Sunflower-Like Nanostructure with Built-In Hotspots for Alpha-Fetoprotein Detection
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Modification of SERS Active Substrate
2.3. Finite-Difference Time-Domain (FDTD) Simulations
2.4. Characterization
3. Results and Discussion
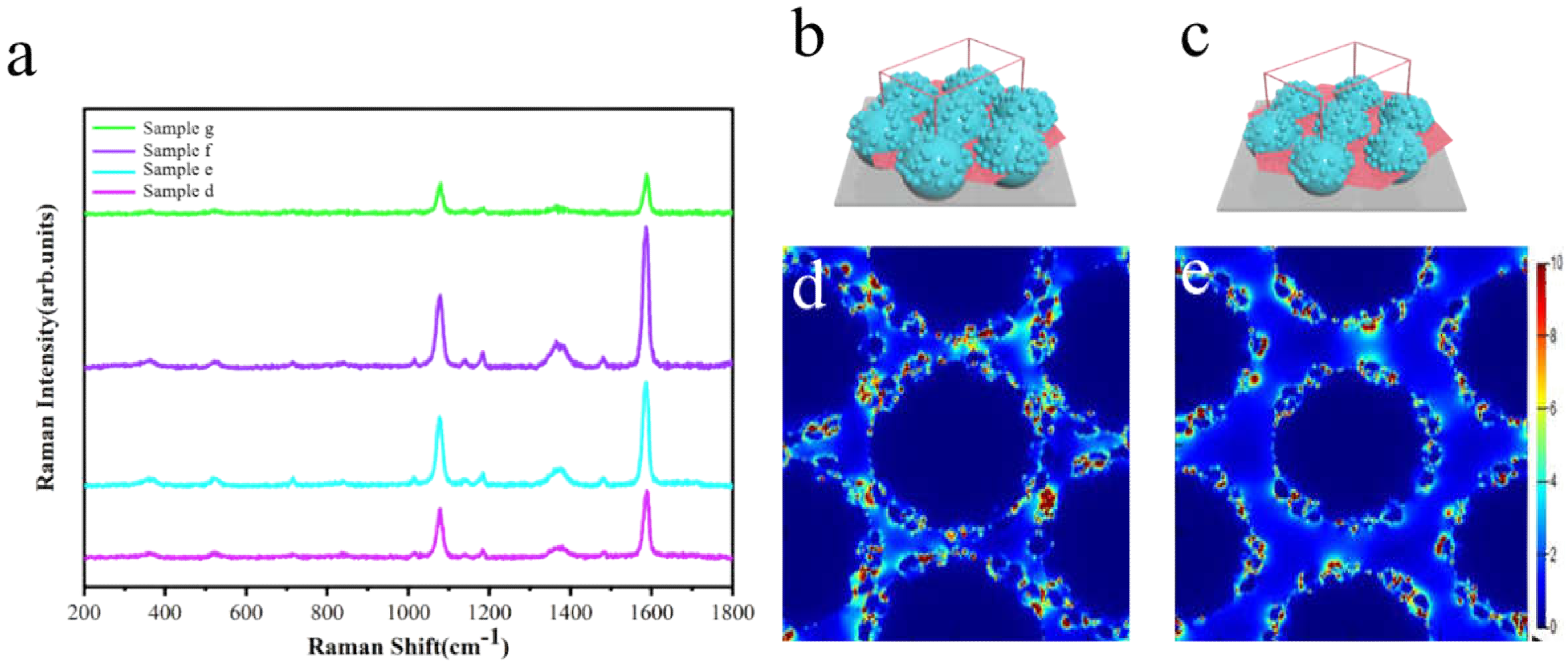
3.1. Construction and Characterization of the Sunflower-Like Nanostructure
3.2. Detection of AFP with Sunflower-Like Structure SERS Active Chips
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wintermark, M.; Reichhart, M.; Thiran, J.P.; Maeder, P.; Chalaron, M.; Schnyder, P.; Bogousslavsky, J.; Meuli, R. Prognostic accuracy of cerebral blood flow measurement by perfusion computed tomography, at the time of emergency room admission, in acute stroke patients. Ann. Neurol. 2002, 51, 417–432. [Google Scholar] [CrossRef]
- Leach, M.O. Application of magnetic resonance imaging to angiogenesis in breast cancer. Breast Cancer Res. 2001, 3, 22–27. [Google Scholar] [CrossRef][Green Version]
- Faletti, R.; Cassinis, M.C.; Fonio, P.; Bergamasco, L.; Pavan, L.J.; Rapellino, A.; David, E.; Gandini, G. Multiparametric Gd-EOB-DTPA magnetic resonance in diagnosis of HCC: Dynamic study, hepatobiliary phase, and diffusion-weighted imaging compared to histology after orthotopic liver transplantation. Abdom. Imaging 2015, 40, 46–55. [Google Scholar] [CrossRef]
- Jiang, N.; Zhuo, X.; Wang, J. Active Plasmonics: Principles, Structures, and Applications. Chem. Rev. 2017, 118, 3054–3099. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Salazar, J.R.; Osvaldo, J.; Oliveira, N. Plasmonic Biosensing Focus Review. Chem. Rev. 2018, 118, 10617–10625. [Google Scholar] [CrossRef]
- Salandrino, A. Plasmonic parametric resonance. Phys. Rev. 2018, 97, 081401. [Google Scholar] [CrossRef]
- Wang, X.; Shi, W.; Jin, Z.; Huang, W.; Lin, J.; Ma, G.; Li, S.; Guo, L. Remarkable SERS Activity Observed from Amorphous ZnO Nanocages. Angew. Chem. 2017, 129, 9983–9987. [Google Scholar] [CrossRef]
- Liow, C.H.; Lu, X.; Tan, C.F.; Chan, K.H.; Zeng, K.; Li, S.; Ho, G.W. Spatially Probed Plasmonic Photothermic Nanoheater Enhanced Hybrid Polymeric-Metallic PVDF-Ag Nanogenerator. Small 2018, 14, 1702268. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Fu, T.; Tang, J.; Pan, D.; Zhang, S.; Xu, H. Routing a Chiral Raman Signal Based on Spin-Orbit Interaction of Light. Phys. Rev. Lett. 2019, 123, 183903. [Google Scholar] [CrossRef]
- Lopez, G.A.; Estevez, M.C.; Soler, M. Recent advances in nanoplasmonic biosensors: Applications and lab-on-a-chip integration. Nanophotonics 2017, 6, 123–136. [Google Scholar] [CrossRef]
- Hu, J.; Wang, C.; Yang, S.; Zhou, F.; Li, Z.; Kan, C. Surface Plasmon Resonance in Periodic Hexagonal Lattice Arrays of Silver Nanodisks. J. Nanomater. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Lin, D.; Wu, Z.; Li, S.; Zhao, W.; Ma, C.; Wang, J.; Jiang, Z.; Zhong, Z.; Zheng, Y.; Yang, X. Large-Area Au-Nanoparticle-Functionalized Si Nanorod Arrays for Spatially Uniform Surface-Enhanced Raman Spectroscopy. ACS Nano 2017, 11, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Krasavin, A.V.; Nasir, M.E.; Dickson, W.; Zayats, A.V. Reactive tunnel junctions in electrically driven plasmonic nanorod metamaterials. Nat. Nanotechnol. 2017, 13, 159–164. [Google Scholar] [CrossRef]
- Liu, B.; Yao, X.; Chen, S.; Lin, H.; Yang, Z.; Liu, S.; Ren, B. Large-Area Hybrid Plasmonic Optical Cavity (HPOC) Substrates for Surface-Enhanced Raman Spectroscopy. Adv. Funct. Mater. 2018, 28, 1802263. [Google Scholar] [CrossRef]
- Wang, A.; Ruan, W.; Song, W.; Chen, L.; Zhao, B.; Jung, Y.M.; Wang, X. Detection of the potential tumor marker of AFP using surface-enhanced Raman scattering-based immunoassay. J. Raman Spectrosc. 2013, 44, 1649–1653. [Google Scholar] [CrossRef]
- Chen, J.; Li, T.; Wang, S.; Zhu, S. Multiplexed Holograms by Surface Plasmon Propagation and Polarized Scattering. Nano Lett. 2017, 17, 5051–5055. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Su, Y.; Du, L.; Wang, R.; Zhang, L.; Zhao, D.; Xie, W. Plasmon-Enhanced Deuteration under Visible-Light Irradiation. ACS Nano 2019, 13, 10754–10760. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, Y.; Yang, N.; You, T.; Chen, H.; Yin, P. Direct determination of the tumor marker AFP via silver nanoparticle enhanced SERS and AFP-modified gold nanoparticles as capturing substrate. Microchim. Acta 2018, 185, 1–6. [Google Scholar] [CrossRef]
- Zheng, H.; Cheng, L.; Zou, B.; Wang, Y.; Zhang, X.; Zhou, S. Gel-assisted synthesis of oleate-modified Fe3O4@Ag composite microspheres as magnetic SERS probe for thiram detection. CrystEngComm 2015, 17, 6393–6398. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, X.; Wang, Y.; Zhu, A.; Gao, R.; Zhao, X.; Chen, L. Controlling the Growth Locations of Ag Nanoparticles at Nanoscale by Shifting LSPR Hotspots. Nanomaterials 2019, 11, 1553. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, C.; Gao, R.; Gao, L.; Li, Q.; Yuan, G.; He, J. A novel electrochemical immunosensor based on the rGO-TEPA-PTC-NH2 and AuPt modified C60 bimetallic nanoclusters for the detection of Vangl1, a potential biomarker for dysontogenesis. Biosens. Bioelectron. 2015, 7, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, J.R.; Birke, R.L. A Unified Approach to Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2008, 112, 5605–5617. [Google Scholar] [CrossRef]
- Yang, S.; Zhanga, F.; Wang, Z.; Liang, Q. A graphene oxide-based label-free electrochemical aptasensor for the detection of alpha-fetoprotein. Biosens. Bioelectron. 2018, 112, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Sun, X.; Chen, L.; Cheng, W.; Han, X.X.; Zhao, B.; He, C. Multiplex Immunochips for High-Accuracy Detection of AFP-L3% Based on Surface-Enhanced Raman Scattering: Implications for Early Liver Cancer Diagnosis. Anal. Chem. 2017, 89, 8877–8883. [Google Scholar] [CrossRef]
- Wang, F.; Yang, M.; Ji, S.; Yang, L.; Zhao, J.; Liu, H.; Sui, Y.; Sun, Y.; Yang, J.; Zhang, X. Boosting spectral response of multi-crystalline Si solar cells with Mn2+ doped CsPbCl3 quantum dots downconverter. J. Power Sources 2018, 395, 85–91. [Google Scholar] [CrossRef]
- Conti, F.; Dall’Agata, M.; Gramenzi, A.; Biselli, M. Biomarkers for the early diagnosis of bacterial infection and the surveillance of hepatocellular carcinoma in cirrhosis. Biosens. Bioelectron. 2015, 9, 1343–1351. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Zhu, A.; Wang, Y.; Zhang, Y.; Zhang, X. Sunflower-Like Nanostructure with Built-In Hotspots for Alpha-Fetoprotein Detection. Molecules 2021, 26, 1197. https://doi.org/10.3390/molecules26041197
Zhao X, Zhu A, Wang Y, Zhang Y, Zhang X. Sunflower-Like Nanostructure with Built-In Hotspots for Alpha-Fetoprotein Detection. Molecules. 2021; 26(4):1197. https://doi.org/10.3390/molecules26041197
Chicago/Turabian StyleZhao, Xiaoyu, Aonan Zhu, Yaxin Wang, Yongjun Zhang, and Xiaolong Zhang. 2021. "Sunflower-Like Nanostructure with Built-In Hotspots for Alpha-Fetoprotein Detection" Molecules 26, no. 4: 1197. https://doi.org/10.3390/molecules26041197
APA StyleZhao, X., Zhu, A., Wang, Y., Zhang, Y., & Zhang, X. (2021). Sunflower-Like Nanostructure with Built-In Hotspots for Alpha-Fetoprotein Detection. Molecules, 26(4), 1197. https://doi.org/10.3390/molecules26041197




