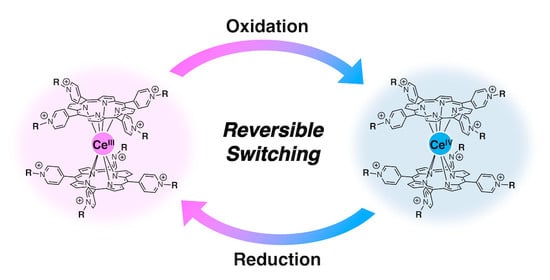
Synthesis of Bis{meso-Tetrakis(4-N-alkylpyridiniumyl)porphyrinato}cerium and Its Redox Switching Behavior †
Abstract
1. Introduction
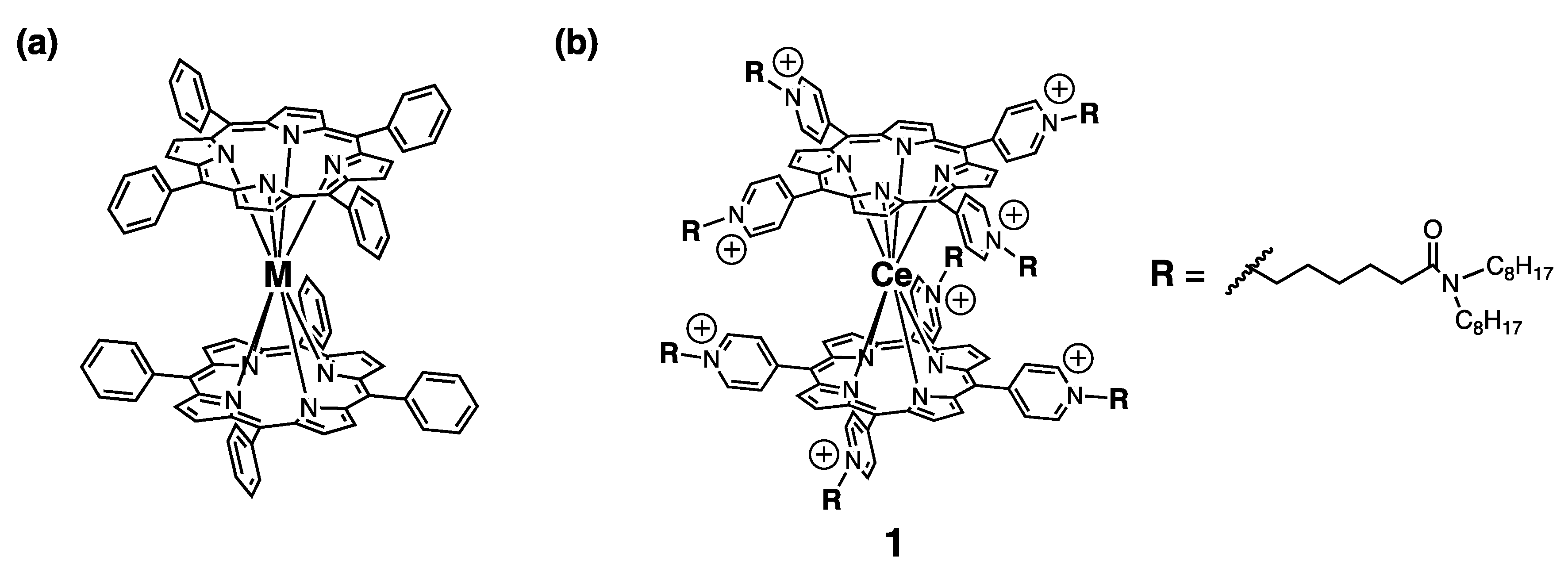
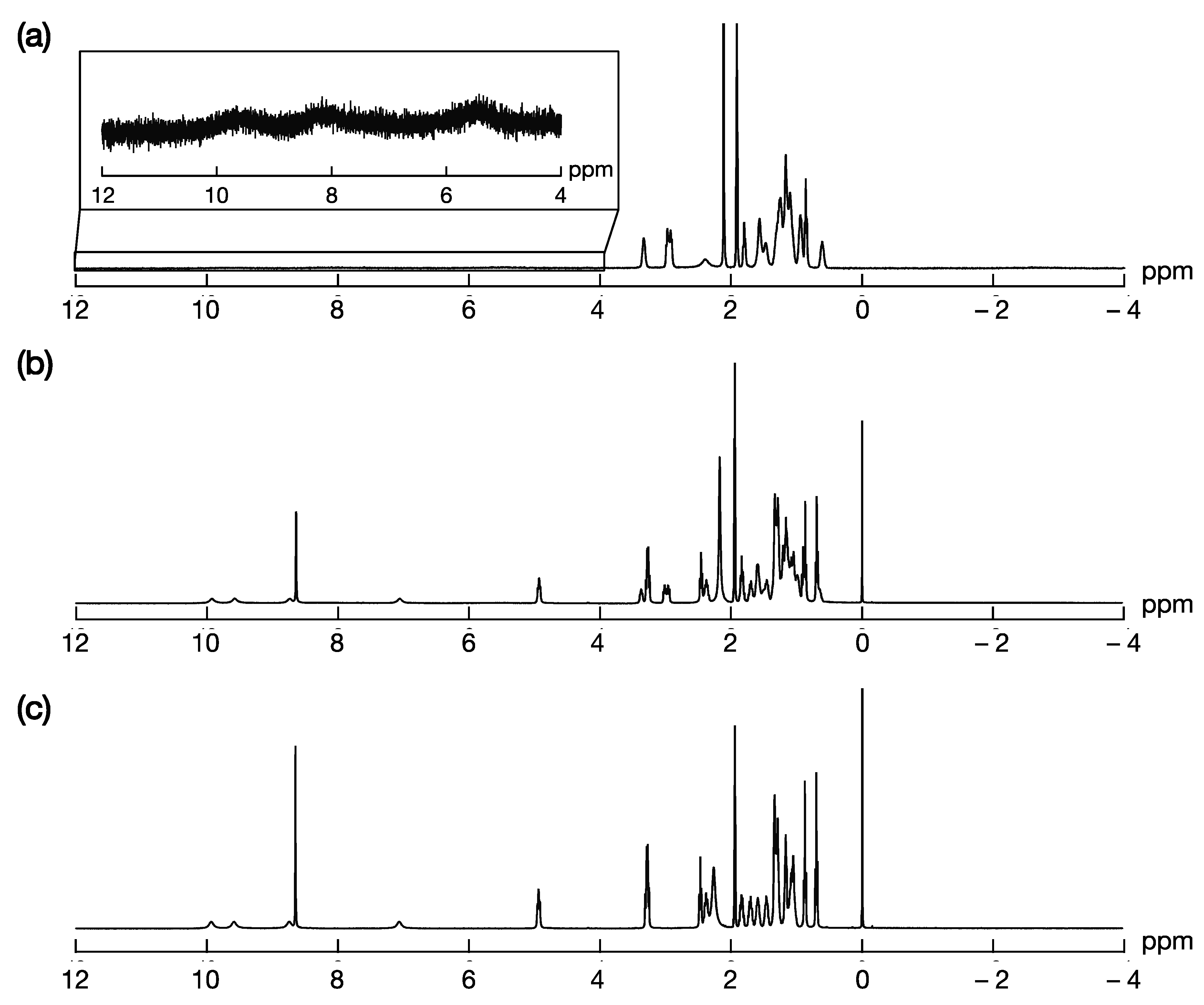
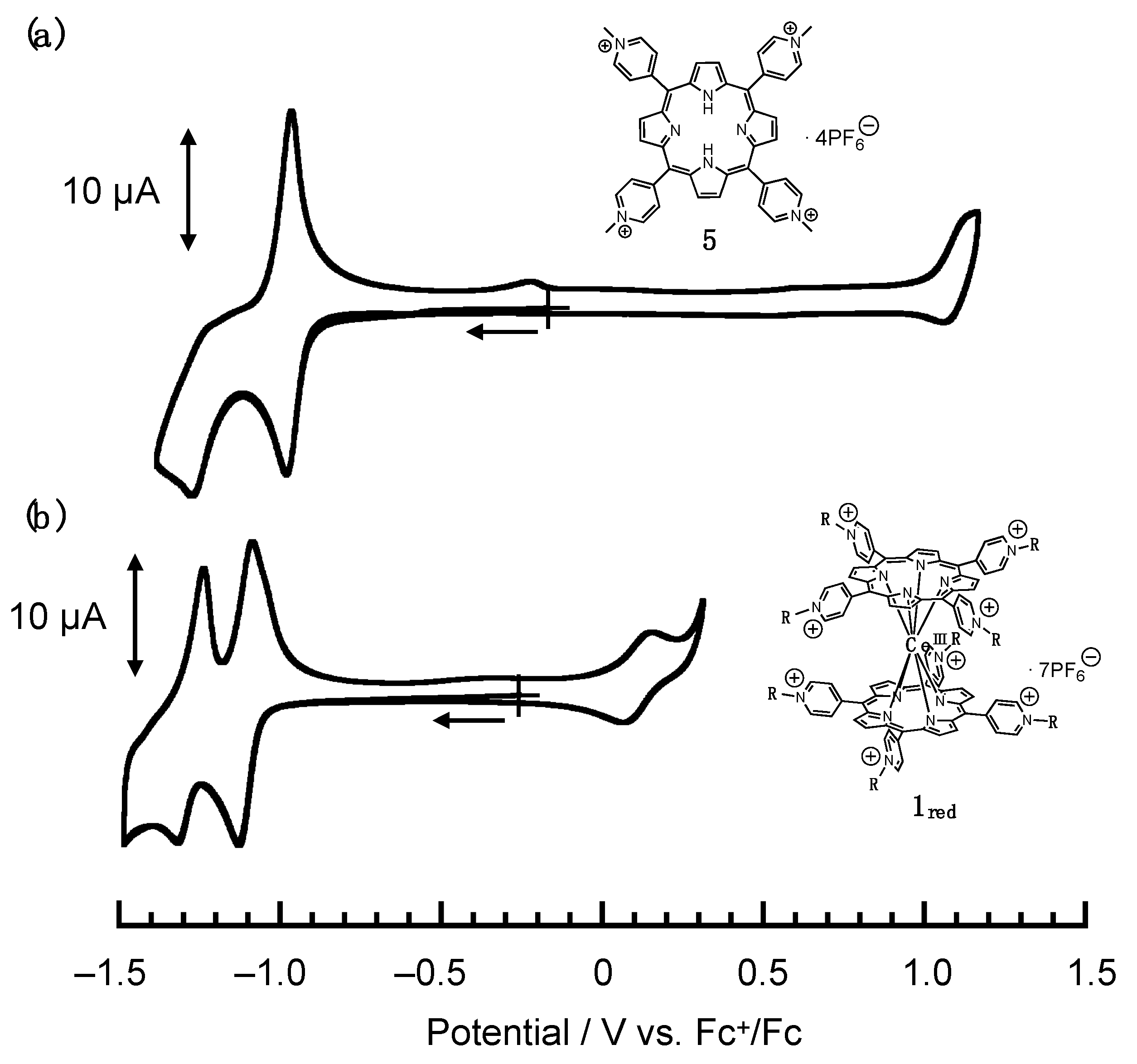
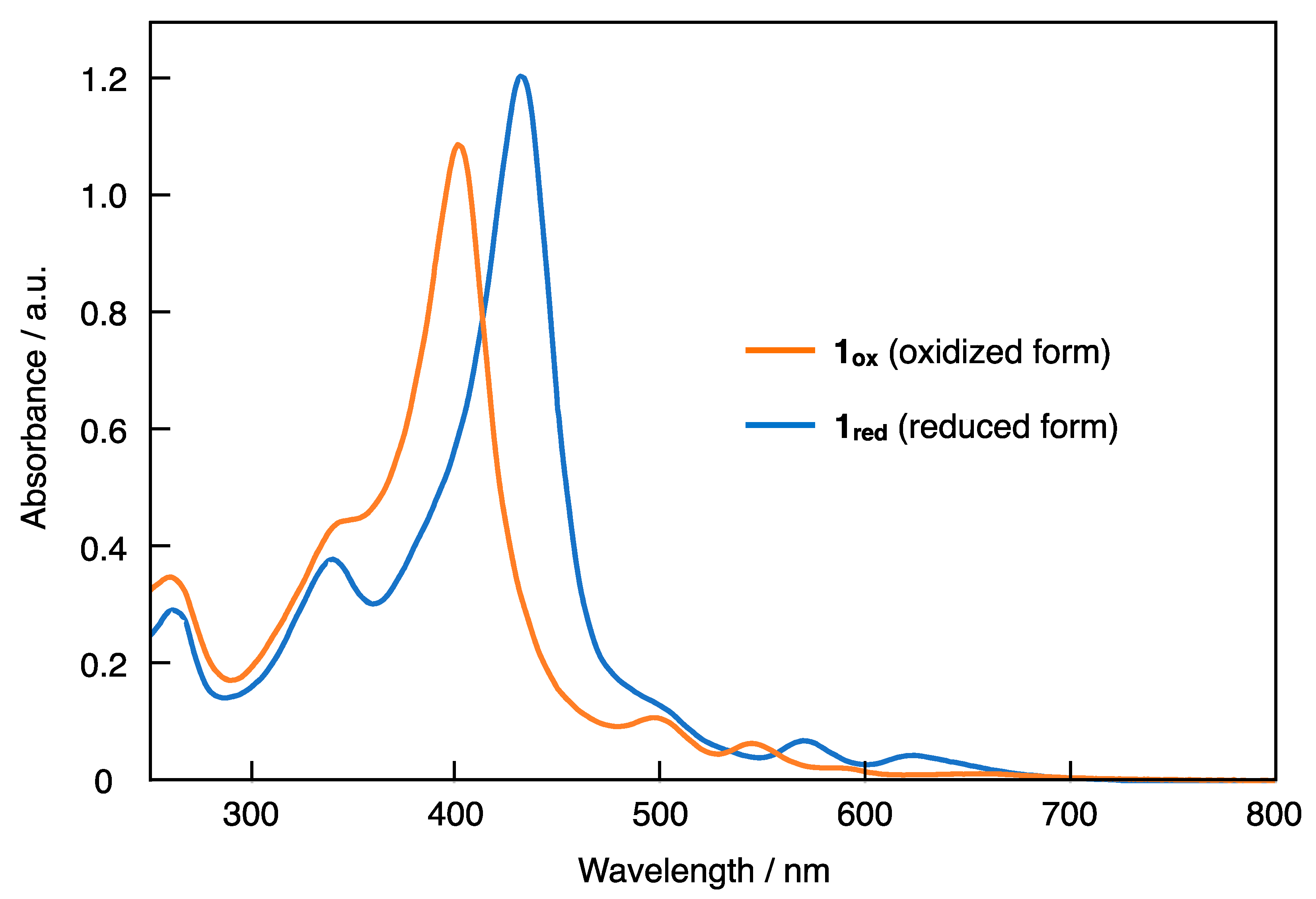
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis
- N,N-Di-n-octyl-6-bromohexanamide
- N,N-Di-n-octyl-6-iodohexanamide (4)
- 1red
- 1ox
- Reduction of 1ox into 1red by treatment with aqueous KI
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kirin, I.S.; Moskalev, P.N.; Makashev, Y.A. Formation of phthalocyanines of rare-earth elements. Zhurnal Neorganicheskoi Khimii 1965, 10, 1951–1953. [Google Scholar]
- Kirin, I.S.; Moskalev, P.N.; Makashev, Y.A. New complex compounds of phthalocyanine with rare earth elements. Zhurnal Neorganicheskoi Khimii 1967, 12, 707–712. [Google Scholar]
- Chabach, D.; Tahiri, M.; Cian, A.D.; Fischer, J.; Weiss, R.; El Malouli Bibout, E. Tervalent-Metal Porphyrin-Phthalocyanine Heteroleptic Sandwich-Type Complexes. Synthesis, Structure, and Spectroscopic Characterization of Their Neutral, Singly-Oxidized, and Singly-Reduced States. J. Am. Chem. Soc. 1995, 117, 8548–8556. [Google Scholar] [CrossRef]
- Jiang, J.; Bian, Y.; Furuya, F.; Liu, W.; Choi, M.T.M.; Kobayashi, N.; Li, H.-W.; Yang, Q.; Mak, T.C.W.; Ng, D.K.P. Synthesis, Structure, Spectroscopic Properties, and Electrochemistry of Rare Earth Sandwich Compounds with Mixed 2,3-Naphthalocyaninato and Octaethylporphyrinato Ligands. Chem. Eur. J. 2001, 7, 5059–5069. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, W.; Cheng, K.-L.; Poon, K.-W.; Ng, D.K.P. Heteroleptic Rare Earth Double-Decker Complexes with Porphyrinato and 2,3-Naphthalocyaninato Ligands − Preparation, Spectroscopic Characterization, and Electrochemical Studies. Eur. J. Inorg. Chem. 2001, 413–417. [Google Scholar] [CrossRef]
- Kadish, K.M.; Nakanishi, T.; Gürek, A.; Ahsen, V.; Yilmaz, I. Electrochemistry of a Double-Decker Lutetium(III) Phthalocyanine in Aqueous Media. The First Evidence for Five Reductions. J. Phys. Chem. B 2001, 105, 9817–9821. [Google Scholar] [CrossRef]
- Bian, Y.; Jiang, J.; Tao, Y.; Choi, M.T.M.; Li, R.; Ng, A.C.H.; Zhu, P.; Pan, N.; Sun, X.; Arnold, D.P.; et al. Tuning the Valence of the Cerium Center in (Na)phthalocyaninato and Porphyrinato Cerium Double-Deckers by Changing the Nature of the Tetrapyrrole Ligands. J. Am. Chem. Soc. 2003, 125, 12257–12267. [Google Scholar] [CrossRef]
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Kishihara, S.; Kaizu, Y. Lanthanide Double-Decker Complexes Functioning as Magnets at the Single-Molecular Level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar] [CrossRef]
- Gonidec, M.; Davies, E.S.; McMaster, J.; Amabilino, D.B.; Veciana, J. Probing the Magnetic Properties of Three Interconvertible Redox States of a Single-Molecule Magnet with Magnetic Circular Dichroism Spectroscopy. J. Am. Chem. Soc. 2010, 132, 1756–1757. [Google Scholar] [CrossRef]
- Komeda, T.; Isshiki, H.; Liu, J.; Zhang, Y.-F.; Lorente, N.; Katoh, K.; Breedlove, B.K.; Yamashita, M. Observation and electric current control of a local spin in a single-molecule magnet. Nat. Commun. 2011, 2, 217. [Google Scholar] [CrossRef]
- Tanaka, D.; Inose, T.; Tanaka, H.; Lee, S.; Ishikawa, N.; Ogawa, T. Proton-induced switching of the single molecule magnetic properties of a porphyrin based TbIII double-decker complex. Chem. Commun. 2012, 48, 7796–7798. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, K.; Tao, J.; Jiang, J. Twist angle perturbation on mixed (phthalocyaninato)(porphyrinato) dysprosium(iii) double-decker SMMs. Chem. Commun. 2012, 48, 2973–2975. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, D.; Sumitani, N.; Inose, H.; Tanaka, H.; Ishikawa, N.; Ogawa, T. Effect of Protonation on the Single-molecule-magnet Behavior of a Mixed (Phthalocyaninato)(porphyrinato)terbium Double-decker Complex. Chem. Lett. 2015, 44, 668–670. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, F.; Chen, X.; Dong, B.; Wang, K.; Jiang, S.; Wang, C.; Chen, X.; Qi, D.; Sun, H.; et al. A New Bis(phthalocyaninato) Terbium Single-Ion Magnet with an Overall Excellent Magnetic Performance. Inorg. Chem. 2017, 56, 13889–13896. [Google Scholar] [CrossRef]
- Horii, Y.; Horie, Y.; Katoh, K.; Breedlove, B.K.; Yamashita, M. Changing Single-Molecule Magnet Properties of a Windmill-Like Distorted Terbium(III) α-Butoxy-Substituted Phthalocyaninato Double-Decker Complex by Protonation/Deprotonation. Inorg. Chem. 2018, 57, 565–574. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, F.; Chen, X.; Zhang, Y.; Wang, H.; Wang, K.; Qi, D.; Sun, H.-L.; Jiang, J. Bis[1,8,15,22-tetrakis(3-pentyloxy)phthalocyaninato]terbium Double-Decker Single-Ion Magnets. Inorg. Chem. 2019, 58, 2422–2429. [Google Scholar] [CrossRef]
- Katoh, K.; Yoshida, Y.; Yamashita, M.; Miyasaka, H.; Breedlove, B.K.; Kajiwara, T.; Takaishi, S.; Ishikawa, N.; Isshiki, H.; Zhang, Y.F.; et al. Direct Observation of Lanthanide(III)-Phthalocyanine Molecules on Au(111) by Using Scanning Tunneling Microscopy and Scanning Tunneling Spectroscopy and Thin-Film Field-Effect Transistor Properties of Tb(III)- and Dy(III)-Phthalocyanine Molecules. J. Am. Chem. Soc. 2009, 131, 9967–9976. [Google Scholar] [CrossRef]
- Katoh, K.; Isshiki, H.; Komeda, T.; Yamashita, M. Molecular Spintronics Based on Single-Molecule Magnets Composed of Multiple-Decker Phthalocyaninato Terbium(III) Complex. Chem. Asian J. 2012, 7, 1154–1169. [Google Scholar] [CrossRef]
- Lu, G.; Kong, X.; Sun, J.; Zhang, L.; Chen, Y.; Jiang, J. Solution-processed single crystal microsheets of a novel dimeric phthalocyanine-involved triple-decker for high-performance ambipolar organic field effect transistors. Chem. Commun. 2017, 53, 12754–12757. [Google Scholar] [CrossRef]
- Tashiro, K.; Konishi, K.; Aida, T. Enantiomeric Resolution of Chiral Metallobis(porphyrin)s: Studies on Rotatability of Electronically Coupled Porphyrin Ligands. Angew. Chem. Int. Ed. Engl. 1997, 36, 856. [Google Scholar] [CrossRef]
- Ogi, S.; Ikeda, T.; Wakabayashi, R.; Shinkai, S.; Takeuchi, M. A Bevel-Gear-Shaped Rotor Bearing a Double-Decker Porphyrin Complex. Chem. Eur. J. 2010, 16, 8285–8290. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, J.; Komatsu, Y.; Kobayashi, D.; Asakawa, M.; Miyake, K. Rotational Libration of a Double-Decker Porphyrin Visualized. J. Am. Chem. Soc. 2010, 132, 6870–6871. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Ikeda, T.; Takeuchi, M.; Sada, K.; Kawai, T. Molecular Rotation in Self-Assembled Multidecker Porphyrin Complexes. ACS Nano 2011, 5, 9575–9582. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Imada, T.; Shinkai, S. A Strong Positive Allosteric Effect in the Molecular Recognition of Dicarboxylic Acids by a Cerium(IV) Bis[tetrakis(4-pyridyl)porphyrinate] Double Decker. Angew. Chem. Int. Ed. 1998, 37, 2096–2099. [Google Scholar] [CrossRef]
- Ikeda, M.; Tanida, T.; Takeuchi, M.; Shinkai, S. Allosteric Silver(I) Ion Binding with Peripheral π Clefts of a Ce(IV) Double Decker Porphyrin. Org. Lett. 2000, 2, 1803–1805. [Google Scholar] [CrossRef]
- Robetson, A.; Ikeda, M.; Takeuchi, M.; Shinkai, S. Allosteric Binding of K+ to Crown Ether Macrocycles Appended to a Lanthanum Double Decker System. Bull. Chem. Soc. Jpn. 2001, 74, 883–888. [Google Scholar] [CrossRef]
- Ikeda, M.; Takeuchi, M.; Shinkai, S.; Tani, F.; Naruta, Y.; Sakamoto, S.; Yamaguchi, K. Allosteric Binding of an Ag+ Ion to Cerium(IV) Bis-porphyrinates Enhances the Rotational Activity of Porphyrin Ligands. Chem. Eur. J. 2002, 8, 5542–5550. [Google Scholar] [CrossRef]
- Ye, T.; Takami, T.; Wang, R.; Jiang, J.; Weiss, P.S. Tuning Interactions between Ligands in Self-Assembled Double-Decker Phthalocyanine Arrays. J. Am. Chem. Soc. 2006, 128, 10984–10985. [Google Scholar] [CrossRef]
- Otsuki, J.; Kawaguchi, S.; Yamakawa, T.; Asakawa, M.; Miyake, K. Arrays of Double-Decker Porphyrins on Highly Oriented Pyrolytic Graphite. Langmuir 2006, 22, 5708–5715. [Google Scholar] [CrossRef]
- Horii, Y.; Kishiue, S.; Damjanović, M.; Katoh, K.; Breedlove, B.K.; Enders, M.; Yamashita, M. Supramolecular Approach for Enhancing Single-Molecule Magnet Properties of Terbium(III)-Phthalocyaninato Double-Decker Complexes with Crown Moieties. Chem. Eur. J. 2018, 24, 4320–4327. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, C.; Ma, F.; Qi, D.; Liu, Q.; Sun, H.-L.; Jiang, J. Fabricating Bis(phthalocyaninato) Terbium SIM into Tetrakis(phthalocyaninato) Terbium SMM with Enhanced Performance through Sodium Coordination. Chem. Eur. J. 2018, 24, 8066–8070. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Yasuda, N.; Damjanović, M.; Wernsdorfer, W.; Breedlove, B.K.; Yamashita, M. Manipulation of the Coordination Geometry along the C4 Rotation Axis in a Dinuclear Tb3+ Triple-Decker Complex via a Supramolecular Approach. Chem. Eur. J. 2020, 26, 4805–4815. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Machida, K.; Yamamoto, E.; Adachi, G. Synthesis and Spectroscopic Properties of Water-Soluble Cerium(III) or Praseodymium(III) Mono[tetra(4-pyridyl)-porphyrinate] and Cerium(IV) Bis[tetra(4-pyridyl)-porphyrinate]. Chem. Lett. 1991, 20, 2035–2038. [Google Scholar] [CrossRef]
- Jiang, J.; Machida, K.; Adachi, G. Synthesis of Water-Soluble Lanthanide Porphyrin Sandwich Complexes: Bis(tetrapyridylporphyrinato) Cerium(IV), [Ce(tpyp)2], and Bis(tetramethylpyridylporphyrinato) Cerium(IV), [Ce(tmpyp)2]. Bull. Chem. Soc. Jpn. 1992, 65, 1990–1992. [Google Scholar] [CrossRef]
- Jiang, J.; Machida, K.; Adachi, G. Synthesis and characterization of water-soluble rare earth porphyrins Ce(tpyp)2 and Ce(tmpyp)2. J. Alloy Compd. 1993, 192, 296–299. [Google Scholar] [CrossRef]
- Buchler, J.W.; Nawra, M. Metal Complexes with Tetrapyrrole Ligands. 67. Synthesis and Spectroscopic Properties of Water-Soluble Cerium Bisporphyrinate Double-Decker Ions. Inorg. Chem. 1994, 33, 2830–2837. [Google Scholar] [CrossRef]
- Spyroulias, G.A.; Montauzon, D.D.; Maisonat, A.; Poilblanc, R.; Coutsolelos, A.G. Synthesis, UV-visible and electrochemical studies of lipophilic and hydrophilic lanthanide(III) bis(porphyrinates). Inorg. Chem. Acta 1998, 275–276, 182–191. [Google Scholar] [CrossRef]
- Yamamoto, K.; Nakazawa, S.; Imaoka, T. Manganese-Cerium Porphyrin Tri-nuclear Complex as a Catalyst for Water Oxidation. Mol. Cryst. Liq. Cryst. 2002, 379, 407–412. [Google Scholar] [CrossRef]
- Tsikalas, G.K.; Coutsolelos, A.G. Synthesis and Characterization of a New Asymmetric Bis-Porphyrinato Lanthanide Complex Presenting Mixed Hydrophilic−Hydrophobic Properties and Its Precursor Form. Inorg. Chem. 2003, 42, 6801–6804. [Google Scholar] [CrossRef]
- Donohoe, R.J.; Duchowski, J.K.; Bocian, D.F. Hole delocalization in oxidized cerium(IV) porphyrin sandwich complexes. J. Am. Chem. Soc. 1988, 110, 6119–6124. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishino, T.; Yamada, Y.; Yamamoto, A.; Tanaka, K. Synthesis of Bis{meso-Tetrakis(4-N-alkylpyridiniumyl)porphyrinato}cerium and Its Redox Switching Behavior. Molecules 2021, 26, 790. https://doi.org/10.3390/molecules26040790
Nishino T, Yamada Y, Yamamoto A, Tanaka K. Synthesis of Bis{meso-Tetrakis(4-N-alkylpyridiniumyl)porphyrinato}cerium and Its Redox Switching Behavior. Molecules. 2021; 26(4):790. https://doi.org/10.3390/molecules26040790
Chicago/Turabian StyleNishino, Toshio, Yasuyuki Yamada, Ayumi Yamamoto, and Kentaro Tanaka. 2021. "Synthesis of Bis{meso-Tetrakis(4-N-alkylpyridiniumyl)porphyrinato}cerium and Its Redox Switching Behavior" Molecules 26, no. 4: 790. https://doi.org/10.3390/molecules26040790
APA StyleNishino, T., Yamada, Y., Yamamoto, A., & Tanaka, K. (2021). Synthesis of Bis{meso-Tetrakis(4-N-alkylpyridiniumyl)porphyrinato}cerium and Its Redox Switching Behavior. Molecules, 26(4), 790. https://doi.org/10.3390/molecules26040790