Coumarin Sulfonamides and Amides Derivatives: Design, Synthesis, and Antitumor Activity In Vitro
Abstract
:1. Introduction
2. Results
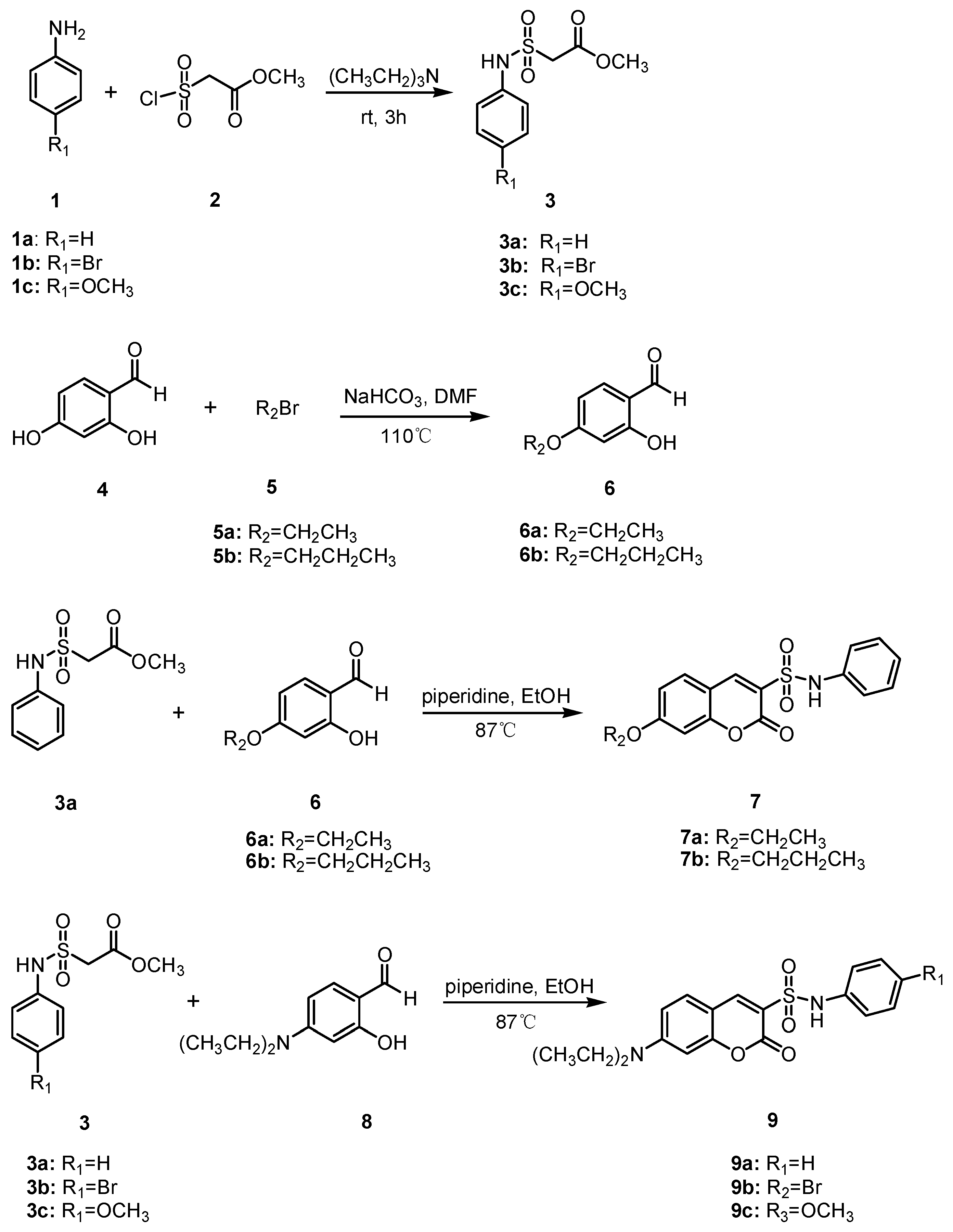
2.1. Chemical Synthesis
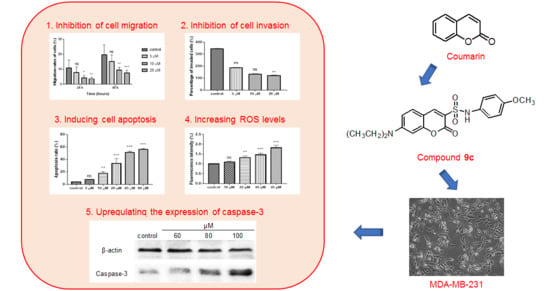
2.2. Cytotoxicity
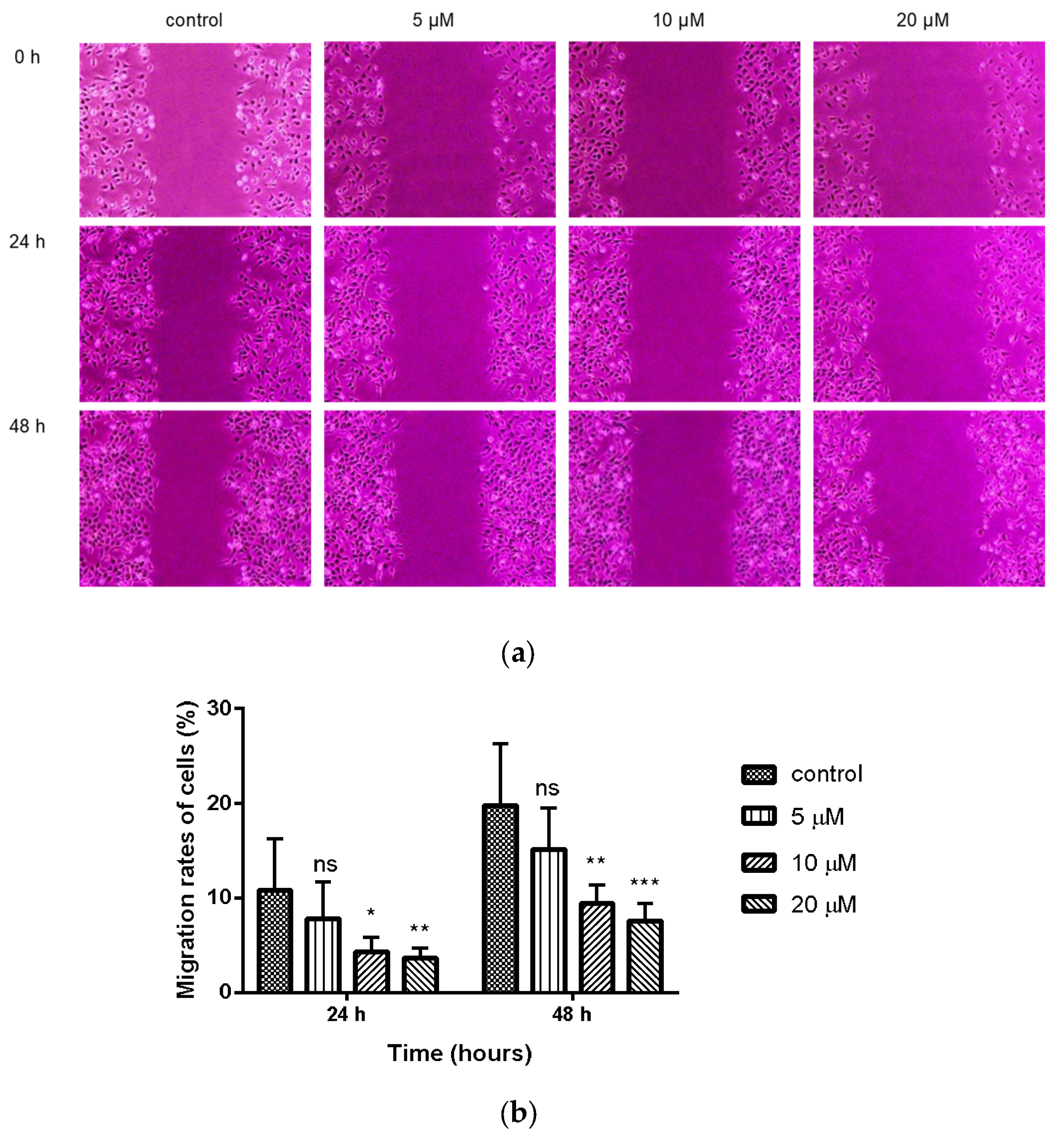
2.3. Inhibition of Wound Healing in MDA-MB-231 Cells by Compound 9c
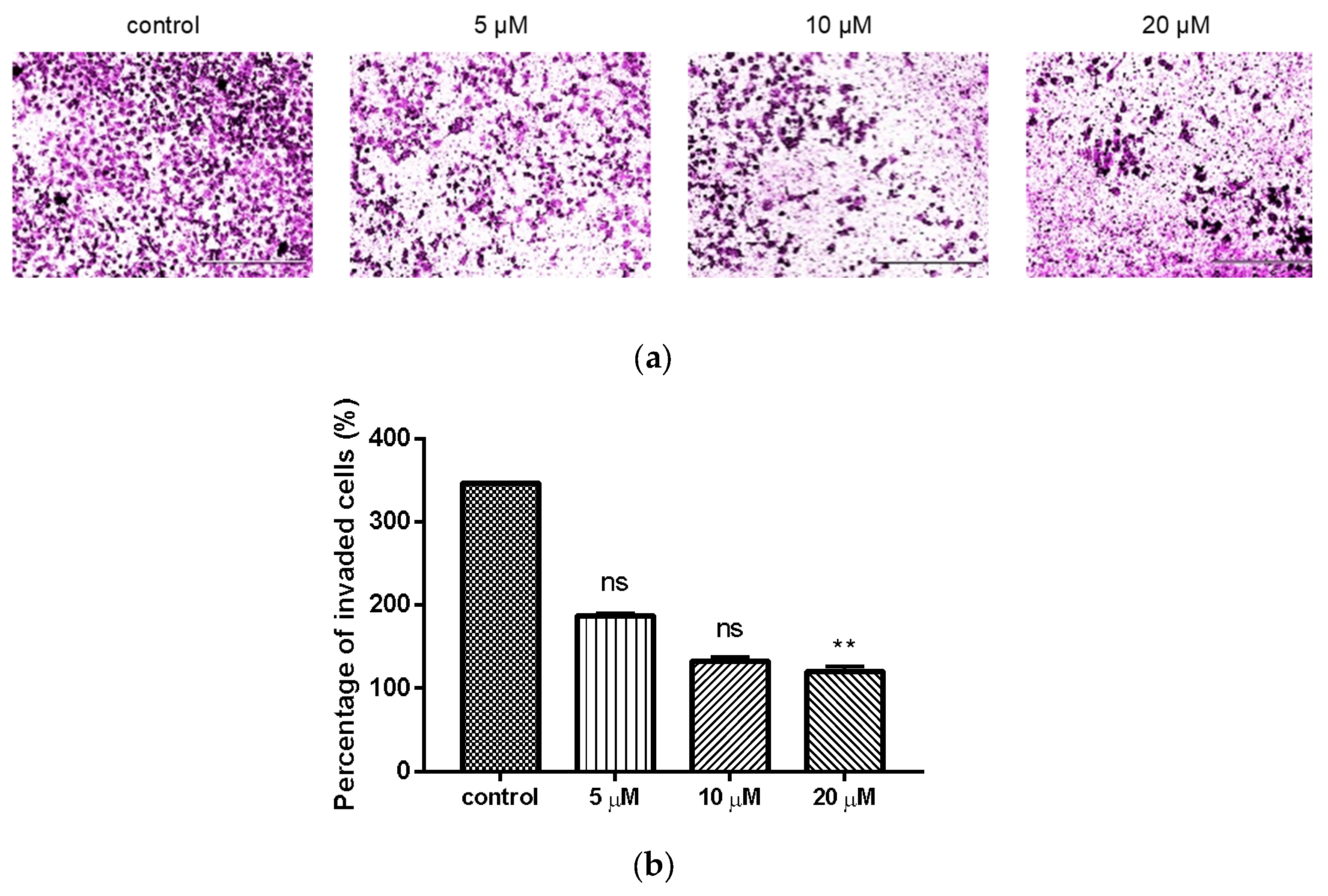
2.4. Inhibition of Compond 9c on the Invasion of MDA-MB-231 Cells
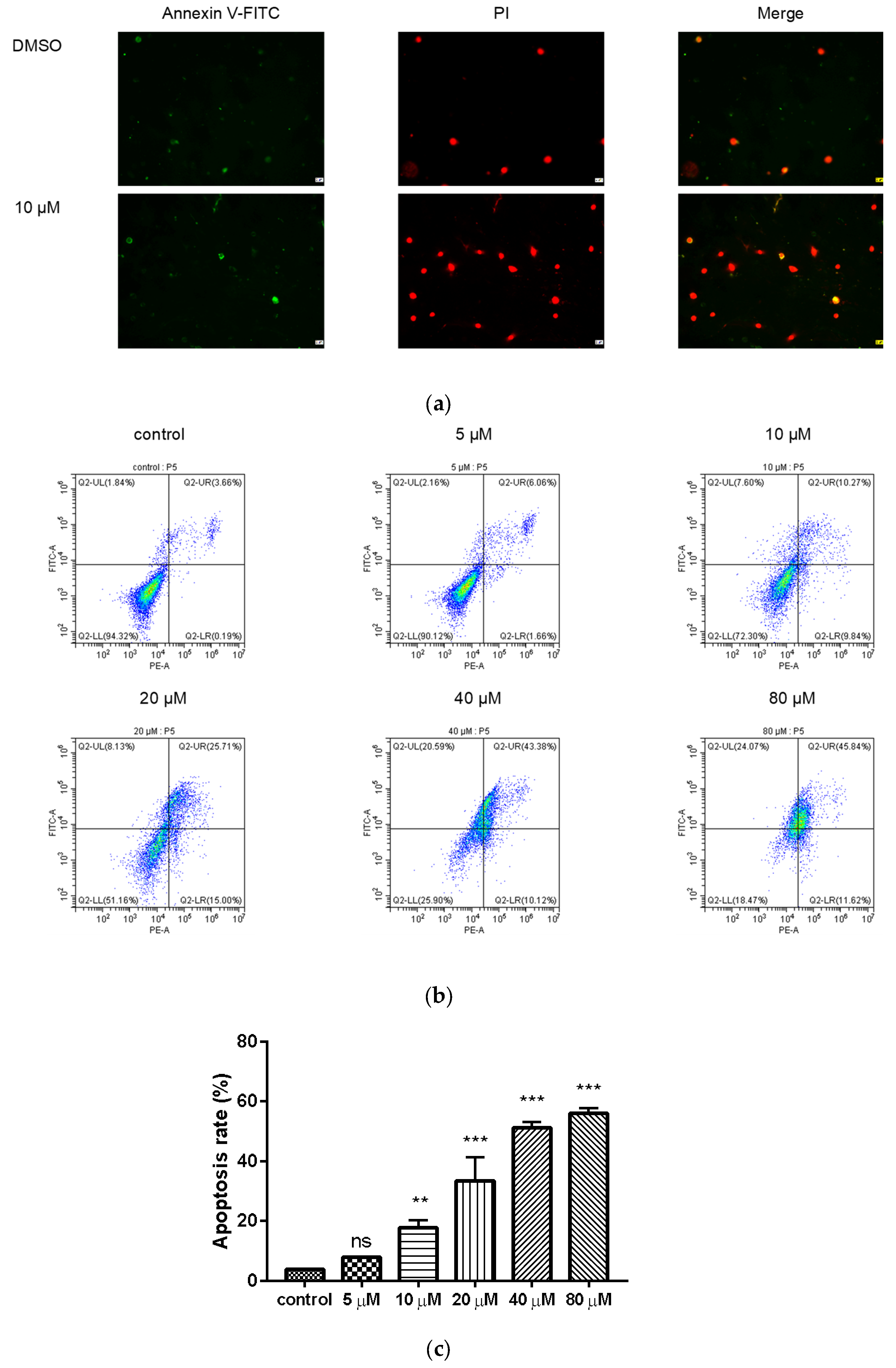
2.5. Compound 9c Induces Apoptosis in MDA-MB-231 Cells
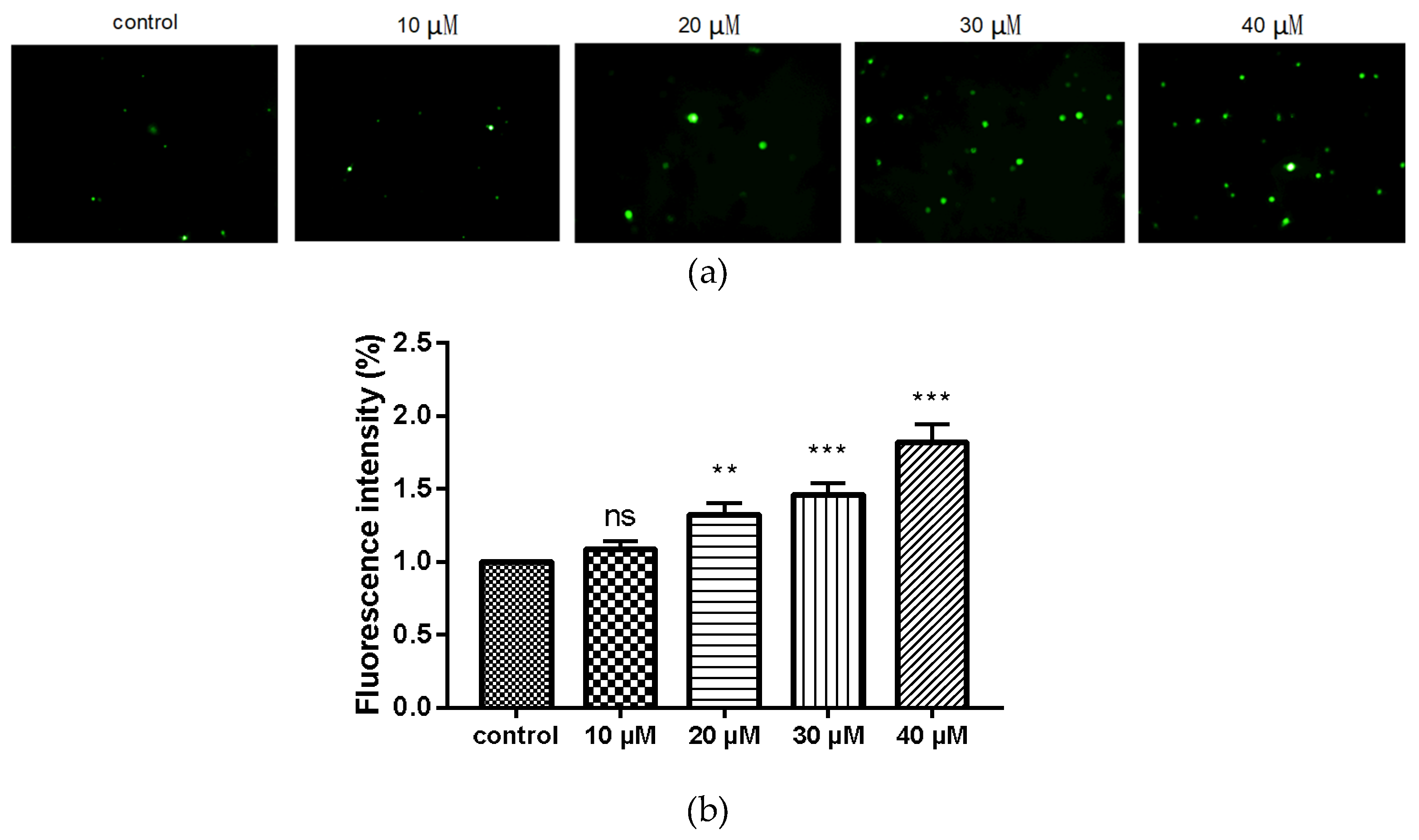
2.6. Compound 9c Increases ROS Production in MDA-MB-231 Cells
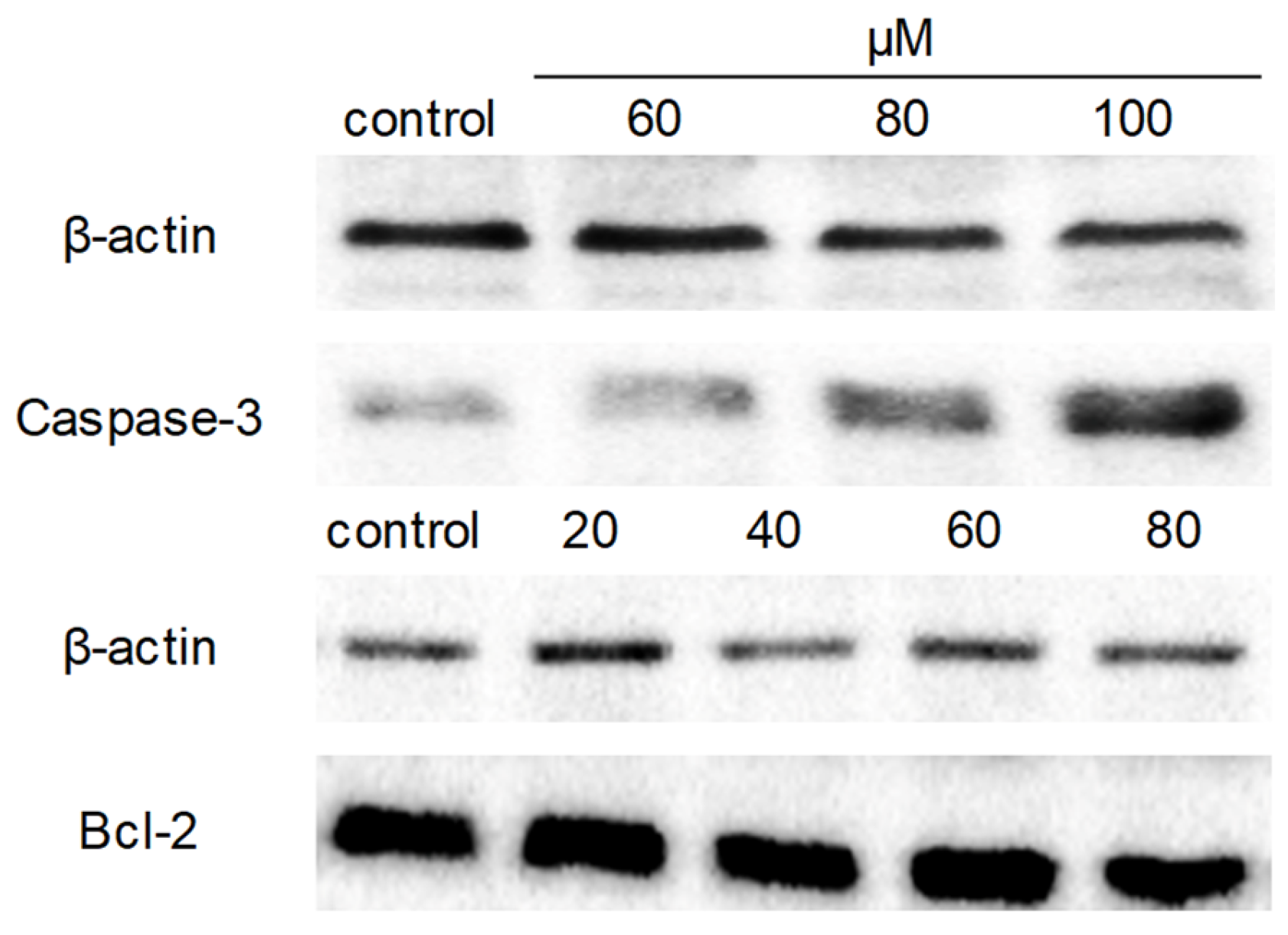
2.7. Western Blot Analysis
3. Discussion
4. Materials and Methods
4.1. Chemical
4.1.1. Syntheses
4.1.2. General Procedure for the Synthesis of Compounds 3a–3c
4.1.3. General Procedure for the Synthesis of Compounds 6a–6b
4.1.4. General Procedure for the Synthesis of Compounds 7a–7b
4.1.5. General Procedure for the Synthesis of Compounds 9a–9c
4.1.6. Synthesis of Compound 11
4.1.7. General Procedure for the Synthesis of Compounds 12a–12c
4.2. Biology
4.2.1. Cell Culture
4.2.2. MTT Assay
4.2.3. Cell Migration Assay
4.2.4. Analysis of Cell Invasion
4.2.5. Apoptosis Analysis and Morphology Observation
4.2.6. Intracellular ROS Level Detection
4.2.7. Western Blotting
4.2.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Varmus, H. The new era in cancer research. Science 2006, 312, 1162–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerru, N.; Singh, P.; Koorbanally, N.; Raj, R.; Kumar, V. Recent advances (2015–2016) in anticancer hybrids. Eur. J. Med. Chem. 2017, 142, 179–212. [Google Scholar] [CrossRef] [PubMed]
- Hoult, J.R.S.; Payá, M. Pharmacological and biochemical actions of simple coumarins: Natural products with therapeutic potential. Gen. Pharmacol. 1996, 27, 713–722. [Google Scholar] [CrossRef]
- Hawryl, M.A.; Soczewinski, E.; Dzido, T.H. Separation of coumarins from Archangelica officinalis in high-performance liquid chromatography and thin-layer chromatography systems. J. Chromatogr. A 2000, 886, 75–81. [Google Scholar] [CrossRef]
- Devulapally, S.; Chandraiah, G.; Pramod, K.D. A review on pharmacological properties of coumarins. Mini Rev. Med. Chem. 2018, 18, 113–141. [Google Scholar]
- Salem, M.A.I.; Marzouk, M.I.; El-Kazak, A.M. Synthesis and characterization of some new coumarins with in vitro antitumor and antioxidant activity and high protective effects against DNA damage. Molecules 2016, 21, 249. [Google Scholar] [CrossRef]
- Emami, S.; Dadashpour, S. Current developments of coumarin-based anti-cancer agents in medicinal chemistry. Eur. J. Med. Chem. 2015, 102, 611–630. [Google Scholar] [CrossRef]
- Kasumbwe, K.; Venugopala, K.N.; Mohanlall, V.; Odhav, B. Synthetic mono/di-halogenated coumarin derivatives and their anticancer properties. Anticancer Agents Med. Chem. 2017, 17, 276–285. [Google Scholar] [CrossRef]
- Mohamed, T.K.; Batran, R.Z.; Elseginy, S.A.; Ali, M.M.; Mahmoud, A.E. Synthesis, anticancer effect and molecular modeling of new thiazolylpyrazolyl coumarin derivatives targeting VEGFR-2 kinase and inducing cell cycle arrest and apoptosis. Bioorg. Chem. 2019, 85, 253–273. [Google Scholar] [CrossRef]
- Elhenawy, A.A.; Al-Harbi, L.M.; El-Gazzar, M.A.; Khowdiary, M.M.; Moustfa, A. Synthesis, molecular properties and comparative docking and QSAR of new 2-(7-hydroxy-2-oxo-2H-chromen-4-yl) acetic acid derivatives as possible anticancer agents. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 218, 248–262. [Google Scholar] [CrossRef]
- Maleki, E.H.; Bahrami, A.R.; Sadeghian, H.; Matin, M.M. Discovering the structure–activity relationships of different O-prenylated coumarin derivatives as effective anticancer agents in human cervical cancer cells. Toxicol. In Vitro 2020, 63, 104745. [Google Scholar] [CrossRef] [PubMed]
- Mamatha, S.V.; Belagali, S.L.; Mahesh, B.; Kumbar, V.M. Design and synthesis of novel coumarin conjugated acetamides as promising anticancer agents: An in silico and in vitro approach. Anticancer Agents Med. Chem. 2020, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Govindaiah, P.; Dumala, N.; Mattan, I.; Grover, P.; Prakash, M.J. Design, synthesis, biological and in silico evaluation of coumarin-hydrazone derivatives as tubulin targeted antiproliferative agents. Bioorg. Chem. 2019, 91, 103143. [Google Scholar] [CrossRef] [PubMed]
- García, S.; Mercado-Sánchez, I.; Bahena, L.; Alcaraz, Y.; García-Revilla, M.A.; Robles, J.; Santos-Martínez, N.; Ordaz-Rosado, D.; García-Becerra, R.; Vazquez, M.A. Design of fluorescent foumarin-hydroxamic acid derivatives as inhibitors of HDACs: Synthesis, anti-proliferative evaluation and docking studies. Molecules 2020, 25, 5134. [Google Scholar] [CrossRef] [PubMed]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touisni, N.; Maresca, A.; McDonald, P.C.; Lou, Y.; Scozzafava, A.; Dedhar, S.; Winum, J.Y.; Supuran, C.T. Glycosyl coumarin carbonic anhydrase IX and XII inhibitors strongly attenuate the growth of primary breast tumors. J. Med. Chem. 2011, 54, 8271–8277. [Google Scholar] [CrossRef] [Green Version]
- Musa, M.A.; Cooperwood, J.S.; Khan, M.O.F. A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr. Med. Chem. 2008, 15, 2664–2679. [Google Scholar] [CrossRef] [Green Version]
- Sabt, A.; Abdelhafez, O.M.; El-Haggar, R.S.; Madkour, H.M.F.; Eldehna, W.M.; El-Khrisy, E.E.D.A.M.; Abdel-Rahman, M.A.; Rashed, L.A. Novel coumarin-6-sulfonamides as apoptotic anti-proliferative agents: Synthesis, in vitro biological evaluation, and QSAR studies. J. Enzyme Inhib. Med. Chem. 2018, 33, 1095–1107. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.M.; Shen, G.; Neckers, L.; Blake, H.; Holzbeierlein, J.; Cronk, B.; Blagg, B.S.J. Hsp90 inhibitors identified from a library of novobiocin analogues. J. Am. Chem. Soc. 2005, 127, 12778–12779. [Google Scholar] [CrossRef]
- Peperidou, A.; Bua, S.; Bozdag, M.; Hadjipavlou-Litina, D.; Supuran, C.T. Novel 6- and 7-substituted coumarins with inhibitory action against lipoxygenase and tumor-associated carbonic anhydrase IX. Molecules 2018, 23, 153. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Tan, Y.; Gan, N.; Zhang, J.; Li, S.; Zheng, X.; Wang, Z.; Yi, W. Synthesis and anticancer activity of new coumarin-3-carboxylic acid derivatives as potential lactatetransportinhibitors. Bioorg. Med. Chem. 2020, 29, 115870. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, J.; Liu, Y.; Zeng, Y.; Wu, G. A review on anti-tumor mechanisms of coumarins. Front. Oncol. 2020, 10, 592853. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Xu, Y.; Zhou, H.F. Esculetin inhibits proliferation, invasion, and migration of laryngeal cancer in vitro and in vivo by inhibiting janus kinas (JAK)-signal transducer and activator of transcription-3 (STAT3) activation. Med. Sci. Monit. 2019, 25, 7853–7863. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Pan, D.; Zhen, Y.; Huang, J.; Wang, J.; Zhang, J.; He, Z. Ferulin C triggers potent PAK1 and p21-mediated anti-tumor effects in breast cancer by inhibiting tubulin polymerization in vitro and in vivo. Pharmacol. Res. 2020, 152, 104605. [Google Scholar] [CrossRef] [PubMed]
- Elshemy, H.A.H.; Zaki, M.A. Design and synthesis of new coumarin hybrids and insight into their mode of antiproliferative action. Bioorg. Med. Chem. 2017, 25, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Hevia, D.; Patchva, S.; Park, B.; Koh, W.; Aggarwal, B.B. Upsides and downsides of reactive oxygen species for cancer: The roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid. Redox Signal. 2012, 16, 1295–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, H.W.; Zheng, C.S.; Chu, S.J.; Sun, W.X.; Han, L.J.; Yang, R.W.; Qi, J.L.; Lu, G.H.; Wang, X.M.; Yang, Y.H. The evaluation of potent antitumor activities of shikonin coumarin-carboxylic acid, PMMB232 through HIF-1α-mediated apoptosis. Biomed. Pharmacother. 2018, 97, 656–666. [Google Scholar] [CrossRef]
- Musa, M.A.; Latinwo, L.M.; Joseph, M.Y.; Badisa, V.L. Identification of 7,8-diacetoxy-3-arylcoumarin derivative as a selective cytotoxic and apoptosis-inducing agent in a human prostate cancer cell line. Anticancer Res. 2017, 37, 6005–6014. [Google Scholar]
- Talmadge, J.E.; Fidler, I.J. AACR centennial series: The biology of cancer metastasis: Historical perspective. Cancer Res. 2010, 70, 5649–5669. [Google Scholar] [CrossRef] [Green Version]
- Parrish, A.B.; Freel, C.D.; Kornbluth, S. Cellular mechanisms controlling caspase activation and function. CSH Perspect. Biol. 2013, 5, a008672. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, D.; Dong, Y.; Fu, J.; Lu, S.; Yuan, C.; Xia, M.; Sun, L.K. Bcl2 inhibitor ABT737 reverses the Warburg effect via the Sirt3-HIF1α axis to promote oxidative stress-induced apoptosis in ovarian cancer cells. Life Sci. 2020, 255, 117846. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; López-Soto, A.; Kumar, S.; Kroemer, G. Caspases connect cell-death signaling to organismal homeostasis. Immunity 2016, 44, 221–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, C.F.A.; Wong-Brown, M.W.; Bowden, N.A. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019, 10, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, S.; Abdelwahab, S.I.; Kamalidehghan, B.; Syam, S.; May, K.S.; Harmal, N.S.M.; Shafifiyaz, N.; Hadi, A.H.A.; Hashim, N.M.; Rahmani, M.; et al. Involvement of NF-κB and Bcl2/Bax signaling pathways in the apoptosis of MCF7 cells induced by a xanthone compound Pyranocycloartobiloxanthone A. Phytomedicine 2012, 19, 1007–1015. [Google Scholar] [CrossRef]
- Vucicevic, K.; Jakovljevic, V.; Colovic, N.; Tosic, N.; Kostic, T.; Glumac, I.; Pavlovic, S.; Karan-Djurasevic, T.; Colovic, M. Association of Bax expression and Bcl2/Bax ratio with clinical and molecular prognostic markers in chronic lymphocytic leukemia. J. Med. Biochem. 2016, 35, 150–157. [Google Scholar] [CrossRef] [Green Version]
- Reddy, N.; Mallireddigari, M.R.; Cosenza, S.; Gumireddy, K.; Bell, S.C.; Reddy, E.P.; Reddy, M.V.R. Synthesis of new coumarin 3-(N-aryl) sulfonamides and their anticancer activity. Bioorg. Med. Chem. Lett. 2004, 14, 4093–4097. [Google Scholar] [CrossRef]
- Yang, F.; Rauch, K.; Kettelhoit, K.; Ackermann, L. Aldehyde-assisted ruthenium (II)-catalyzed CH oxygenations. Angew. Chem. Int. Ed. 2014, 126, 11467–11470. [Google Scholar] [CrossRef]
- Huang, K.; Liu, M.; Wang, X.; Cao, D.; Gao, F.; Zhou, K.; Wang, W.; Zeng, W. Cascade reaction and FRET-based fluorescent probe for the colorimetric and ratiometric signaling of hydrogen sulfide. Tetrahedron Lett. 2015, 56, 3769–3773. [Google Scholar] [CrossRef]


| Compd | Structure | Compd | Structure |
|---|---|---|---|
| 7a | 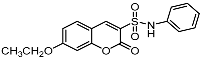 | 9c | 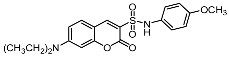 |
| 7b | 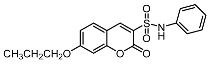 | 12a | 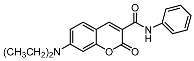 |
| 9a |  | 12b | 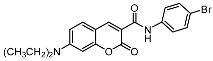 |
| 9b | 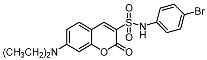 | 12c | 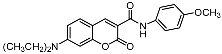 |
| Compd | IC50 (μM) | ||
|---|---|---|---|
| MDA-MB-231 | KB | HCT-116 | |
| 7a | >100 | 79.46 ± 1.62 | >100 |
| 7b | >100 | 20.21 ± 0.42 | >100 |
| 9a | >100 | 34.94 ± 2.83 | 15.51 ± 1.59 |
| 9b | 17.13 ± 0.68 | 10.78 ± 0.13 | 17.26 ± 2.00 |
| 9c | 9.33 ± 1.81 | 13.66 ± 1.33 | >100 |
| 12a | 13.33 ± 1.26 | >100 | >100 |
| 12b | 11.63 ± 0.98 | 14.22 ± 1.64 | >100 |
| 12c | 17.01 ± 1.04 | 97.14 ± 2.43 | >100 |
| 5-Fu | 8.59 ± 0.52 | 94.15 ± 2.76 | 15.28 ± 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Tan, Y.; Li, G.; Chen, L.; Nie, M.; Wang, Z.; Ji, H. Coumarin Sulfonamides and Amides Derivatives: Design, Synthesis, and Antitumor Activity In Vitro. Molecules 2021, 26, 786. https://doi.org/10.3390/molecules26040786
Zhang J, Tan Y, Li G, Chen L, Nie M, Wang Z, Ji H. Coumarin Sulfonamides and Amides Derivatives: Design, Synthesis, and Antitumor Activity In Vitro. Molecules. 2021; 26(4):786. https://doi.org/10.3390/molecules26040786
Chicago/Turabian StyleZhang, Jing, Yaling Tan, Guorong Li, Lexian Chen, Minyi Nie, Zhaohua Wang, and Hong Ji. 2021. "Coumarin Sulfonamides and Amides Derivatives: Design, Synthesis, and Antitumor Activity In Vitro" Molecules 26, no. 4: 786. https://doi.org/10.3390/molecules26040786
APA StyleZhang, J., Tan, Y., Li, G., Chen, L., Nie, M., Wang, Z., & Ji, H. (2021). Coumarin Sulfonamides and Amides Derivatives: Design, Synthesis, and Antitumor Activity In Vitro. Molecules, 26(4), 786. https://doi.org/10.3390/molecules26040786