New Polyenes from the Marine-Derived Fungus Talaromyces cyanescens with Anti-Neuroinflammatory and Cytotoxic Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Elucidation
2.2. Bioactivities
3. Experimental Methods
3.1. General Experimental Procedures
3.2. Fungal Material and Fermentation
3.3. Extraction and Isolation of Metabolites
3.3.1. Talacyanol A (1)
3.3.2. Talacyanol B (2)
3.3.3. Talacyanol C (3)
3.4. MTPA Esterification of Compounds 1–3
3.4.1. Bis-S-MTPA Ester (1a) of Talacyanol A (1)
3.4.2. Bis-R-MTPA Ester (1b) of Talacyanol A (1)
3.4.3. Bis-S-MTPA Ester (2a) of Talacyanol B (2)
3.4.4. Bis-R-MTPA Ester (2b) of Talacyanol B (2)
3.4.5. Tri-S-MTPA Ester (3a) of Talacyanol C (3)
3.4.6. Tri-R-MTPA Ester (3b) of Talacyanol C (3)
3.5. Cytotoxicity Test by SRB Assay and Anti-Neuroinflammatory Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Klemm, E.J.; Wong, V.K.; Dougan, G. Emergence of dominant multidrug-resistant bacterial clades: Lessons from history and whole-genome sequencing. Proc. Natl. Acad. Sci. USA 2018, 115, 12872–12877. [Google Scholar] [CrossRef] [PubMed]
- Mathers, C.; Stevens, G.; Hogan, D.; Mahanani, W.R.; Ho, J. Global and Regional Causes of Death: Patterns and Trends, 2000–2015. In Disease Control Priorities: Improving Health and Reducing Poverty, 3rd ed.; Jamison, D.T., Gelband, H., Horton, S., Jha, P., Laxminarayan, R., Mock, C.N., Nugent, R., Eds.; The World Bank: Washington, DC, USA, 2017; Volume 9, pp. 69–104. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Malve, H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioallied. Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef]
- Rateb, M.E.; Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290–344. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.M.; Li, J.; Jiang, C.X.; Shi, Y.P.; Di, D.L.; Crews, P.; Wu, Q.X. The bioactive secondary metabolites from Talaromyces species. Nat. Prod. Bioprospect. 2016, 6, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; He, L.; Chen, D.; Cai, R.; Long, Y.; Lu, Y.; She, J. Talaramide A, an unusual alkaloid from the mangrove endophytic fungus Talaromyces sp. (HZ-YX1) as an inhibitor of mycobacterial PknG. New J. Chem. 2017, 41, 4273–4276. [Google Scholar] [CrossRef]
- Meng, L.H.; Li, X.M.; Zhang, F.Z.; Wang, Y.N.; Wang, B.G. Talascortenes A–G, highly oxygenated diterpenoid acids from the sea-anemone-derived endozoic fungus Talaromyces scorteus AS-242. J. Nat. Prod. 2020, 83, 2528–2536. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Ohlendorf, B.; Oesker, V.; Wiese, J.; Malien, S.; Schmaljohann, R.; Imhoff, J.F. Acetylcholinesterase inhibitors from a marine fungus Talaromyces sp. Strain LF458. Mar. Biotechnol. 2015, 17, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Küppers, L.; Ebrahim, W.; El-Neketi, M.; Özkaya, F.C.; Mándi, A.; Kurtán, T.; Orfali, R.S.; Müller, W.E.G.; Hartmann, R.; Lin, W.; et al. Lactones from the sponge-derived fungus Talaromyces rugulosus. Mar. Drugs 2017, 15, 359. [Google Scholar] [CrossRef] [PubMed]
- Noinart, J.; Buttachon, S.; Dethoup, T.; Gales, L.; Pereira, J.A.; Urbatzka, R.; Freitas, S.; Lee, M.; Silva, A.M.S.; Pinto, M.M.M.; et al. A new ergosterol analog, a new bis-anthraquinone and anti-obesity activity of anthraquinones from the marine sponge-associated fungus Talaromyces stipitatus KUFA 0207. Mar. Drugs 2017, 15, 139. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, H.; Shao, C.; Huang, H.; Jiang, J.; Zhu, X.; Liu, Y.; Liu, L.; Lu, Y.; Li, M.; et al. Cytotoxic norsesquiterpene peroxides from the endophytic fungus Talaromyces flavus isolated from the mangrove plant Sonneratia apetala. J. Nat. Prod. 2011, 74, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Li, X.M.; Li, X.; Wang, C.Y.; Liu, H.; Kassack, M.U.; Meng, L.H.; Wang, B.G. Antioxidant hydroanthraquinones from the marine algal-derived endophytic fungus Talaromyces islandicus EN-501. J. Nat. Prod. 2017, 80, 162–168. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 9 December 2020).
- Ardura-Fabregat, A.; Boddeke, E.W.G.M.; Boza-Serrano, A.; Brioschi, S.; Castro-Gomez, S.; Ceyzeriat, K.; Dansokho, C.; Dierkes, T.; Gelders, G.; Heneka, M.T.; et al. Targeting Neuroinflammation to Treat Alzheimer’s Disease. CNS Drugs 2017, 31, 1057–1082. [Google Scholar] [CrossRef] [PubMed]
- Freire, F.; Seco, J.M.; Quiñoá, E.; Riguera, R. Determining the absolute stereochemistry of secondary/secondary diols by 1H NMR: Basis and applications. J. Org. Chem. 2005, 70, 3778–3790. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Kim, H.; Shin, Y.; Kim, B.Y.; Lee, S.K.; Oh, K.-B.; Shin, J.; Oh, D.-C. Separacenes A–D, novel polyene polyols from the marine Actinomycete, Streptomyces sp. Mar. Drugs 2013, 11, 2882–2893. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.T.; Shi, X.; Xian, P.J.; Feng, Z.; Yang, J.; Yang, X.L. A new fusicoccane diterpene and a new polyene from the plant endophytic fungus Talaromyces pinophilus and their antimicrobial activities. Nat. Prod. Res. 2019, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Stodola, F.H.; Cabot, C.; Benjamin, C.R. Structure of ramulosin, a metabolic product of the fungus Pestalotia ramulosa. Biochem. J. 1964, 93, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Stierle, D.B.; Stierle, A.A.; Kunz, A. Dihydroramulosin from Botrytis sp. J. Nat. Prod. 1998, 61, 1277–1278. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xia, G.; Chen, S.; Liu, Y.; Li, H.; She, Z. Eurothiocin A and B, sulfur-containing benzofurans from a soft coral-derived fungus Eurotium rubrum SH-823. Mar. Drugs 2014, 12, 3669–3680. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.-E.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef]
- Smyrniotopoulos, V.; Firsova, D.; Fearnhead, H.; Grauso, L.; Mangoni, A.; Tasdemir, D. Density Functional Theory (DFT)-Aided Structure Elucidation of Linear Diterpenes from the Irish Brown Seaweed Bifurcaria bifurcata. Mar. Drugs 2021, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.W.; Won, Y.J.; Hong, S.; Kong, H.J.; Lee, E.S. Prediction of Cancer Incidence and Mortality in Korea. Cancer Res. Treat. 2020, 52, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.K.; Jo, S.H.; Choi, D.K.; Trinh, P.T.H.; Lee, H.S.; Cao, V.A.; Van, T.T.T.; Shin, H.J. Anti-neuroinflammatory agent, restricticin B, from the marine-derived fungus Penicillium janthinellum and its inhibitory activity on the no production in bv-2 microglia cells. Mar. Drugs 2020, 18, 465. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-K.; Lee, H.-S.; Kang, J.S.; Shin, H.J. Dokdolipids A–C, hydroxylated rhamnolipids from the marine-derived Actinomycete Actinoalloteichus hymeniacidonis. Mar. Drugs 2019, 17, 237. [Google Scholar] [CrossRef] [PubMed]
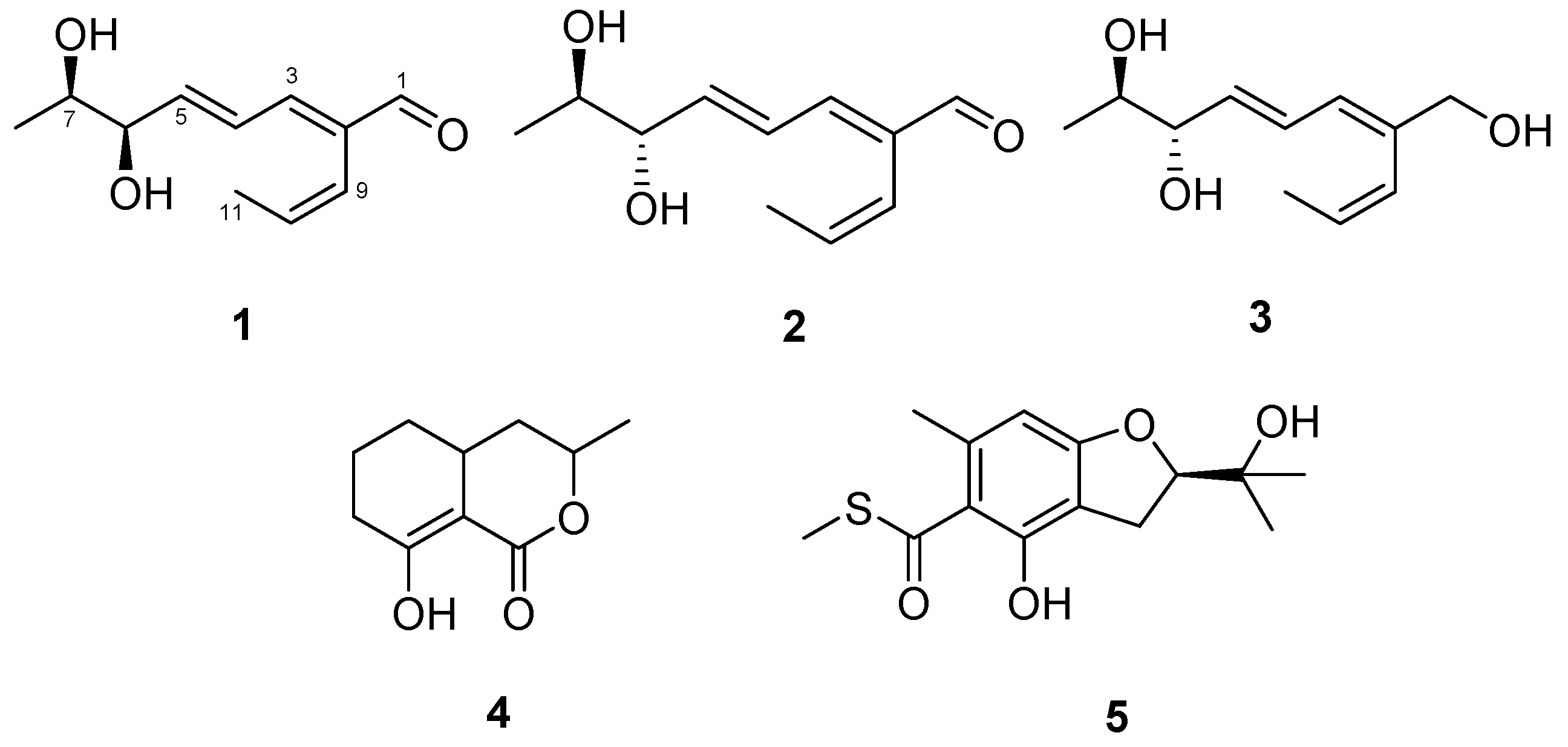



| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 9.46, s | 196.0 | 9.46, s | 196.0 | 4.05, s | 66.4 |
| 2 | 139.1 | 139.1 | 139.8 | |||
| 3 | 7.13 (d, 11.2) | 150.2 | 7.13 (d, 11.2) | 150.3 | 6.20 (d, 11.0) | 126.6 |
| 4 | 6.66 (dd, 11.3, 15.3) | 128.8 | 6.63 (dd, 11.2, 15.3) | 128.6 | 6.33 (dd, 11.0, 15.3) | 130.7 |
| 5 | 6.44 (dd, 5.6, 15.3) | 146.0 | 6.49 (dd, 5.6, 15.3) | 146.3 | 5.80 (dd, 7.0, 15.4) | 134.1 |
| 6 | 4.09 (t, 5.5) | 77.0 | 4.07 (t, 5.4) | 77.0 | 3.93 (t, 5.5) | 77.7 |
| 7 | 3.72, m | 71.4 | 3.71, m | 71.5 | 3.67, m | 71.7 |
| 8 | 1.14 (d, 6.4) | 18.7 | 1.17 (dd, 1.0, 6.4) | 18.9 | 1.13 (d, 6.4) | 18.6 |
| 9 | 5.98 (d, 11.8) | 122.0 | 5.98 (d, 11.8) | 122.0 | 5.90 (d, 11.5) | 127.1 |
| 10 | 5.92, m | 132.5 | 5.92, m | 132.4 | 5.75, m | 129.9 |
| 11 | 1.54 (d, 6.6) | 15.7 | 1.54 (d, 6.6) | 15.7 | 1.61 (dd, 1.8, 6.9) | 15.4 |
| Cell Line | GI50, μM | ADR a |
|---|---|---|
| HCT-15 | 64.3 | <0.5 |
| NUGC-3 | 62.2 | <0.5 |
| NCI-H23 | 70.9 | <0.5 |
| ACHN | 44.4 | <0.5 |
| PC-3 | 54.1 | <0.5 |
| MDA-MB-231 | 91.8 | <0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, H.J.; Anh, C.V.; Cho, D.-Y.; Choi, D.-K.; Kang, J.S.; Trinh, P.T.H.; Choi, B.-K.; Lee, H.-S. New Polyenes from the Marine-Derived Fungus Talaromyces cyanescens with Anti-Neuroinflammatory and Cytotoxic Activities. Molecules 2021, 26, 836. https://doi.org/10.3390/molecules26040836
Shin HJ, Anh CV, Cho D-Y, Choi D-K, Kang JS, Trinh PTH, Choi B-K, Lee H-S. New Polyenes from the Marine-Derived Fungus Talaromyces cyanescens with Anti-Neuroinflammatory and Cytotoxic Activities. Molecules. 2021; 26(4):836. https://doi.org/10.3390/molecules26040836
Chicago/Turabian StyleShin, Hee Jae, Cao Van Anh, Duk-Yeon Cho, Dong-Kug Choi, Jong Soon Kang, Phan Thi Hoai Trinh, Byeoung-Kyu Choi, and Hwa-Sun Lee. 2021. "New Polyenes from the Marine-Derived Fungus Talaromyces cyanescens with Anti-Neuroinflammatory and Cytotoxic Activities" Molecules 26, no. 4: 836. https://doi.org/10.3390/molecules26040836
APA StyleShin, H. J., Anh, C. V., Cho, D.-Y., Choi, D.-K., Kang, J. S., Trinh, P. T. H., Choi, B.-K., & Lee, H.-S. (2021). New Polyenes from the Marine-Derived Fungus Talaromyces cyanescens with Anti-Neuroinflammatory and Cytotoxic Activities. Molecules, 26(4), 836. https://doi.org/10.3390/molecules26040836







