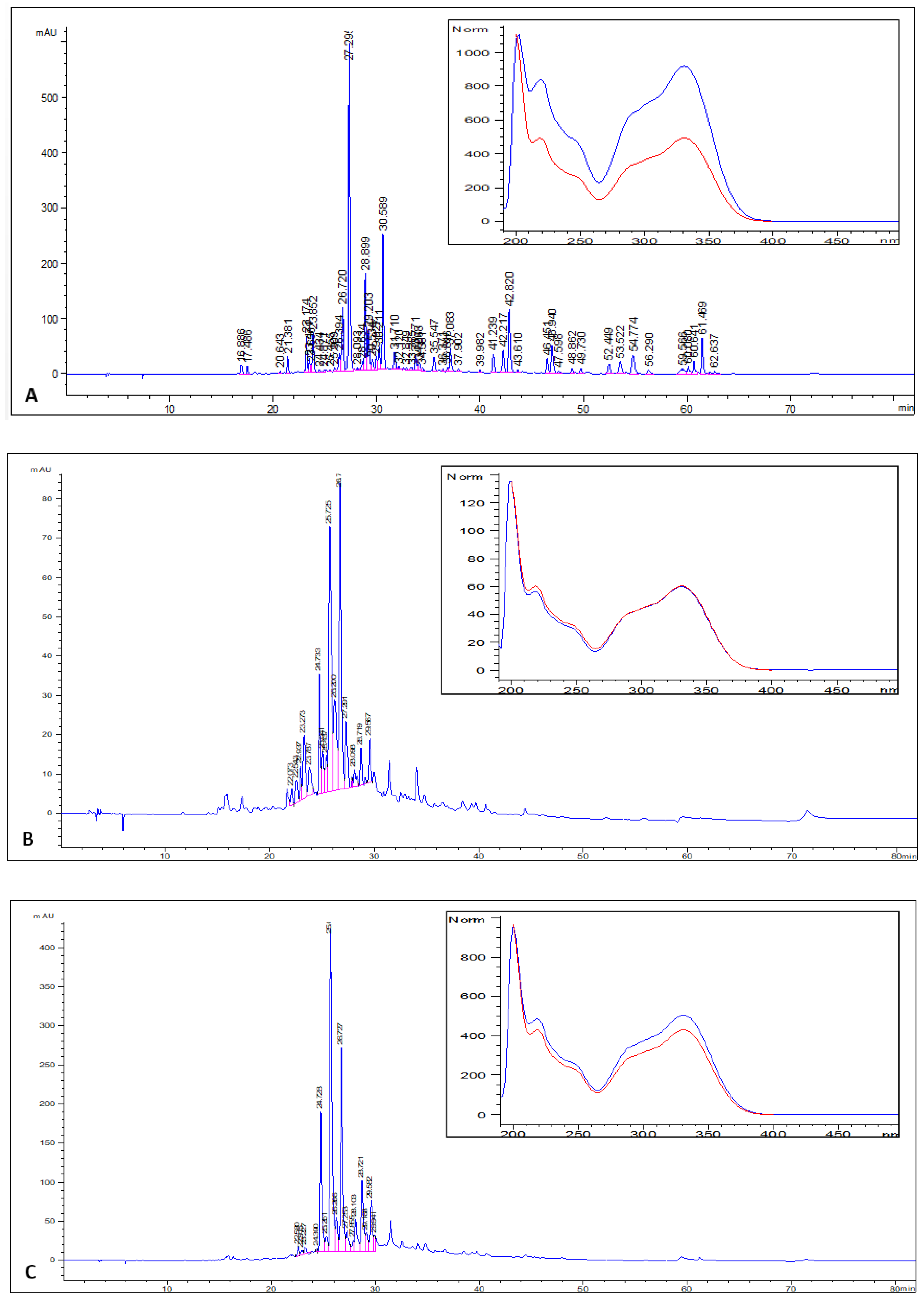
2.4. Determination of the HPLC-PDA (Photodiode Array Detector) Polyphenol Profile Leaf, Stem, and Root Extracts
2.5. Structural Elucidation
3. Materials and Methods
3.1. Plant Materials
3.2. Reagent and Solvents
3.3. Determination of Phytochemical Classes
3.4. Extract Preparation for the Determination of Total Phenolic and Flavonoid Contents and Tests for Biological Activity
3.5. Determination of the Total Phenolic Content
3.6. Determination of the Total Flavonoid Content
3.7. Antioxidant Activity
3.7.1. DPPH Radical Scavenging Assay
3.7.2. Reducing Power
3.7.3. β-Carotene Blanching Test
3.8. Anti-Malarial Activity
3.9. Statistical Analysis of Biological Data
3.10. Extraction, Isolation, and Characterization of Compounds
3.11. General Procedure for the Determination of Compounds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ngugi, P.M.; Kimuni, N.; Ngeranwa, N.; Orinda, O.; Njagi, M.; Maina, D.; Agyirifo, S.; Gathumbi, K.; King’e, W.; Eliud, E.N. Antidiabetic and Safety of Lantana rhodesiensis in Alloxan Induced Diabetic Rats. J. Dev. Drugs 2015, 4, 1–10. [Google Scholar]
- Fratkin, E. Traditional medicine and concepts of healing among Samburu pastoralists of Kenya. J. Ethnobiol. 1996, 16, 63–97. [Google Scholar]
- Sawadogo, W.R.; Maciuk, A.; Banzouzi, J.T.; Champy, P.; Figadere, B.; Guissou, I.P.; Nacoulma, O.G. Mutagenic effect, antioxidant and anticancer activities of six medicinal plants from Burkina Faso. Nat. Prod. Res. 2012, 26, 575–579. [Google Scholar] [CrossRef]
- Jean, B.M.; Nâg-Tiero, M.R.; Martin, K.; Germaine, N.O.; Norma, A.-A. A review on Lantana rhodesiensis Moldenke: Traditional uses, phytochemical constituents and pharmacological activities. Int. J. Phytomed. 2017, 9, 1–9. [Google Scholar] [CrossRef][Green Version]
- Edeoga, H.O.; Okwu, D.E.; Mbaebie, B.O. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 2005, 4, 685–688. [Google Scholar] [CrossRef]
- Ruffo, C.K.; Birnie, A.; Tengnäs, B. Edible Wild Plants of Tanzania; Regional Land Management Unit/Sida: Nairobi, Kenya, 2002; ISBN 0-947643-51-6. [Google Scholar]
- Jean, B.M.; Alfonso, R.-M.; Hervé, C.T.; Véronique, M.; Germaine, N.O. Phytochemistry investigation and antioxidant activity of four edible Verbenaceae of Burkina Faso. Int. J. Phytomed. 2017, 9, 655. [Google Scholar] [CrossRef][Green Version]
- Seyoum, A.; Killeen, G.F.; Kabiru, E.W.; Knols, B.G.J.; Hassanali, A. Field efficacy of thermally expelled or live potted repellent plants against African malaria vectors in western Kenya. Trop. Med. Int. Health 2003, 8, 1005–1011. [Google Scholar] [CrossRef]
- Nea, F.; Amenan, E.; Leon, E.; Kenne, T.; Genva, M.; Saive, M.; Felix, Z.; Fauconnier, M. A new chemotype of Lantana rhodesiensis Moldenke essential oil from Côte d’Ivoire: Chemical composition and biological activities. Ind. Crops Prod. 2019, 141, 111766. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Bangou, M.J.; Kiendrebeogo, M.; Compaoré, M.; Coulibaly, A.Y.; Meda, N.-T.R.; Abarca, N.A.; Zeba, B.; Millogo-Rasolodimby, J.; Nacoulma, O.G. Enzyme Inhibition Effect and Polyphenolic Content of Medicinal Plant Extracts from Burkina Faso. J. Biol. Sci. 2011, 31–38. [Google Scholar] [CrossRef][Green Version]
- Sawadogo, W.R.; Cerella, C.; Al-mourabit, A.; Moriou, C.; Teiten, M.; Guissou, I.P.; Dicato, M.; Diederich, M. Cytotoxic, Antiproliferative and Pro-Apoptotic Effects of 5-Hydroxyl-6,7,3′,4′,5′-Pentamethoxyflavone Isolated from Lantana ukambensis. Nutrients 2015, 7, 10388–10397. [Google Scholar] [CrossRef]
- Kumar, S.; Sandhir, R.; Ojha, S. Evaluation of antioxidant activity and total phenol in different varieties of Lantana camara leaves. BMC Res. Notes 2014, 7, 1–9. [Google Scholar] [CrossRef]
- Amiot, M.J.; Riollet, C.; Landrier, J.F. Polyphénols et syndrome métabolique. Med. Mal. Metab. 2009, 3, 476–482. [Google Scholar] [CrossRef]
- Barros, L.M.; Duarte, A.E.; Waczuk, E.P.; Roversi, K.; Da Cunha, F.A.B.; Rolon, M.; Coronel, C.; Gomez, M.C.V.; De Menezes, I.R.A.; Da Costa, J.G.M.; et al. Safety assessment and antioxidant activity of lantana montevidensis leaves: Contribution to its phytochemical and pharmacological activity. EXCLI J. 2017, 16, 566–582. [Google Scholar]
- Mansoori, A.; Singh, N.; Dubey, S.K.; Thakur, T.K.; Alkan, N.; Das, S.N.; Kumar, A. Phytochemical Characterization and Assessment of Crude Extracts From Lantana camara L. for Antioxidant and Antimicrobial Activity. Front. Agron. 2020, 2, 1–14. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.-H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- McDonald, S.; Prenzler, P.D.; Antolovich, M.; Robards, K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001, 73, 13–84. [Google Scholar] [CrossRef]
- Skotti, E.; Anastasaki, E.; Kanellou, G.; Polissiou, M.; Tarantilis, P.A. Total phenolic content, antioxidant activity and toxicity of aqueous extracts from selected Greek medicinal and aromatic plants. Ind. Crops. Prod. 2014, 53, 46–54. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Oriakhi, K.; Oikeh, E.I.; Ezeugwu, N.; Anoliefo, O.; Aguebor, O. Comparative Antioxidant Activities of Extracts of Vernonia amygdalina and Ocimum gratissimum Leaves. J. Agric. Sci. 2014, 6, 13–20. [Google Scholar] [CrossRef]
- Uyoh, E.A.; Chukwurah, P.N.; David, I.A.; Bassey, A.C. Evaluation of Antioxidant Capacity of Two Ocimum Species Consumed Locally as Spices in Nigeria as a Justification for Increased Domestication. Am. J. Plant Sci. 2013, 04, 222–230. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. Leb. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant activity of medicinal and aromatic plants. A review. Flavour Fragr. J. 2010, 25, 291–312. [Google Scholar] [CrossRef]
- Kulisic, T.; Radonic, A.; Katalinic, V.; Milos, M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004, 85, 633–640. [Google Scholar] [CrossRef]
- Taubert, D.; Breitenbach, T.; Lazar, A.; Censarek, P.; Harlfinger, S.; Berkels, R.; Klaus, W.; Roesen, R. Reaction rate constants of superoxide scavenging by plant antioxidants. Free Radic. Biol. Med. 2003, 35, 1599–1607. [Google Scholar] [CrossRef]
- Zin, Z.M.; Abdul Hamid, A.; Osman, A.; Saari, N. Antioxidative activities of chromatographic fractions obtained from root, fruit and leaf of Mengkudu (Morinda citrifolia L.). Food Chem. 2006, 94, 169–178. [Google Scholar] [CrossRef]
- Wallace, G.; Fry, S.C. Phenolic components of the primary cell wall. Inter. Rev. Cytol. 1994, 151, 229–267. [Google Scholar]
- Binns, A.N.; Chen, R.H.; Wood, H.N.; Lynn, D.G. Cell division promoting activity of naturally occurring dehydrodiconiferyl glucosides: Do cell wall components control cell division? Proc. Natl. Acad. Sci. USA 1987, 84, 980–984. [Google Scholar] [CrossRef]
- Martial-didier, A.K.; Hubert, K.K.; Eugène, K.; Parfait, J.; Kablan, T. Phytochemical Properties and Proximate Composition of Papaya (Carica papaya L. var solo 8) Peels. Turk. J. Agric. Food Sci. Technol. 2017, 5, 676–680. [Google Scholar] [CrossRef]
- Arbaayah, H.; Umi Kalsom, Y. Antioxidant properties in the oyster mushrooms (Pleurotus spp.) and split gill mushroom (Schizophyllum commune) ethanolic extracts. Mycosphere 2013, 4, 661–673. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Kader, A.A. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California. J. Agric. Food Chem. 2002, 50, 4976–4982. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli, S.; Djomeh, Z.E. Total phenols, antioxidant potential and antimicrobial activity of methanol extract of rosemary (Rosmarinus officinalis L.). Glob. Vet. 2011, 7, 337–341. [Google Scholar]
- Bergonzi, M.C.; Bilia, A.R.; Gallori, S.; Guerrini, D.; Vincieri, F.F. Variability in the content of the constituents of Hypericum perforatum L. and some commercial extracts. Drug Dev. Ind. Pharm. 2001, 27, 491–497. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zheng, W. Effect of plant growth temperature on antioxidant capacity in strawberry. J. Agric. Food Chem. 2001, 49, 4977–4982. [Google Scholar] [CrossRef] [PubMed]
- Casassa, I.A.F.; Sari, I.A.S.; Avagnina, E.S.; Díaz, T.G.M.; Jofré, L.Q.V.; Fanzone, L.B.M.; Catania, I.A.C. Influence de deux techniques de maceration sur la composition phenolique et aromatique et les caractéristiques orgnoleptiques de vins cv. merlot. Rev. Internet Vitic. Oenol. 2007, 25, 1–12. [Google Scholar]
- Yang, D.J.; Hwang, L.S.; Lin, J.T. Effects of different steeping methods and storage on caffeine, catechins and gallic acid in bag tea infusions. J. Chromatogr. A 2007, 1156, 312–320. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef]
- Venditti, E.; Bacchetti, T.; Tiano, L.; Carloni, P.; Greci, L.; Damiani, E. Hot vs. cold water steeping of different teas: Do they affect antioxidant activity? Food Chem. 2010, 119, 1597–1604. [Google Scholar] [CrossRef]
- Bouyahya, A.; Abrini, J.; Bakri, Y.; Dakka, N. Screening phytochimique et évaluation de l’activité antioxydante et antibactérienne des extraits d’Origanum compactum. Phytotherapie 2017, 15, 379–383. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Marco, R.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan Honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Gursoy, N.; Sarikurkcu, C.; Cengiz, M.; Solak, M.H. Antioxidant activities, metal contents, total phenolics and flavonoids of seven Morchella species. Food Chem. Toxicol. 2009, 47, 2381–2388. [Google Scholar] [CrossRef]
- Compaoré, M.; Lamien-Meda, A.; Mogoşan, C.; Lamien, C.E.; Kiendrebeogo, M.; Voştinaru, O.; Vlase, L.; Ionescu, C.; Nacoulma, O.G. Antioxidant, diuretic activities and polyphenol content of Stereospermum kunthianum Cham. (Bignoniaceae). Nat. Prod. Res. 2011, 25, 1777–1788. [Google Scholar] [CrossRef]
- Jonville, M. Etude de la Composition Chimique et des Potentialités Antipaludiques de Plantes Utilisées en Médecine Traditionnelle au Cambodge et dans L’archipel des Mascareignes. Ph.D. Thesis, Université de Liège, Liège, Belgium, 2011. [Google Scholar]
- Clarkson, C.; Maharaj, V.J.; Crouch, N.R.; Grace, O.M.; Pillay, P.; Matsabisa, M.G.; Bhagwandin, N.; Smith, P.J.; Folb, P.I. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J. Ethnopharmacol. 2004, 92, 177–191. [Google Scholar] [CrossRef]
- Bashige-Chiribagula, V.; Bakari-Amuri, S.; Mbuyi-Kalonji, S.; Kahumba-Byanga, J.; Duez, P.; Lumbu-Simbi, J.B. Étude ethnobotanique, phytochimique et évaluation de l’activité antiplasmodiale de 13 plantes réputées antipaludéennes dans la commune du Kenya (Lubumbashi, RDC). Phytotherapie 2017, 1–10. [Google Scholar] [CrossRef]
- Esseh, K.; Afanyibo, Y.-G.; Ahama-Esseh, K.Y.S.; Idoh, K.; Koudouvo, K.; Agbonon, A.; Gbeassor, M. Screening Phytochimique, Étude Toxicologique, Évaluation des Activités Antiplasmodiale et Antiradicalaire de la Tige Feuillée de Senna occidentalis Linn (Fabaceae). Eur. Sci. J. ESJ 2019, 15, 411–433. [Google Scholar]
- Nassirou, R.; Ibrahim, M.; Ilagouma, A.; Mahamadou, A.; Mamoudou, M.; Abdoulaye, A.; Oukem-Boyer, O.; Ikhiri, K. Évaluation in vitro de l’activité antiplasmodiale d’extraits de plantes issues de la pharmacopée traditionnelle du Niger. J. Appl. Biosci. 2015, 89, 8291–8300. [Google Scholar] [CrossRef][Green Version]
- Baba, G.; Adewumi, A.A.J.; Aina, V.O. Phytochemical Characterization and in-vivo Anti- Malaria Activity of Lantana camara Leaf Extract. Br. J. Pharmacol. Toxicol. 2011, 2, 277–282. [Google Scholar]
- Chiou, W.F.; Lin, L.C.; Chen, C.F. Acteoside protects endothelial cells against free radical-induced oxidative stress. J. Pharm. Pharmacol. 2004, 56, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lin, Y.; Chien, M.; Hou, W.; Hu, M. Antioxidant and antihypertensive activities of acteoside and its analogs. Biochemistry 2012, 53, 421–429. [Google Scholar]
- Lee, H.D.; Kim, J.H.; Pang, Q.Q.; Jung, P.M.; Cho, E.J.; Lee, S. Antioxidant activity and acteoside analysis of abeliophyllum distichum. Antioxidants 2020, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jang, T.W.; Choi, J.S.; Park, J.H. Protective and inhibitory effects of acteoside from Abeliophyllum distichum Nakai against oxidative DNA damage. Mol. Med. Rep. 2020, 22, 2076–2084. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Woo, E.R.; Choi, C.Y.; Shin, D.W.; Lee, D.G.; You, H.J.; Jeong, H.G. Protective Effect of Acteoside on Carbon Tetrachloride-Induced Hepatotoxicity. Life Sci. 2004, 74, 1051–1064. [Google Scholar] [CrossRef]
- Nair, A.G.R.; Kotiyal, J.P.; Bhardwaj, D.K. Myricetin 7,4′-dimethyl ether and its 3-galactoside from Rhus lancea. Phytochemistry 1983, 22, 318–319. [Google Scholar] [CrossRef]
- Nagao, T.; Abe, F.; Kinjo, J.; Okabe, H. Antiproliferative constituents in plants 10. Flavones from the leaves of Lantana montevidensis Briq. and consideration of structure-activity relationship. Biol. Pharm. Bull. 2002, 25, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Rwangabo, P.C.; Claeys, M.; Pieters, L.; Corthout, J.; Vanden Berghe, D.A.; Vlietinck, A.J. Umuhengerin, a new antimicrobially active flavonoid from lantana trifolia. J. Nat. Prod. 1988, 51, 966–968. [Google Scholar] [CrossRef]
- Akkal, S.; Benayache, F.; Bentamene, A.; Medjroubi, K.; Seguin, E.; Tillequin, F. Flavonoid aglycones from Centaurea napifolia. Chem. Nat. Compd. 2003, 39, 165–166. [Google Scholar] [CrossRef]
- Servi, H.; Goren, N. Chemistry of endemic Tanacetum mucroniferum Hub.-Mor. & Grierson extracts and three new sesquiterpene lactones. Turk. J. Chem. 2019, 43, 352–358. [Google Scholar]
- Abu-Niaaj, L.; Katampe, I. Isolation and characterization of flavones from Artemisia monosperma. Pharmacogn. J. 2018, 10, 1018–1023. [Google Scholar] [CrossRef]
- Itoh, T.; Ohguchi, K.; Iinuma, M.; Nozawa, Y.; Akao, Y. Inhibitory effects of polymethoxy flavones isolated from Citrus reticulate on degranulation in rat basophilic leukemia RBL-2H3: Enhanced inhibition by their combination. Bioorg. Med. Chem. 2008, 16, 7592–7598. [Google Scholar] [CrossRef]
- Yang, L.N.; Xing, J.G.; He, C.H.; Wu, T. The phenolic compounds from Dracocephalum moldavica L. Biochem. Syst. Ecol. 2014, 54, 19–22. [Google Scholar] [CrossRef]
- Belkacem, S.; Belbache, H.; Boubekri, C.; Mosset, P.; Rached-Mosbah, O.; Marchioni, E.; Benayache, S.; Benayache, F. Chemical Constituents from Centaurea parviflora Desf. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 1275–1279. [Google Scholar]
- Polatoǧlu, K.; Karakoç, Ö.C.; Demirci, F.; Gökçe, A.; Gören, N. Chemistry and biological activities of Tanacetum chiliophyllum var. oligocephalum extracts. J. AOAC Int. 2013, 96, 1222–1227. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M. Food Phenolics: Sources, Chemistry, Effects and Applications. Food Chem. 1995, 57, 481–482. [Google Scholar]
- Barkat Malika. Imène Laib Composition chimique et activité antioxydante de l’huile essentielle des fleurs sèches de Lavandula officinalis. Rev. Génie Ind. 2011, 6, 46–54. [Google Scholar]
- Rebey, I.B.; Wannes, W.A.; Kaab, S.B.; Bourgou, S.; Tounsi, M.S.; Ksouri, R.; Fauconnier, M.L. Bioactive compounds and antioxidant activity of Pimpinella anisum L. accessions at different ripening stages. Sci. Hortic. 2019, 246, 453–461. [Google Scholar] [CrossRef]
- Bettaieb Rebey, I.; Bourgou, S.; Saidani Tounsi, M.; Fauconnier, M.L.; Ksouri, R. Phytochemical composition and antioxidant activity of Lavandula dentate extracts Etude de la composition chimique et de l’activité antioxydante des différents extraits de la Lavande dentée (Lavandula dentata). J. New Sci. Agric. Biotechnol. 2017, 39, 2096–2105. [Google Scholar]
- Lamia, S.A.; Moussa, B.; Marie-laure, F.; Georges, L. Chemical Composition and Antioxidant Activity of Thymus fontanesii Essential Oil from Algeria. Nat. Prod. J. 2018, 8, 1–7. [Google Scholar]
- Ledoux, A.; St-gelais, A.; Cieckiewicz, E.; Jansen, O.; Illien, B.; Di Giovanni, N.; Marvilliers, A.; Hoareau, F. Antimalarial Activities of Alkyl Cyclohexenone Derivatives Isolated from the Leaves of Poupartia borbonica. J. Nat. Prod. 2017, 80, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Delemarre, J.M.; Van Der Kaay, H.J. Plasmodium falciparum malaria contracted in the natural way in the Netherlands. Ned. Tijdschr. Geneeskd. 1979, 123, 1981–1982. [Google Scholar]
- Murebwayire, S.; Frédérich, M.; Hannaert, V.; Jonville, M.C.; Duez, P. Antiplasmodial and antitrypanosomal activity of Triclisia sacleuxii (Pierre) Diels. Phytomedicine 2008, 15, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Makler, M.T.; Ries, J.M.; Williams, J.A.; Bancroft, J.E.; Piper, R.C.; Gibbins, B.L.; Hinrichs, D.J. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am. J. Trop. Med. Hyg. 1993, 48, 739–741. [Google Scholar] [CrossRef] [PubMed]
- Makler, M.T.; Hinrichs, D.J. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am. J. Trop. Med. Hyg. 1993, 48, 205–210. [Google Scholar] [CrossRef] [PubMed]

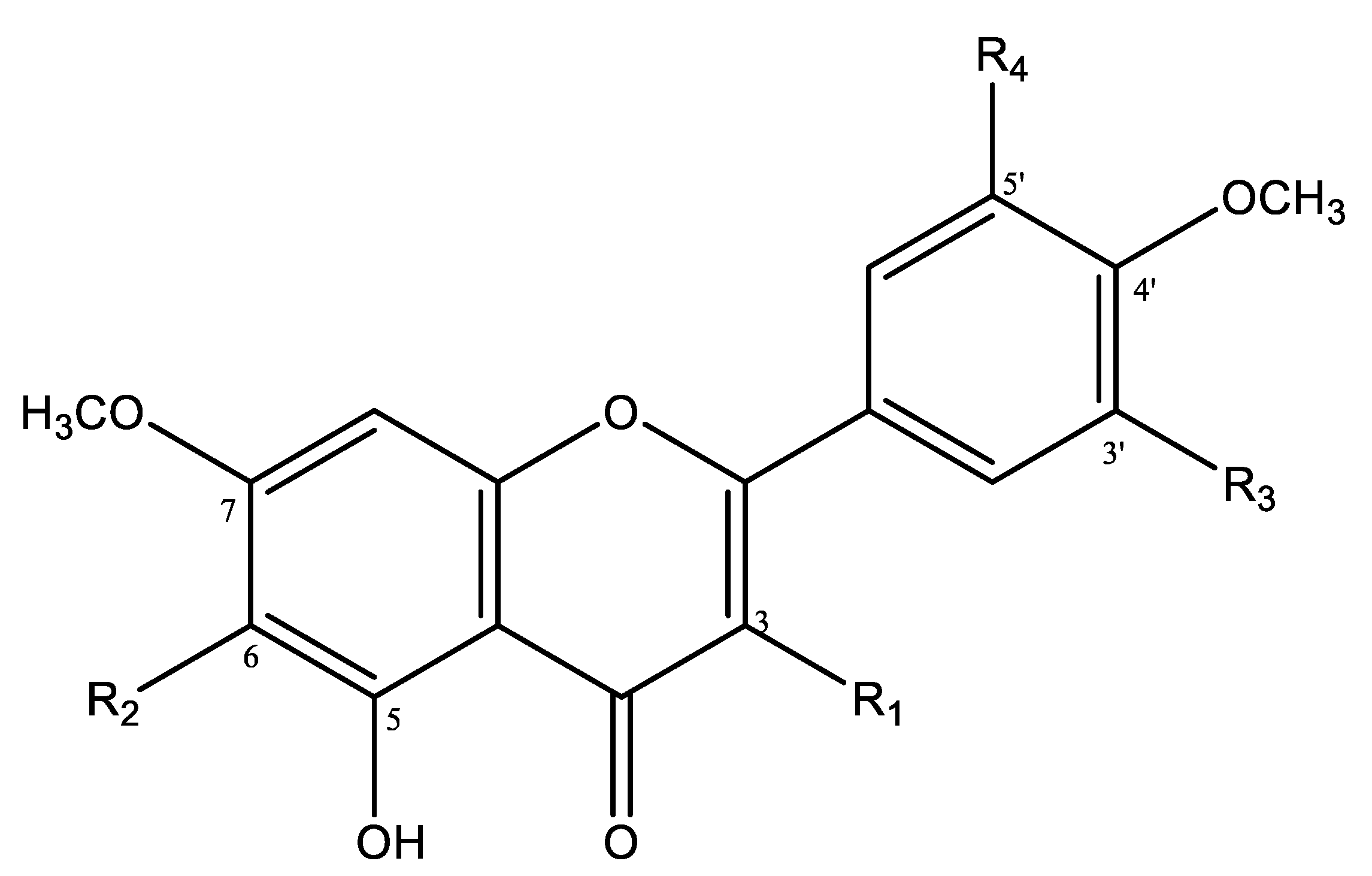
| Compounds | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 1 | H | OH | OH | OH |
| 2 | H | OCH3 | OCH3 | OCH3 |
| 3 | H | OCH3 | OCH3 | H |
| 4 | H | OH | OH | H |
| Phytochemical Classes | Test Performed | Leaves | Stems | Roots | |
|---|---|---|---|---|---|
| Polyphenols | Iron chloride 2% | +++ | ++ | + | |
| Flavonoids | Cyanidin | +++ | + | + | |
| Terpenes/sterols | Lieberman and Bürchard | ++ | + | ++ | |
| Tannins | catechin | Stiasny | ++ | + | + |
| gallic | Stiasny | ++ | ++ | + | |
| Saponins | Foam formation | + | + | ++ | |
| Alkaloids | Dragendorff | + | + | + | |
| Leuco-anthocyanins | Cyanidin | - | - | - | |
| Anthocyanins | Cyanidin | - | - | - | |
| Polyphenolic Compound Contents | ||
|---|---|---|
| Total Polyphenols (mg GAE/g Extract) | Total Flavonoids (mg QE/g Extract) | |
| Leaves | 273.27 ± 0.48 | 110.54 ± 0.46 |
| Stems | 206.06 ± 0.87 | 52.95 ± 0.64 |
| Roots | 153.37 ± 0.61 | 34.87 ± 0.34 |
| Extract (MeOH/H2O) | 3D7, IC50 (µg/mL) |
|---|---|
| Leaves | 12.5 ± 2.5 |
| Stems | ˃100 |
| Roots | ˃100 |
| Artemisinin | 0.004 ± 0.001 |
| 1 | 2 | 3 | 4 | |||||
|---|---|---|---|---|---|---|---|---|
| δH m (J in Hz) | δC | δH m (J in Hz) | δC | δH m (J in Hz) | δC | δH m (J in Hz) | δC | |
| 2 | 165.1 | 164.1 | 165.3 | 165.0 | ||||
| 3 | 6.59, s | 102.5 | 6.64, s | 105.3 | 6.76, s | 104.1 | 6.67, s | 102.4 |
| 4 | 182.8 | 182.8 | 182.6 | 182.9 | ||||
| 5 | 150.9 | 153.4 | 151.8 | 150.6 | ||||
| 6 | 130.0 | 132.7 | 131.7 | 130.0 | ||||
| 7 | 154.4 | 159.1 | 159.4 | 154.4 | ||||
| 8 | 6.81, s | 90.4 | 6.61, s | 91.0 | 6.89, s | 92.7 | 6.85, s | 90.6 |
| 9 | 150.6 | 152.7 | 154.9 | 150.6 | ||||
| 10 | 105.2 | 106.2 | 105.4 | 105.2 | ||||
| 1’ | 121.4 | 126.5 | 122.0 | 122.4 | ||||
| 2’ | 7.12, s | 101.7 | 7.12, s | 103.9 | 7.14, d (8.5) | 111.1 | 7.52, d (2.13) | 109.2 |
| 3’ | 138.5 | 153.7 | 152.5 | 150.7 | ||||
| 4’ | 148.5 | 141.5 | 151.0 | 148.1 | ||||
| 5’ | 145.7 | 153.7 | 7.55, d (2.2) | 108.8 | 6.94, d (8.3) | 115.4 | ||
| 6’ | 7.12, s | 107.3 | 7.12, s | 103.9 | 7.68, dd (8.5–2.2) | 119.9 | 7.54, dd (8.3–2.13) | 120.4 |
| 6-OCH3 | 4.00, s | 61.1 | 3.85, s | 61.1 | ||||
| 7-OCH3 | 3.99, s | 55.5 | 3.93, s | 56,5 | 4.00, s | 57.0 | 4.02, s | 55.6 |
| 3’-OCH3 | 3.98, s | 56,5 | 3.93, s | 56.8 | ||||
| 4’-OCH3 | 3.95, s | 55.5 | 3.94, s | 60.9 | 3.96, s | 56.6 | 3.99, s | 55.3 |
| 5’-OCH3 | 3.98 | 56,5 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nea, F.; Bitchi, M.B.; Genva, M.; Ledoux, A.; Tchinda, A.T.; Damblon, C.; Frederich, M.; Tonzibo, Z.F.; Fauconnier, M.-L. Phytochemical Investigation and Biological Activities of Lantana rhodesiensis. Molecules 2021, 26, 846. https://doi.org/10.3390/molecules26040846
Nea F, Bitchi MB, Genva M, Ledoux A, Tchinda AT, Damblon C, Frederich M, Tonzibo ZF, Fauconnier M-L. Phytochemical Investigation and Biological Activities of Lantana rhodesiensis. Molecules. 2021; 26(4):846. https://doi.org/10.3390/molecules26040846
Chicago/Turabian StyleNea, Fatimata, Michel Boni Bitchi, Manon Genva, Allison Ledoux, Alembert Tiabou Tchinda, Christian Damblon, Michel Frederich, Zanahi Félix Tonzibo, and Marie-Laure Fauconnier. 2021. "Phytochemical Investigation and Biological Activities of Lantana rhodesiensis" Molecules 26, no. 4: 846. https://doi.org/10.3390/molecules26040846
APA StyleNea, F., Bitchi, M. B., Genva, M., Ledoux, A., Tchinda, A. T., Damblon, C., Frederich, M., Tonzibo, Z. F., & Fauconnier, M.-L. (2021). Phytochemical Investigation and Biological Activities of Lantana rhodesiensis. Molecules, 26(4), 846. https://doi.org/10.3390/molecules26040846