Lignins Isolated via Catalyst-Free Organosolv Pulping from Miscanthus x giganteus, M. sinensis, M. robustus and M. nagara: A Comparative Study
Abstract
1. Introduction
2. Biomass Leaf-To-Stem Ratio, Chemical Composition, Lignin and Ash Content
2.1. Leave-To-Stem Ratio and Chemical Composition of the Miscanthus Biomass
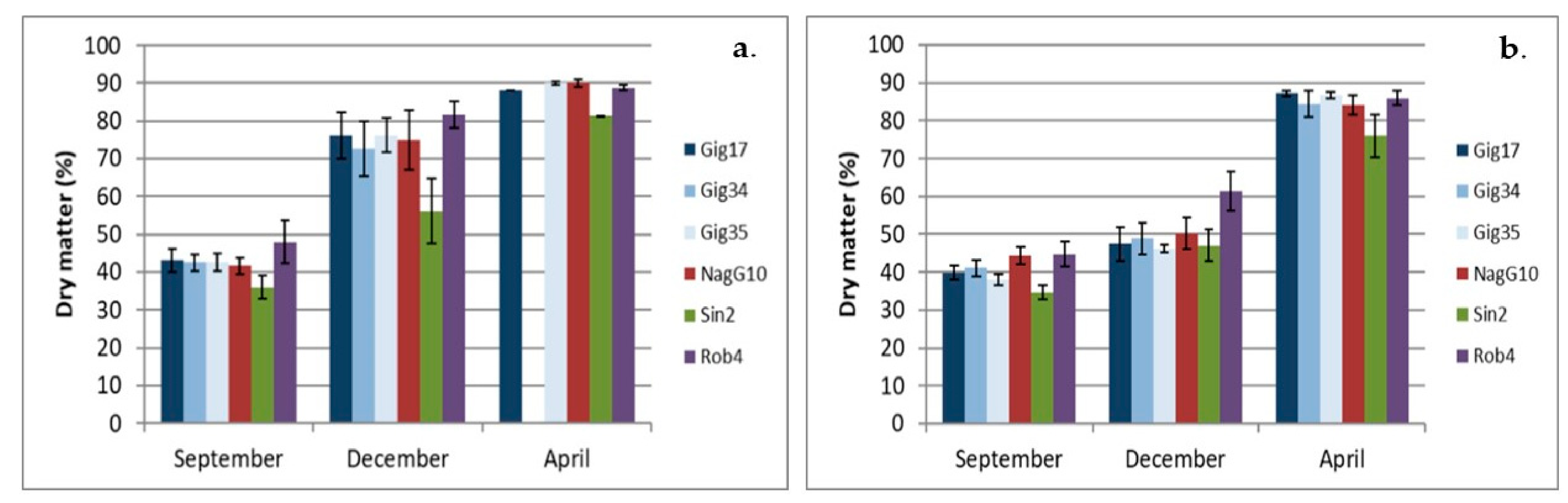
2.2. Dry Matter
2.3. Ash Content
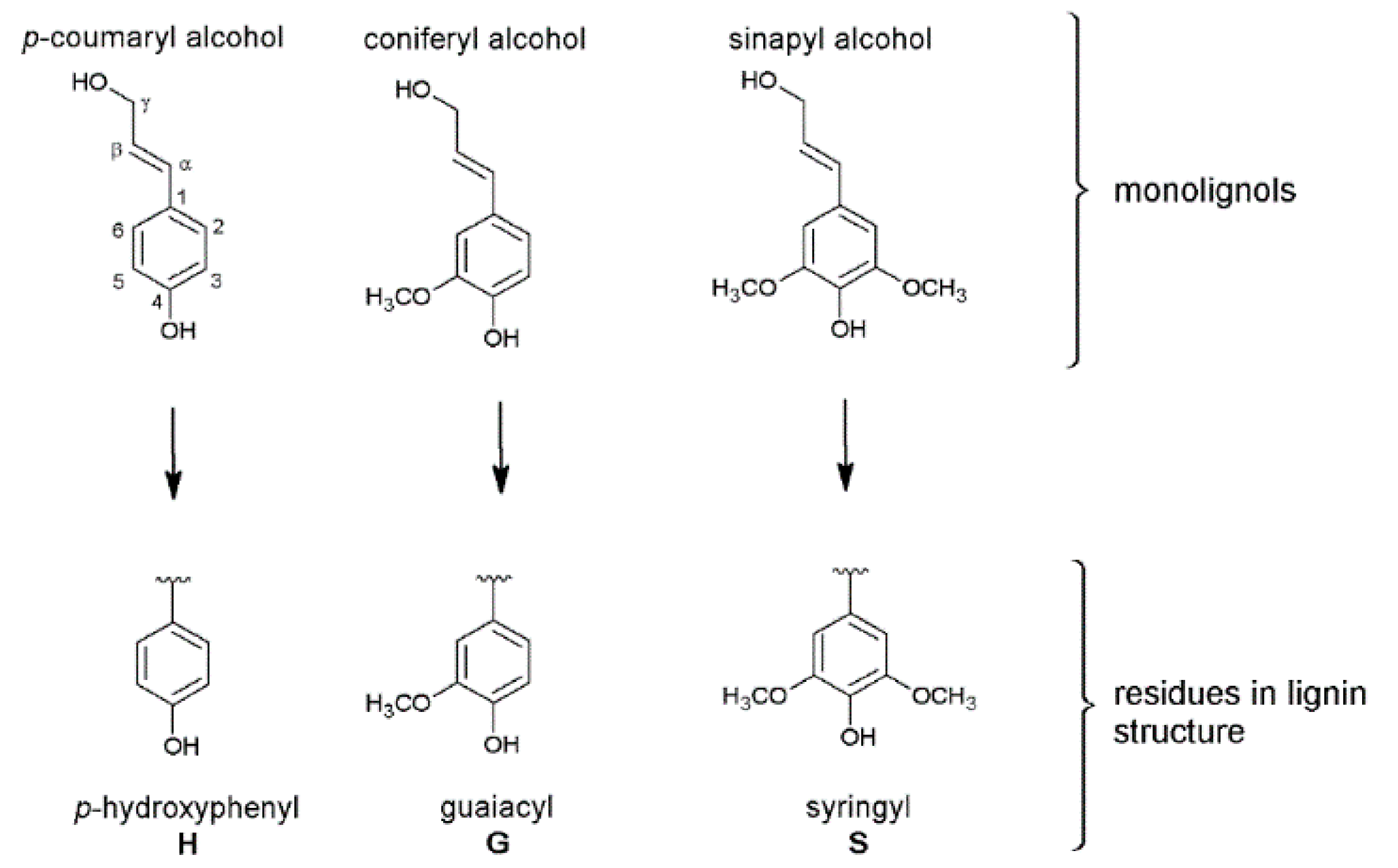
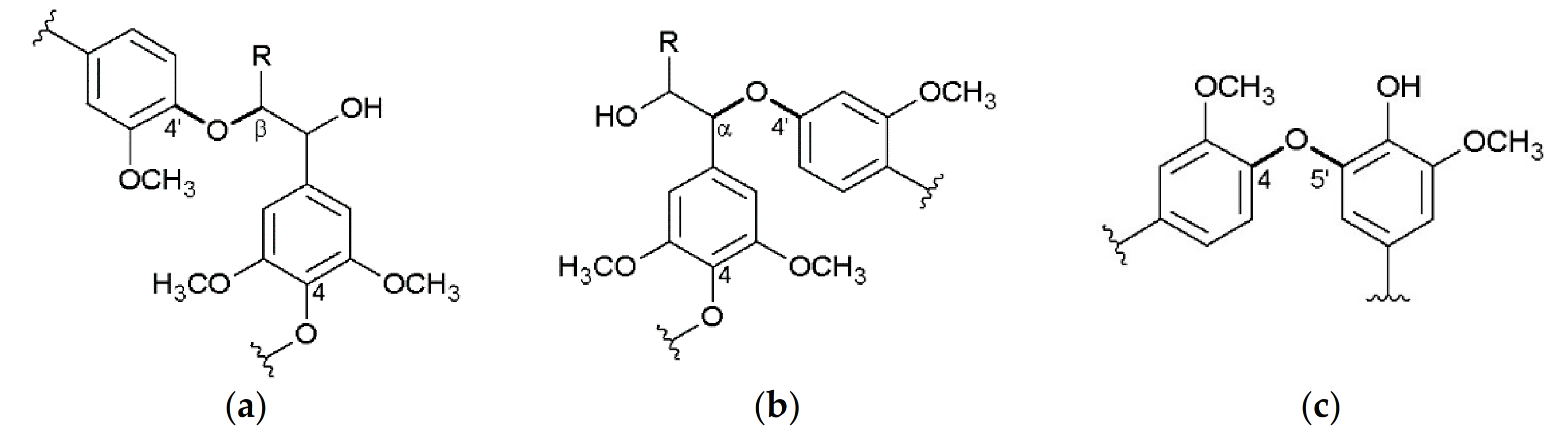
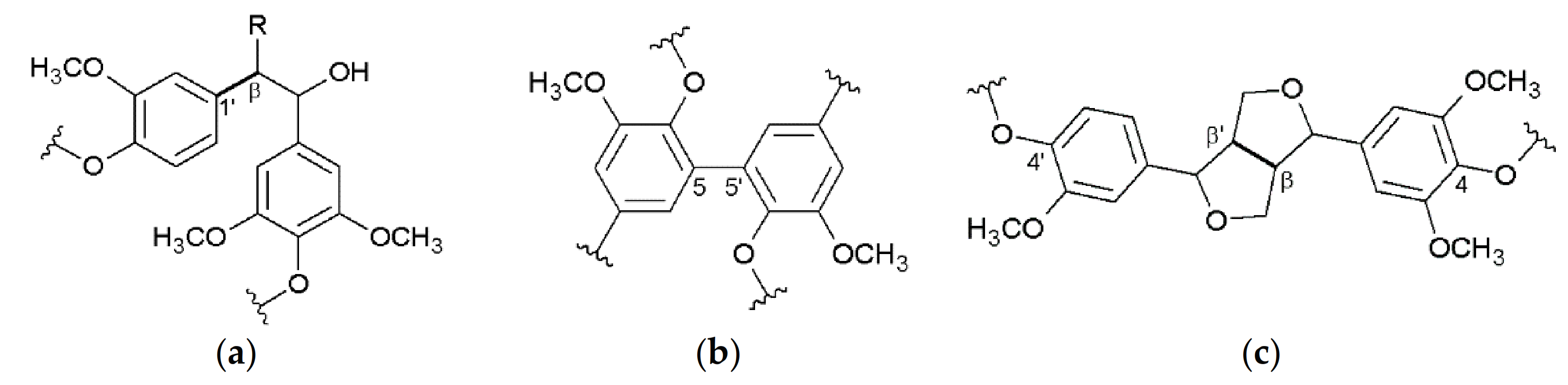
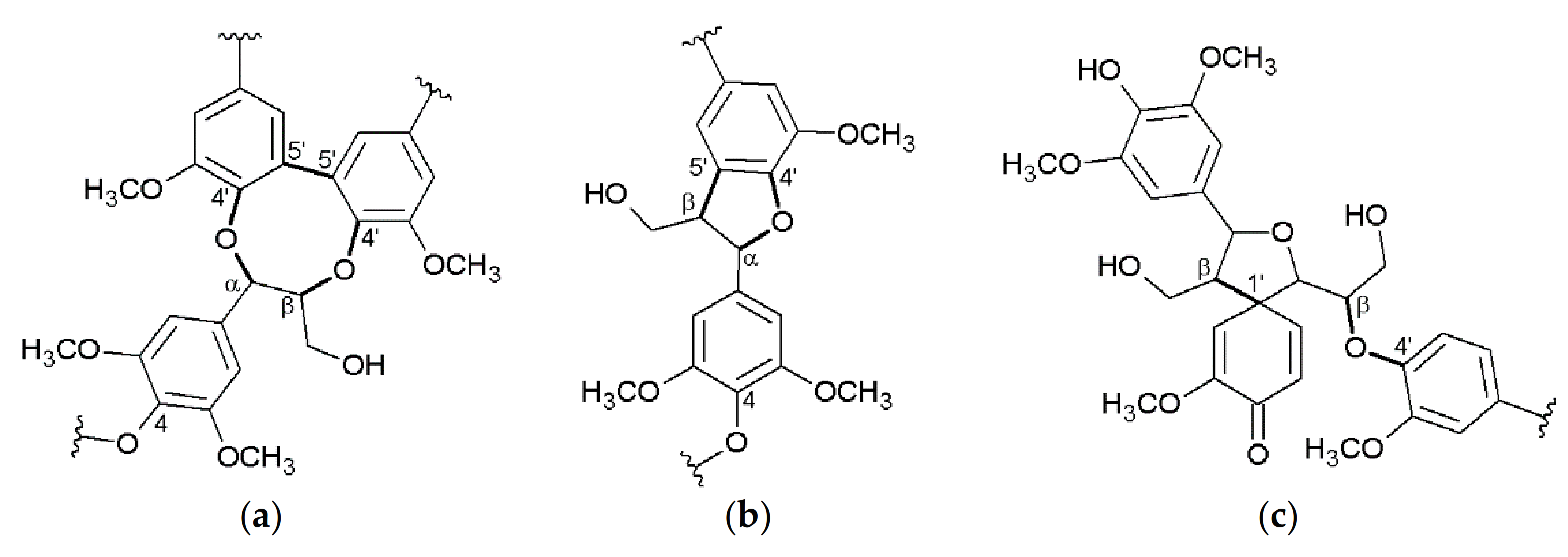
3. Structure of the Isolated Miscanthus-Derived Lignins
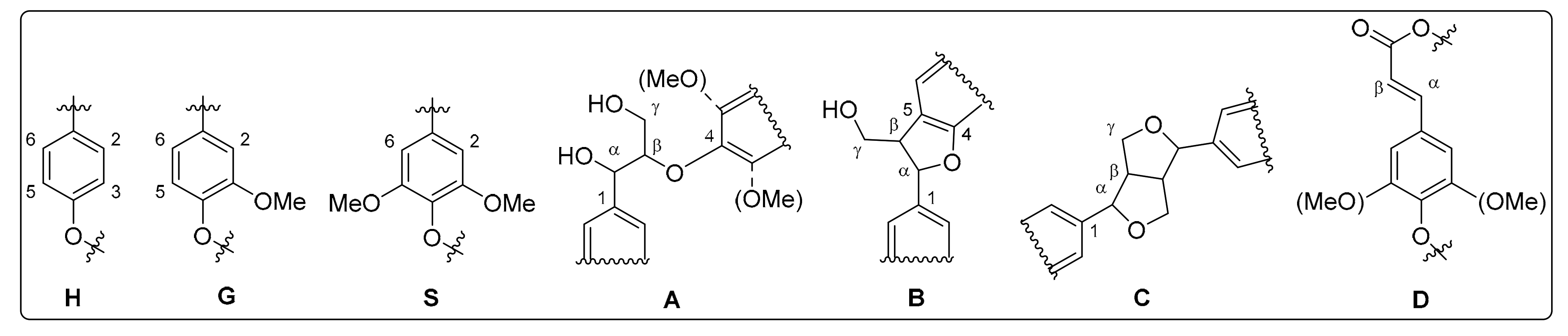
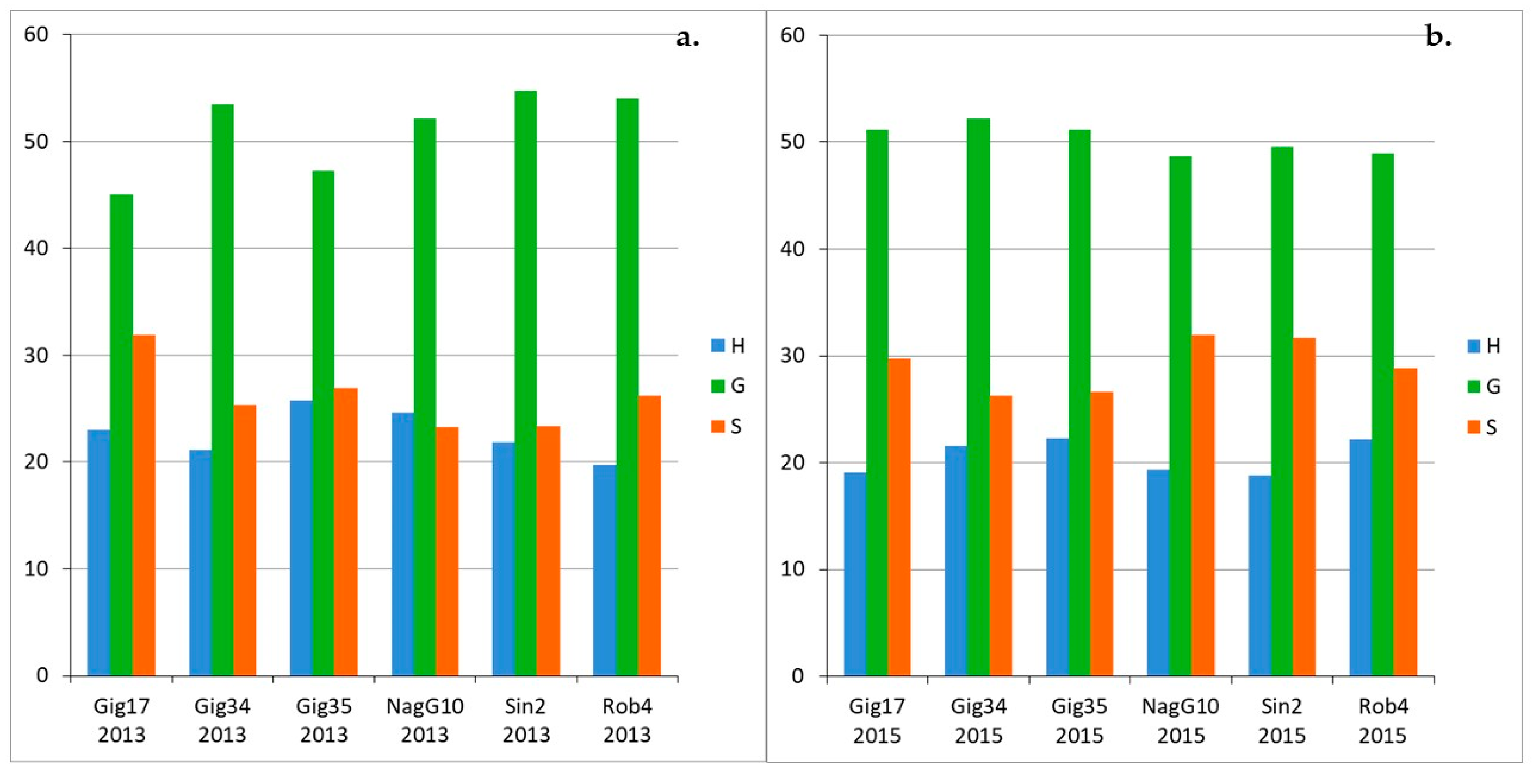
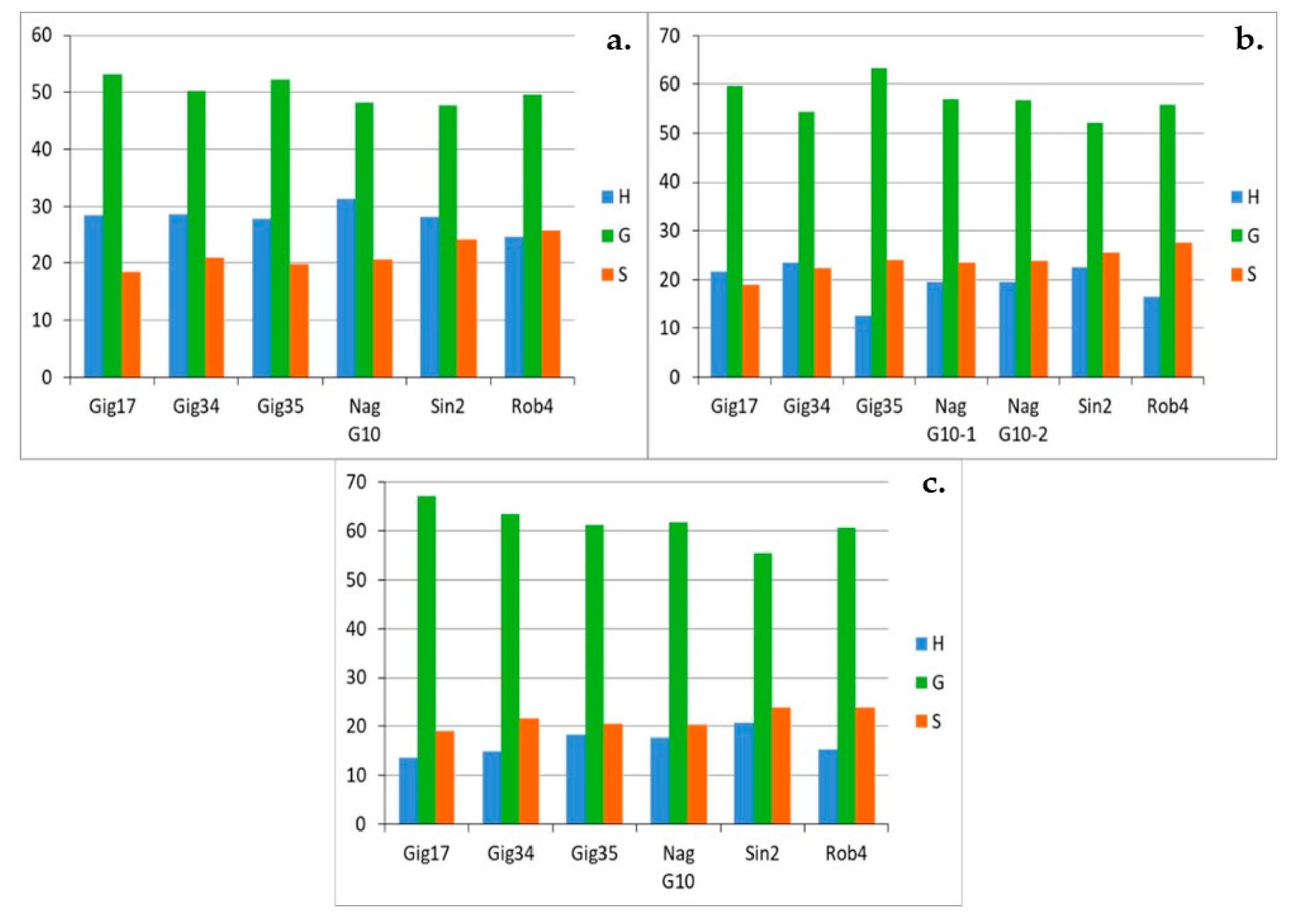
3.1. Monolignol Ratio Accoroding to NMR Spectroscopy
3.2. Interunit Linkages Accoroding to NMR-Spectroscopy
4. Materials and Methods
4.1. Lignin Isolation using a Catalyst-free Organosolv Process
4.2. Chemical Composition of the Biomasses
4.3. HSQC NMR Analyses
5. Conclusions
6. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Winkler, B.; Mangold, A.; von Cossel, M.; Clifton-Brown, J.; Pogrzeba, M.; Lewandowski, I.; Iqbald, Y.; Kiesel, A. Implementing miscanthus into farming systems: A review of agronomic practices, capital and labour demand. Renew. Sust. Energy Rev. 2020, 132, 110053. [Google Scholar] [CrossRef]
- GRACE. GRowing Advanced Industrial Crops on Marginal Lands for Biorefineries. Available online: https://www.grace-bbi.eu/project/ (accessed on 25 October 2020).
- Von Cossel, M.; Lewandowski, I.; Elbersen, B.; Staritsky, I.; Van Eupen, M.; Iqbal, Y.; Mantel, S.; Scordia, D.; Testa, G.; Cosentino, S.L.; et al. Marginal Agricultural Land Low-Input Systems for Biomass Production. Energies 2019, 12, 3123. [Google Scholar] [CrossRef]
- Ben Fradja, N.; Rozakis, S.; Borzęcka, M.; Matyka, M. Miscanthus in the European bio-economy: A network analysis. Ind. Crops Prod. 2020, 148, 112281. [Google Scholar] [CrossRef]
- von Cossel, M.; Mangold, A.; Iqbal, Y.; Hartung, J.; Lewandowski, I.; Kiesel, A. How to Generate Yield in the First Year—A Three-Year Experiment on Miscanthus (Miscanthus x giganteus) Establishment under Maize (Zea mays L.). Agronomy 2019, 9, 237. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Lignocellulosic Biorefineries in Europe: Current State and Prospects. Trend Biotechnol. 2018. [Google Scholar] [CrossRef]
- Vanhamaki, S.; Medkova, K.; Malamakis, A.; Kontogianni, S.; Marisova, E.; Huisman-Dellago, D.; Moussiopoulos, N. Bio-based Circular Economy in European National and Regional Strategies. Int. J. Sustain. Dev. Plan. 2019, 14, 31–43. [Google Scholar] [CrossRef]
- Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. A Sustainable Bioeconomy for Europe: Strengthening the Connection between Economy, Society and the Environment; Reference No. 11.10.2018 COM; The European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Pude, R.; Treseler, C.H.; Noga, G. Morphological, chemical and technical parameters of Miscanthus genotypes. J. Appl. Bot. 2004, 78, 58–63. [Google Scholar]
- Moll, L.; Wever, C.; Völkering, G.; Pude, R. Increase of Miscanthus Cultivation with New Roles in Materials Production—A Review. Agronomy 2020, 10, 308. [Google Scholar] [CrossRef]
- Lewandowski, I.; Clifton-Brown, J.; Trindade, L.M.; Van Der Linden, G.C.; Schwarz, K.-U.; Müller-Sämann, K.; Anisimov, A.; Chen, C.-L.; Dolstra, O.; Donnison, I.S.; et al. Progress on optimizing miscanthus biomass production for the European bioeconomy: Results of the EU FP7 project OPTIMISC. Front. Plant Sci. 2016, 7, 1620. [Google Scholar] [CrossRef]
- Iqbal, Y.; Kiesel, A.; Wagner, M.; Nunn, C.; Kalinina, O.; Hastings, A.F.S.J.; Clifton-Brown, J.C.; Lewandowski, I. Harvest Time Optimization for Combustion Quality of Different Miscanthus Genotypes across Europe. Front. Plant Sci. 2017, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Y.; Lewandowski, I. Inter-annual variation in biomass combustion quality traits over five years in fifteen Miscanthus genotypes in south Germany. Fuel Process. Technol. 2014, 121, 47–55. [Google Scholar] [CrossRef]
- Felten, D.; Froba, N.; Fries, J.; Emmerling, C. Energy balances and CO2-mitigation potentials of bioenergy crops (Miscanthus, rapeseed, maize) based on farming conditions in Western Germany. Renew. Energy 2013, 55, 160–174. [Google Scholar] [CrossRef]
- Greef, J.M.; Deuter, M.; Jung, C.; Schondelmaier, J. Genetic diversity of European Miscanthus species revealed by AFLP fingerprinting. Genet. Resour. Crop Evol. 1997, 44, 185–195. [Google Scholar] [CrossRef]
- Monti, A.; Zegada-Lizarazu, W.; Zanetti, F.; Casler, M. Chapter Two—Nitrogen Fertilization Management of Switchgrass, Miscanthus and Giant Reed: A Review. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 153, pp. 87–119. [Google Scholar]
- Villaverde, J.J.; Ligero, P.; Vega, A.D. Miscanthus x giganteus as a Source of Biobased Products Through Organosolv Fractionation: A Mini Review. TOASJ 2010, 4, 102–110. [Google Scholar] [CrossRef]
- Emmerling, C.; Pude, R. Introducing Miscanthus to the greening measures of the EU Common Agricultural Policy. GCB Bioenergy 2017, 9, 274–279. [Google Scholar] [CrossRef]
- Verordnung (EU) 2017/2393 des Europäischen Parlaments und des Rates vom 13. Dezember 2017 zur Änderung der Verordnungen (EU) Nr. 1305/2013 über die Förderung der ländlichen Entwicklung durch den Europäischen Landwirtschaftsfonds die Entwicklung des ländlichen Raums (ELER), (EU) Nr. 1306/2013 über die Finanzierung, die Verwaltung und das Kontrollsystem der Gemeinsamen Agrarpolitik, (EU) Nr. 1307/2013 mit Vorschriften über Direktzahlungen an Inhaber landwirtschaftlicher Betriebe im Rahmen von Stützungsregelungen der Gemeinsamen Agrarpolitik, (EU) Nr. 1308/2013 über eine gemeinsame Marktorganisation für landwirtschaftliche Erzeugnisse und (EU) Nr. 652/2014 mit Bestimmungen für die Verwaltung der Ausgaben in den Bereichen Lebensmittelkette, Tiergesundheit und Tierschutz sowie Pflanzengesundheit und Pflanzenvermehrungsmaterial Amtsblatt der Europäischen Union vom 29.12.2017, L350, 15–49. Available online: https://eur-lex.europa.eu/legal-content/DE/TXT/PDF/?uri=CELEX:32017R2393&from=DE (accessed on 25 October 2020).
- Muthusamy, S.K.; Lenka, S.K.; Katiyar, A.; Chinnusamy, V.; Singh, A.K.; Bansal, K.C. Genome-Wide Identification and Analysis of Biotic and Abiotic Stress Regulation of C4 Photosynthetic Pathway Genes in Rice. Appl. Biochem. Biotechnol. 2018. [Google Scholar] [CrossRef]
- Kellogg, E.A. C4 photosynthesis. Curr. Biol. 2013, 23, R594–R599. [Google Scholar] [CrossRef] [PubMed]
- Teese, P. Intraspecific variation for CO2 compensation point and differential growth among variants in a C3-C4 intermediate plant. Oecologia 1995, 102, 371–376. [Google Scholar] [CrossRef]
- Van der Weijde, T.; Kiesel, A.; Iqbal, Y.; Muylle, H.; Dolstra, O.; Visser, R.G.F.; Lewandowski, I.; Trindade, L.M. Evaluation of Miscanthus sinensis biomass quality as feedstock for conversion into different bioenergy products. GCB Bioenergy 2016, 9, 176–190. [Google Scholar] [CrossRef]
- da Costa, R.M.F.; Lee, S.J.; Allison, G.G.; Hazen, S.P.; Winters, A.; Bosch, M. Genotype, development and tissue-derived variation of cell-wall properties in the lignocellulosic energy crop Miscanthus. Ann. Bot. 2014, 114, 1265–1277. [Google Scholar] [CrossRef]
- Baker, P.W.; Winters, A.; Hale, M.D.C. Biodegradation of Different Genotypes of Miscanthus by Wood Rot Fungi. BioResources 2016, 11, 4379–4391. [Google Scholar] [CrossRef]
- Sonnenberg, A.M.; Baars, J.J.P.; Visser, M.H.M.; Lavrijssen, B.; Hendrickx, P.M. Evaluation of shiitake strains (Lentinula edodes) on selective lignin degradation in Miscanthus x giganteus, TKI T&U: KV 1310-032, PPO/PRI report 2016-3. 2016. [CrossRef]
- Li, Z.; Zhao, C.; Zha, Y.; Wan, C.; Si, S.; Liu, F.; Zhang, R.; Li, F.; Yu, B.; Yi, Z.; et al. The minor wall-networks between monolignols and interlinked-phenolics predominantly affect biomass enzymatic digestibility in Miscanthus. PLoS ONE 2014, 9, e105115. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, H.; Akiyama, T.; Tamai, A.; Nawawi, D.S.; Syafii, W.; Yokoyama, T.; Matsumoto, Y. Variation of the contents of biphenyl structures in lignins among wood species. Holzforschung 2019. [Google Scholar] [CrossRef]
- Schäfer, J.; Sattler, M.; Iqbal, Y.; Lewandowski, I.; Bunzel, M. Characterization of Miscanthus cell wall polymers. GCB Bioenergy 2019, 11, 191–205. [Google Scholar] [CrossRef]
- Bergs, M.; Do, X.T.; Rumpf, J.; Völkering, G.; Pude, R.; Monakhova, Y.; Konow, C.; Schulze, M. Harvesting Season Influence on Monolignol Ratio and Linkage in six different Miscanthus genotypes. RSC Adv. 2020, 10, 10740–10751. [Google Scholar] [CrossRef]
- Rumpf, J.; Do, X.T.; Burger, R.; Monakhova, Y.; Schulze, M. Extraction of High-Purity Lignins via Catalyst-free Organosolv Pulping from Low-Input Crops. Biomacromolecules 2020, 21, 1929–1942. [Google Scholar] [CrossRef]
- Mazar, A.; Jemaa, N.; Wafa Al Dajani, W.; Marinova, M.; Perrier, M. Optimization of Lignin Recovery from the Pre-Hydrolysate of Kraft-Based Dissolving Pulp Production Processes. Appl. Sci. 2021, 11, 454. [Google Scholar] [CrossRef]
- El Hage, R.; Brosse, N.; Chrusciel, L.; Sanchez, C.; Sannigrahi, P.; Ragauskas, A. Characterization of milled wood lignin and ethanol organosolv lignin from miscanthus. Polym. Degrad. Stab. 2009, 94, 1632–1638. [Google Scholar] [CrossRef]
- El Hage, R.; Perrin, D.; Brosse, N. Effect of the pre-treatment severity on the antioxidant properties of ethanol organosolv Miscanthus x giganteus lignin. Nat. Resour. 2012, 3, 29–34. [Google Scholar] [CrossRef]
- El Hage, R.; Brosse, N.; Sannigrahi, P.; Ragauskas, A. Effects of process severity on the chemical structure of Miscanthus ethanol organosolv lignin. Polym. Degrad. Stab 2010, 95, 997–1003. [Google Scholar] [CrossRef]
- Chan, J.M.W.; Bauer, S.; Sorek, H.; Sreekumar, S.; Wang, K.; Toste, F.D. Studies on the vanadium-catalyzed nonoxidative depolymerization of Miscanthus giganteus-derived lignin. ACS Catal. 2013, 3, 1369–1377. [Google Scholar] [CrossRef]
- Luo, H.; Klein, I.M.; Jiang, Y.; Zhu, H.; Liu, B.; Kenttämaa, H.I.; Abu-Omar, M.M. Total Utilization of Miscanthus Biomass, Lignin and Carbohydrates, Using Earth Abundant Nickel Catalyst. ACS Sustain. Chem. Eng. 2016, 4, 2316–2322. [Google Scholar] [CrossRef]
- Vanderghem, C.; Richel, A.; Jacquet, N.; Blecker, C.; Paquot, M. Impact of formic/acetic acid and ammonia pre-treatments on chemical structure and physico-chemical properties of Miscanthus x giganteus lignins. Polym. Degrad. Stab. 2011, 96, 1761–1770. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Abeywickrama, C.J.; Rakshit, S.K.; Brosse, N. Effect of different pretreatments on delignification pattern and enzymatic hydrolysability of miscanthus, oil palm biomass and typha grass. Bioresour. Technol. 2013, 135, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Groenewold, G.S.; Johnson, K.M.; Fox, S.C.; Rae, C.; Zarzana, C.A.; Kersten, B.R.; Rowe, S.M.; Westover, T.L.; Gresham, G.L.; Emerson, R.M. Pyrolysis Two-Dimensional GC-MS of Miscanthus Biomass: Quantitative Measurement using an Internal Standard Method. Energy Fuels 2017, 31, 1620–1630. [Google Scholar] [CrossRef]
- del Río, J.C.; Rencoret, J.; Gutiérrez, A.; Elder, T.; Kim, H.; Ralph, J. Lignin Monomers from beyond the Canonical Monolignol Biosynthetic Pathway: Another Brick in the Wall. ACS Sust. Chem. Eng. 2020, 8, 4997–5012. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef]
- Tolbert, A.; Akinosho, H.; Khunsupat, R.; Naskar, A.K.; Ragauskas, A.J. Characterization and analysis of the molecular weight of lignin for biorefining studies. Biofuels Bioprod. Bioref. 2014, 8, 836–856. [Google Scholar] [CrossRef]
- Bergs, M. Einfluss von Miscanthus-Genotyp und Erntezeit auf Gehalt und Struktur von Lignin aus Organosolv-Verfahren. Ph.D. Thesis, Rheinische Friedrich Wilhelms University Bonn, Bonn, Germany, 20 December 2018. Available online: https://nbn-resolving.org/urn:nbn:de:hbz:5n-53211 (accessed on 27 November 2020).
- Bergs, M.; Völkering, G.; Kraska, T.; Do, X.; Monakhova, Y.; Konow, C.; Pude, R.; Schulze, M. Miscanthus x giganteus Stem versus Leave-derived Lignins Differing in Monolignol Ratio and Linkage. Int. J. Mol. Sci. 2019, 20, 1200. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.R.D.; Lancefield, C.S.; Miles-Barrett, D.M.; Ackermann, K.; Bode, B.E.; Westwood, N.J.; Lebl, T. Fractionation and DOSY NMR as Analytical Tools: From Model Polymers to a Technical Lignin. ACS Omega 2017, 2, 8466. [Google Scholar] [CrossRef]
- Monakhova, Y.; Diehl, B.W.K.; Do, X.T.; Witzleben, S.; Schulze, M. Novel method for the determination of average molecular weight of natural polymers based on 2D DOSY NMR and chemometrics: Example of heparin. J. Pharm. Biomed. Anal. 2018, 149, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Wahid, R.; Nielsen, S.F.; Hernandez, V.M.; Ward, A.J.; Gislum, R.; Jørgensen, U.; Møller, H.B. Methane production potential from Miscanthus sp: Effect of harvesting time, genotypes and plant fractions. Biosyst. Eng. 2015, 133, 71–80. [Google Scholar] [CrossRef]
- Scagline-Mellor, S.; Griggs, T.; Skousen, J.; Wolfrum, E.; Holásková, I. Switchgrass and Giant Miscanthus Biomass and Theoretical Ethanol Production from Reclaimed Mine Lands. Bioenerg. Res. 2018, 11, 562–573. [Google Scholar] [CrossRef]
- Hafez, I.; Hassan, E.B. Rapid liquefaction of giant miscanthus feedstock in ethanol–water system for production of biofuels. Energy Conv. Manag. 2015, 91, 219–224. [Google Scholar] [CrossRef]
- Sørensen, A.; Teller, P.J.; Hilstrøm, T.; Ahring, B.K. Hydrolysis of Miscanthus for bioethanol production using dilute acid presoaking combined with wet explosion pre-treatment and enzymatic treatment. Bioresour. Technol. 2008, 99, 6602–6607. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.-L.; An, G.H.; Yang, J.; Moon, Y.-H.; Yu, G.-D.; Ahn, J.-W. Bioethanol production from Miscanthus using thermotolerant Saccharomyces cerevisiae mbc 2 isolated from the respiration-deficient mutants. Renew. Energ. 2015, 80, 259–265. [Google Scholar] [CrossRef]
- Lupoi, J.S.; Singh, S.; Parthasarathi, R.; Simmons, B.A.; Henry, R.J. Recent innovations in analytical methods for the qualitative and quantitative assessment of lignin. Renew. Sustain. Energy. Rev. 2015, 49, 871–906. [Google Scholar] [CrossRef]
- Giraudeau, P. Quantitative 2D liquid-state NMR. Magn. Res.Chem. 2014, 52, 259–272. [Google Scholar] [CrossRef]
- Du, X.; Pérez-Boada, M.; Fernández, C.; Rencoret, J.; del Río, J.C.; Jiménez-Barbero, J.; Li, J.; Gutiérrez, A.; Martínez, A.T. Analysis of lignin-carbohydrate and lignin-lignin linkages after hydrolase treatment of xylan-lignin, glucomannan-lignin and glucan-lignin complexes from spruce wood. Planta 2014, 239, 1079–1090. [Google Scholar] [CrossRef]
- Miscanthus nagara. Available online: www.tinplant-gmbh.de (accessed on 18 October 2009).
- Deuter, M. Miscanthus Plant Named “MBS 7001”. U.S. Patent PP22,033 P2, 19 July 2011. [Google Scholar]
- Heaton, E.A.; Dohleman, F.G.; Miguez, A.F.; Juvik, J.A.; Lozovaya, V.; Widholm, J.; Long, S.P. Miscanthus: A Promising Biomass Crop. Adv. Bot. Res. 2010, 56, 75–135. [Google Scholar] [CrossRef]
- Friesen, P.C.; Peixoto, M.M.; Busch, F.A.; Johnson, D.C.; Sage, R.F. Chilling and frost tolerance in Miscanthus and Saccharum genotypes bred for cool temperate climates. J. Exp. Bot. 2014, 65, 3749–3758. [Google Scholar] [CrossRef] [PubMed]
- de Melo Peixoto, M.; Friesen, P.C.; Sage, R.F. Winter cold-tolerance thresholds in field-grown Miscanthus hybrid rhizomes. J. Exp. Bot. 2015, 66, 4415–4425. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.L.; Allen, D.J.; Barney, J.N. Yield potential and stand establishment for 20 candidate bioenergy feedstocks. Biomass Bioenergy 2015, 73, 145–154. [Google Scholar] [CrossRef]
- Dong, H.; Green, S.V.; Nishiwaki, A.; Yamada, T.; Stewart, J.R.; Deuter, M.; Sacks, E.J. Winter hardiness of Miscanthus (I): Overwintering ability and yield of new Miscanthus x giganteus genotypes in Illinois and Arkansas. GCB Bioenergy 2019, 11, 691–705. [Google Scholar] [CrossRef]
- Bauer, S.; Sorek, H.; Mitchell, V.D.; Ibáñez, A.B.; Wemmer, D.E. Characterization of Miscanthus giganteus lignin isolated by ethanol organosolv process under reflux condition. J. Agric. Food Chem. 2012, 60, 8203–8212. [Google Scholar] [CrossRef] [PubMed]
- Gschwend, F.J.V.; Malaret, F.; Shinde, S.; Brandt-Talbot, A.; Hallett, J.P. Rapid pretreatment of Miscanthus using the low-cost ionic liquid triethylammonium hydrogen sulfate at elevated temperatures. Green Chem. 2018, 20, 3486–3498. [Google Scholar] [CrossRef]
- Brandt, L.; Chen, B.E.; van Dongen, T.; Welton, J.P. Structural changes in lignins isolated using an acidic ionic liquid water mixture. Green Chem. 2015, 17, 5019–5034. [Google Scholar] [CrossRef]
- Meng, X.; Parikh, A.; Seemala, B.; Kumar, R.; Pu, Y.; Christopher, P.; Wyman, C.E.; Cai, C.M.; Ragauskas, A.J. Fractionation of Organosolv Lignin Using Acetone:Water and Properties of the Obtained Fractions. ACS Sustain. Chem. Eng. 2017, 5, 580–587. [Google Scholar] [CrossRef]
- Cheng, K.; Sorek, H.; Zimmermann, H.; Wemmer, D.E.; Pauly, M. Solution-State 2D NMR Spectroscopy of Plant Cell Walls Enabled by a Dimethylsulfoxide-d6/1-Ethyl-3-methylimidazolium Acetate Solvent. Anal. Chem. 2013, 85, 3213–3221. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, C.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Wolfe, J. Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples; NREL Technical Report No. NREL/TP-510-42621; National Renewable Energy Laboratory: Golden, CO, USA, 2008; pp. 1–6. [Google Scholar]
- Pude, R.; Treseler, C.H.; Trettin, R.; Noga, G. Suitability of Miscanthus genotypes for lightweight concrete. Die Bodenkultur 2005, 56, 61–69. [Google Scholar]
- Kim, G.-H.; Um, B.-H. Fractionation and characterization of lignins from Miscanthus via organosolv and soda pulping for biorefinery applications. Int. J. Biol. Macromol. 2020, 158, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Walbrück, K.; Maeting, F.; Witzleben, S.; Stephan, D. Natural Fiber-Stabilized Geopolymer Foams—A Review. Materials 2020, 13, 3198. [Google Scholar] [CrossRef] [PubMed]
- Kraska, T.; Kleinschmidt, B.; Weinand, J.; Pude, R. Cascading use of as growing substrate in soilless Miscanthus cultivation of vegetables (tomatoes, cucumbers) and subsequent direct combustion. Scientia Hort. 2018, 235, 205–213. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef] [PubMed]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Alzagameem, A.; El Khaldi-Hansen, B.; Kamm, B.; Schulze, M. Lignocellulosic Biomass for Energy, Biofuels, Biomaterials, and Chemicals. In Biomass and Green Chemistry, 1st ed.; Vaz, S., Jr., Ed.; Springer International Publishing: Basel, Switzerland, 2018; pp. 95–132. ISBN 978-3-319-66736-2. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Nechifor, M.; Tanasă, F.; Zănoagă, M.; McLoughlin, A.; Stróżyk, M.A.; Culebras, M.; Teacă, C.-A. Valorization of lignin in polymer and composite systems for advanced engineering applications—A review. Int. J. Biol. Macromol. 2019, 131, 828–849. [Google Scholar] [CrossRef] [PubMed]
- Lignin Market—Forecasts from 2018 to 2023; Knowledge Sourcing Intelligence LLP: Noida, India, 2018; 104 p; Lignin Market Analysis by Product (Lignosulphonates, Kraft Lignin, Organosolv Lignin) by Application (Macromolecules, Aromatics), by Region (North America, Europe, APAC, Central & South America, MEA), and Segment Forecasts, 2014–2025; ID: 4240413 Report; Grand View Research: San Francisco, CA, USA, 2017; 110p, Available online: https://www.businesswire.com/news/home/20180427005948/en/1-Billion-Lignin-Market---Forecasts-from-2018-to-2023---ResearchAndMarkets.com (accessed on 25 October 2020).
- Zieglowski, M.; Trosien, S.; Rohrer, J.; Mehlhase, S.; Weber, S.; Bartels, K.; Siegert, G.; Trellenkamp, T.; Albe, K.; Biesalski, M.; et al. Reactivity of Isocyanate-Functionalized Lignins: A Key Factor for the Preparation of Lignin-Based Polyurethanes. Front. Chem. 2019, 7, 562. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.E.; Rumpf, J.; Kusch, P.; Albach, R.; Rehahn, M.; Witzleben, S.; Schulze, M. Utilization of Unmodified Kraft Lignin for the Preparation of Highly Flexible and Transparent Polyurethane Coatings. RSC Adv. 2018, 8, 40765–40777. [Google Scholar] [CrossRef]
- Klein, S.E.; Rumpf, J.; Rehahn, M.; Witzleben, S.; Schulze, M. Biobased Flexible Polyurethane Coatings Prepared from Kraft Lignin: One-Pot Synthesis and Antioxidant Activity. J. Coat. Technol. Res. 2019, 16, 1543–1552. [Google Scholar] [CrossRef]
- Klein, S.E.; Alzagameem, A.; Rumpf, J.; Korte, I.; Kreyenschmidt, J.; Schulze, M. Antimicrobial Activity of Lignin-Derived Polyurethane Coatings Prepared from Unmodified and Demethylated Lignins. Coatings 2019, 9, 494. [Google Scholar] [CrossRef]
- Kalami, S.; Arefmanesh, M.; Master, E.; Nejad, M. Replacing 100 % of phenol in phenolic adhesive formulations with lignin. J. Appl. Polym. Sci. 2017, 45124. [Google Scholar] [CrossRef]
- Alzagameem, A.; El Khaldi-Hansen, B.; Büchner, D.; Larkins, M.; Kamm, B.; Witzleben, S.; Schulze, M. Environmentally Benign Lignin-based Antioxidants isolated from Lignocellulose Feedstock. Molecules 2018, 23, 2664. [Google Scholar] [CrossRef] [PubMed]
- Alzagameem, A.; Klein, S.E.; Bergs, M.; Do, X.T.; Korte, I.; Dohlen, S.; Kreyenschmidt, J.; Kamm, B.; Larkins, M.; Schulze, M. Antimicrobial Activity of Lignin and Lignin-Derived Cellulose and Chitosan Composites Against Selected Pathoge nic and Spoilage Microorganisms. Polymers 2019, 11, 670. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Labidi, J.; Gullón, P. Assessment of Green Approaches for the Synthesis of Physically Crosslinked Lignin Hydrogels. J. Ind. Eng. Chem. 2020, 81, 475–487. [Google Scholar] [CrossRef]
- Witzler, M.; Vermeeren, S.; Kolevatov, R.; Haddad, R.; Gericke, M.; Heinze, T.; Schulze, M. Evaluation of the Release Kinetics from Alginate Beads Coated with Polyelectrolyte Layers for Sustained Drug Delivery. ACS Biomacromol. 2020. submmitted. [Google Scholar]
- Liu, R.; Dai, L.; Xu, C.; Wang, K.; Zheng, C.; Chuanling, S. Lignin-Based Micro- and Nanomaterials and their Composites in Biomedical Applications. ChemSusChem 2020, 13, 4266–4283. [Google Scholar] [CrossRef] [PubMed]
- Rumpf, J.; Do, X.T.; Burger, R.; Monakhova, Y.; Schulze, M. Lignin Types and Properties. In Lignin-Based Materials for Biomedical Applications: Preparation, Characterization and Implementation; Hélder, A.S., Tavares Figueiredo, P.I., Eds.; Elsevier: Amsterdam, The Netherlands. (in print)
- Witzler, M.; Alzagameem, A.; Bergs, M.; El Khaldi-Hansen, B.; Klein, S.E.; Hielscher, D.; Kamm, B.; Kreyenschmidt, J.; Tobiasch, E.; Schulze, M. Lignin-Derived Biomaterials for Drug Release and Tissue Engineering. Molecules 2018, 23, 1885. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Yue, F.; Rencoret, J.; Del Río, J.C.; Boerjan, W.; Lu, F.; Ralph, J. Elucidating Tricin-Lignin Structures: Assigning Correlations in HSQC Spectra of Monocot Lignins. Polymers 2018, 10, 916. [Google Scholar] [CrossRef] [PubMed]
- Boeriu, C.G.; Fitigau, F.I.; Gosselink, R.J.A.; Frissen, A.E.; Stoutjesdijk, J.; Peter, F. Fractionation of five technical lignins by selective extraction in green solvents and characterization of isolated fractions. Ind. Crops Prod. 2014, 62, 481–490. [Google Scholar] [CrossRef]
- Lancefield, C.S.; Constant, S.; de Peinder, P.; Bruijnincx, P.C.A. Linkage Abundance and Molecular Weight Characteristics of Technical Lignins by Attenuated Total Reflection-FTIR Spectroscopy Combined with Multivariate Analysis. ChemSusChem 2019, 12, 1139–1146. [Google Scholar] [CrossRef]
- Li, X.; Wei, Y.; Xu, J.; Xu, N.; He, Y. Quantitative visualization of lignocellulose components in transverse sections of moso bamboo based on FTIR macro- and micro-spectroscopy coupled with chemometrics. Biotechnol. Biofuels 2018, 11, 263–279. [Google Scholar] [CrossRef]
- Uddin, M.N.; Nayeem, J.; Islam, M.S.; Jahan, M.S. Rapid determination method of dissolving pulp properties by spectroscopic data and chemometrics. Biomass Convers. Biorefin. 2019, 9, 585–592. [Google Scholar] [CrossRef]
- Colares, C.J.G.; Pastore, T.C.M.; Coradin, V.T.R.; Camargos, J.A.A.; Moreira, A.C.O.; Rubimb, J.C.; Braga, J.W.B. Exploratory Analysis of the Distribution of Lignin and Cellulose in Woods by Raman Imaging and Chemometrics. J. Braz. Chem. Soc. 2015, 1–10. [Google Scholar] [CrossRef]
- Aguilera-Saeza, L.M.; Arrabal-Camposa, F.M.; Callejon-Ferreb, A.J.; Suarez Medina, M.D.; Fernandez, I. Use of multivariate NMR analysis in the content prediction of hemicellulose, cellulose and lignin in greenhouse crop residues. Phytochemistry 2019, 158, 110–119. [Google Scholar] [CrossRef]
- Mancini, M.; Duca, D.; Toscano, G. Laboratory customized online measurements for the prediction of the key-parameters of biomass quality control. J. Near Infrared Spectr. 2019, 1–11. [Google Scholar] [CrossRef]
- Christou, C.; Agapiou, A.; Kokkinofta, R. Use of FTIR spectroscopy and chemometrics for the classification of carobs origin. J. Adv. Res. 2018, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]


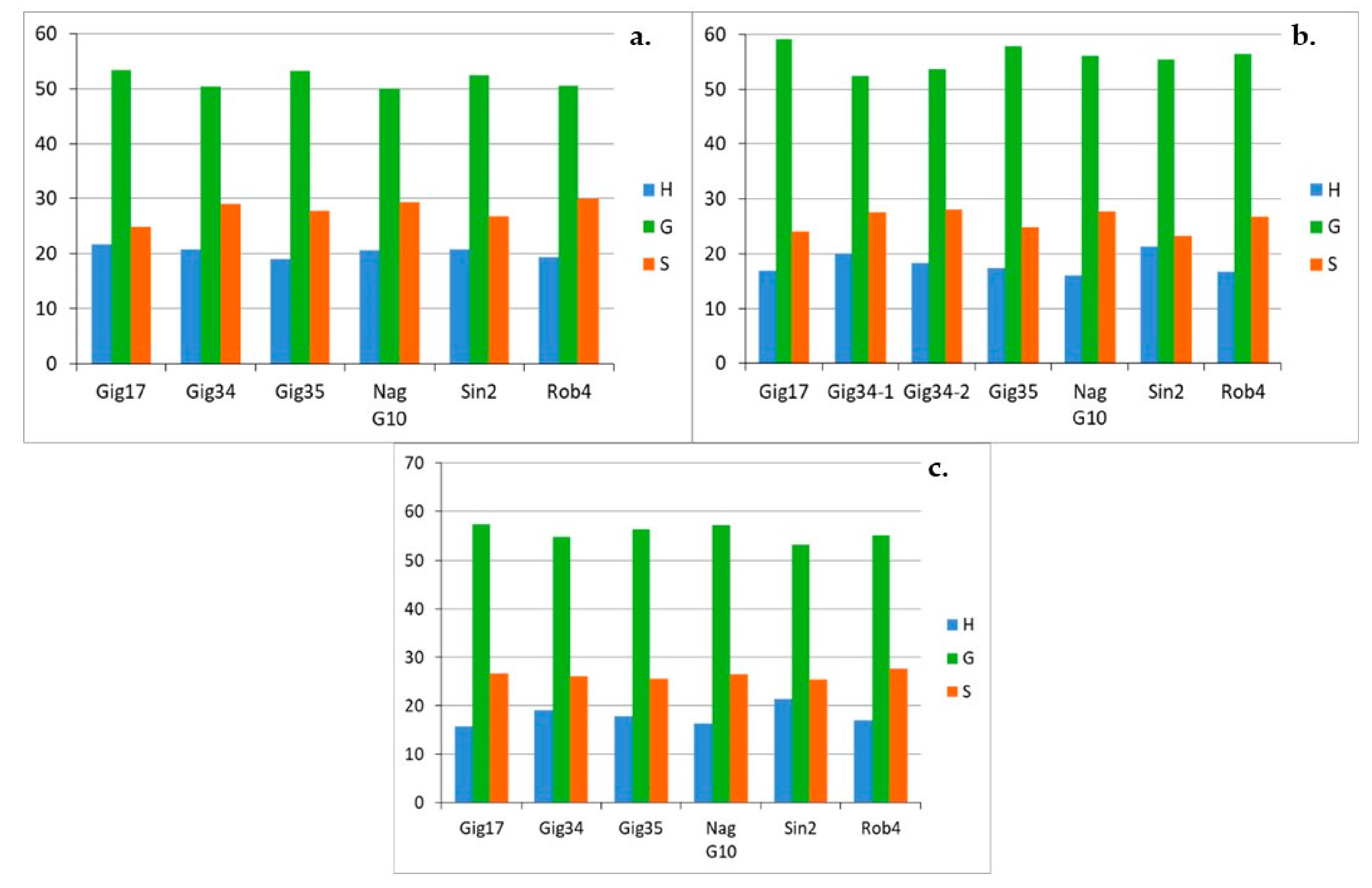
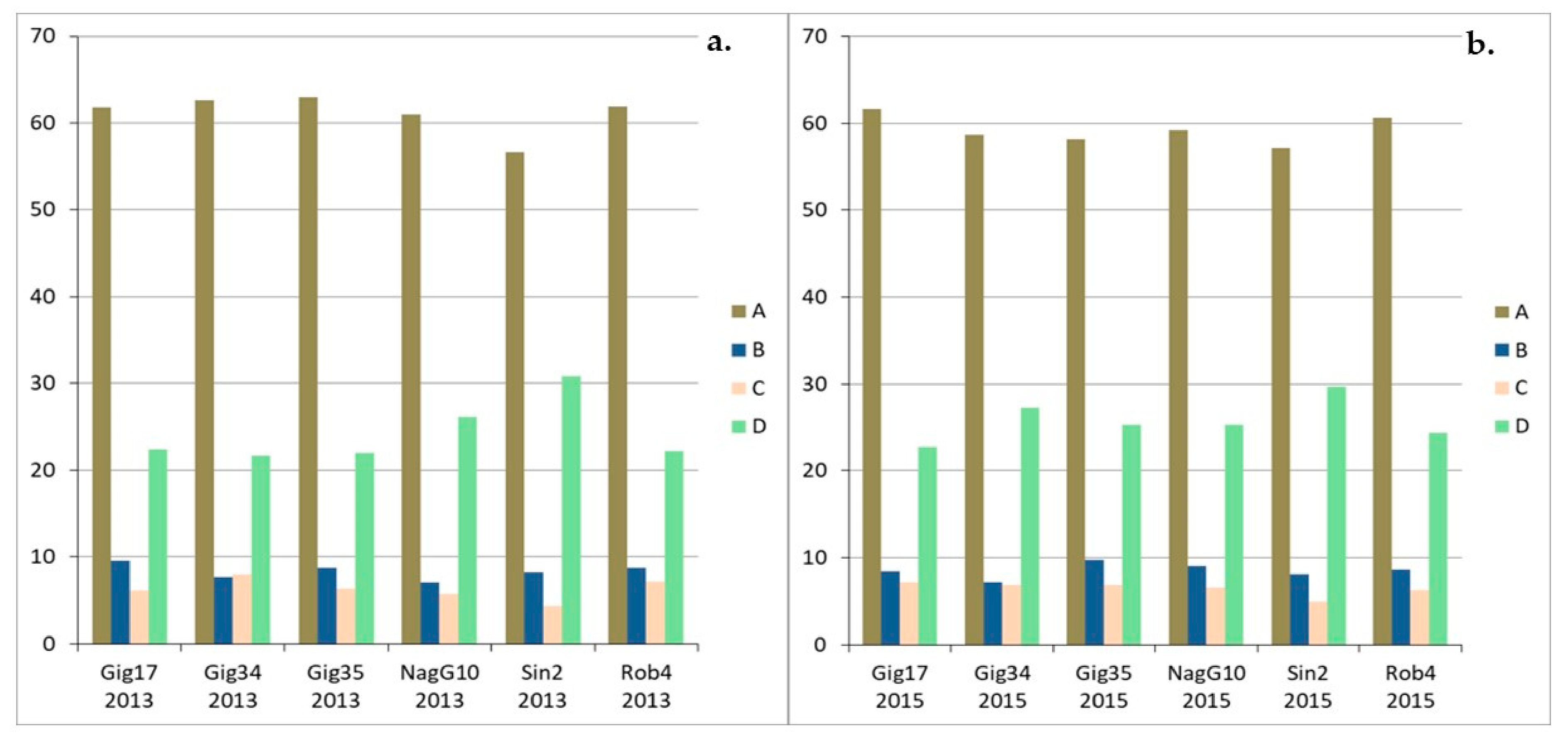
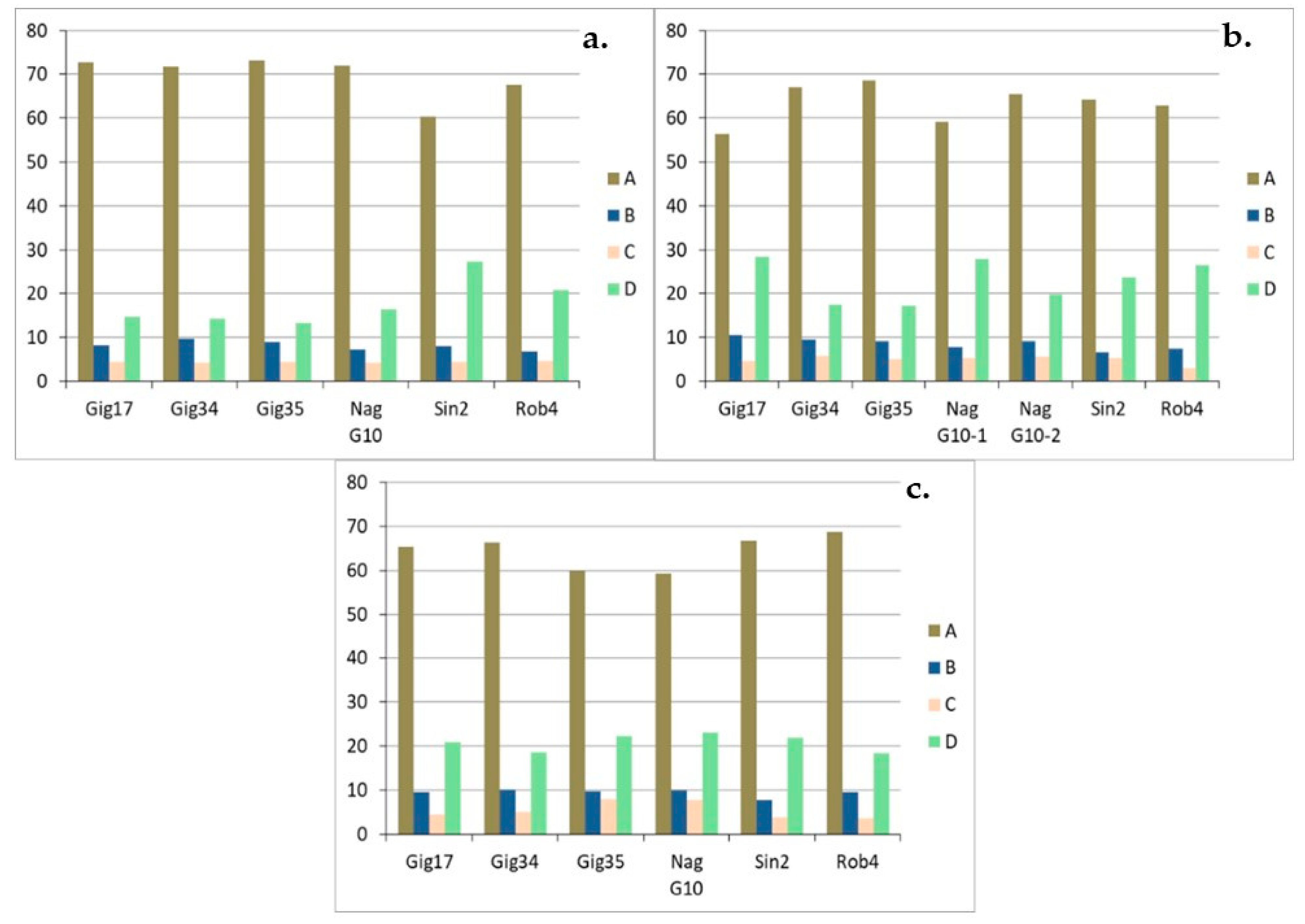
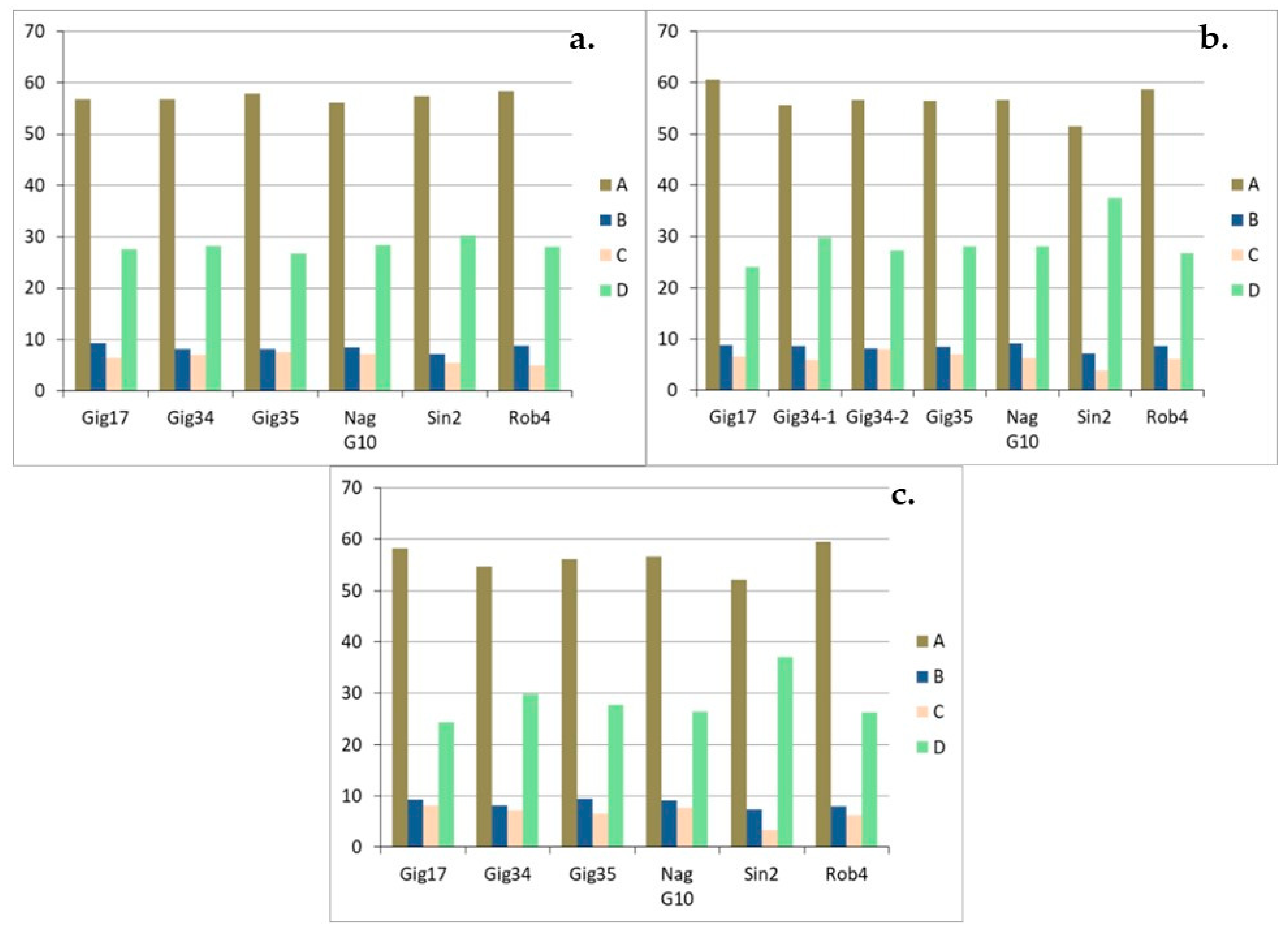
| Genotyp | Gig17 | Gig34 | Gig35 | NagG10 | Sin2 | Rob4 |
|---|---|---|---|---|---|---|
| AIL (%) | 20.6 ± 0.5 | 21.1 ± 0.3 | 19.4 ± 0.9 | 17.6 ± 0.4 | 18.6 ± 0.0 | 18.8 ± 0.1 |
| ASL (%) | 5.1 ± 0.3 | 4.1 ± 0.0 | 5.1 ± 0.0 | 4.9 ± 0.0 | 6.0 ± 0.2 | 5.7 ± 0.0 |
| AIR (%) | 21.8 ± 0.5 | 22.1 ± 0.4 | 20.2 ± 1.2 | 19.2 ± 0.2 | 19.5 ± 0.0 | 19.8 ± 0.1 |
| Total lignin (%) | 25.5 ± 0.5 | 25.3 ± 0.5 | 24.6 ± 0.8 | 22.5 ± 0.4 | 24.6 ± 0.2 | 24.5 ± 0.1 |
| Ash (%) | 1.2 ± 0.1 | 1.1 ± 0.2 | 0.8 ± 0.2 | 1.6 ± 0.2 | 0.9 ± 0.0 | 1.0 ± 0.1 |
| Glucan (%) | 44.8 ± 1.5 | 45.0 ± 2.3 | 48.5 ± 1.1 | 46.3 ± 2.5 | 43.6 ± 0.3 | 41.54 ± 0.2 |
| Xylan (%) | 28.4 ± 1.8 | 29.5 ± 0.6 | 29.4 ± 0.6 | 29.6 ± 1.1 | 28.2 ± 0.4 | 26.5 ± 0.5 |
| Galactan (%) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.4 ± 0.6 | 1.1 ± 0.0 |
| Arabinan (%) | 3.1 ± 0.0 | 4.1 ± 1.6 | 3.6 ± 0.3 | 4.7 ± 0.1 | 4.4 ± 0.0 | 3.6 ± 0,0 |
| Mannan (%) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 2.3 ± 0.0 | 2.3 ± 0,0 |
| Dry matter (%) | 92.5 | 91.2 | 91.2 | 91.4 | 91.9 | 92.6 |
| Total ash (%) | 4.5 | 6.8 | 5.2 | 6.4 | 5.4 | 5.8 |
| Genotype | Gig17 | Gig34 | Gig35 | NagG10 | Sin2 | Rob4 |
|---|---|---|---|---|---|---|
| AIL (%) | 21.2 ± 0.0 | 21.0 ± 0.4 | 22.0 ± 0.1 | 21.3 ± 0.1 | 19.2 ± 0.1 | 19.2 ± 0.1 |
| ASL (%) | 4.7 ± 0.3 | 4.3 ± 0.0 | 5.0 ± 0.1 | 4.5 ± 0.1 | 5.7 ± 0.0 | 5.3 ± 0.2 |
| AIR (%) | 22.4 ± 0.2 | 22.3 ± 0.6 | 22.5 ± 0.1 | 21.8 ± 0.1 | 19.8 ± 0.2 | 19.8 ± 0.1 |
| Total lignin (%) | 26.0 ± 0.4 | 25.2 ± 0.4 | 27.0 ± 0.2 | 25.8 ± 0.1 | 24.9 ± 0.1 | 24.5 ± 0.1 |
| Ash (%) | 1.3 ± 0.1 | 1.0 ± 0.0 | 0.6 ± 0.0 | 0.5 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 |
| Glucan (%) | 50 ± 0.6 | 50.5 ± 0.9 | 49.6 ± 0.4 | 47.1 ± 1.4 | 48.2 ± 3.0 | 45.7 ± 0.3 |
| Xylan (%) | 27.4 ± 2.8 | 26.2 ± 0.5 | 23.6 ± 0.0 | 23.9 ± 0.5 | 28.7 ± 1.7 | 25.6 ± 0.3 |
| Galactan (%) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.4 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Arabinan (%) | 1.9 ± 0.5 | 2.0 ± 0.3 | 1.8 ± 0.4 | 1.8 ± 1.6 | 2.9 ± 0.2 | 2.1 ± 0.2 |
| Mannan (%) | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.1 ± 1.6 | 0.0 ± 0.0 | 2.3 ± 0.1 | 2.3 ± 0.0 |
| Dry matter (%) | 92.2 | 92.2 | 92.1 | 93.0 | 92.8 | 92.7 |
| Total ash (%) | 2.5 | 3.1 | 2.4 | 1.8 | 3.4 | 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergs, M.; Monakhova, Y.; Diehl, B.W.; Konow, C.; Völkering, G.; Pude, R.; Schulze, M. Lignins Isolated via Catalyst-Free Organosolv Pulping from Miscanthus x giganteus, M. sinensis, M. robustus and M. nagara: A Comparative Study. Molecules 2021, 26, 842. https://doi.org/10.3390/molecules26040842
Bergs M, Monakhova Y, Diehl BW, Konow C, Völkering G, Pude R, Schulze M. Lignins Isolated via Catalyst-Free Organosolv Pulping from Miscanthus x giganteus, M. sinensis, M. robustus and M. nagara: A Comparative Study. Molecules. 2021; 26(4):842. https://doi.org/10.3390/molecules26040842
Chicago/Turabian StyleBergs, Michel, Yulia Monakhova, Bernd W. Diehl, Christopher Konow, Georg Völkering, Ralf Pude, and Margit Schulze. 2021. "Lignins Isolated via Catalyst-Free Organosolv Pulping from Miscanthus x giganteus, M. sinensis, M. robustus and M. nagara: A Comparative Study" Molecules 26, no. 4: 842. https://doi.org/10.3390/molecules26040842
APA StyleBergs, M., Monakhova, Y., Diehl, B. W., Konow, C., Völkering, G., Pude, R., & Schulze, M. (2021). Lignins Isolated via Catalyst-Free Organosolv Pulping from Miscanthus x giganteus, M. sinensis, M. robustus and M. nagara: A Comparative Study. Molecules, 26(4), 842. https://doi.org/10.3390/molecules26040842








