Enhancing the Potentiality of Trichoderma harzianum against Pythium Pathogen of Beans Using Chamomile (Matricaria chamomilla, L.) Flower Extract
Abstract
1. Introduction
2. Results and Discussion
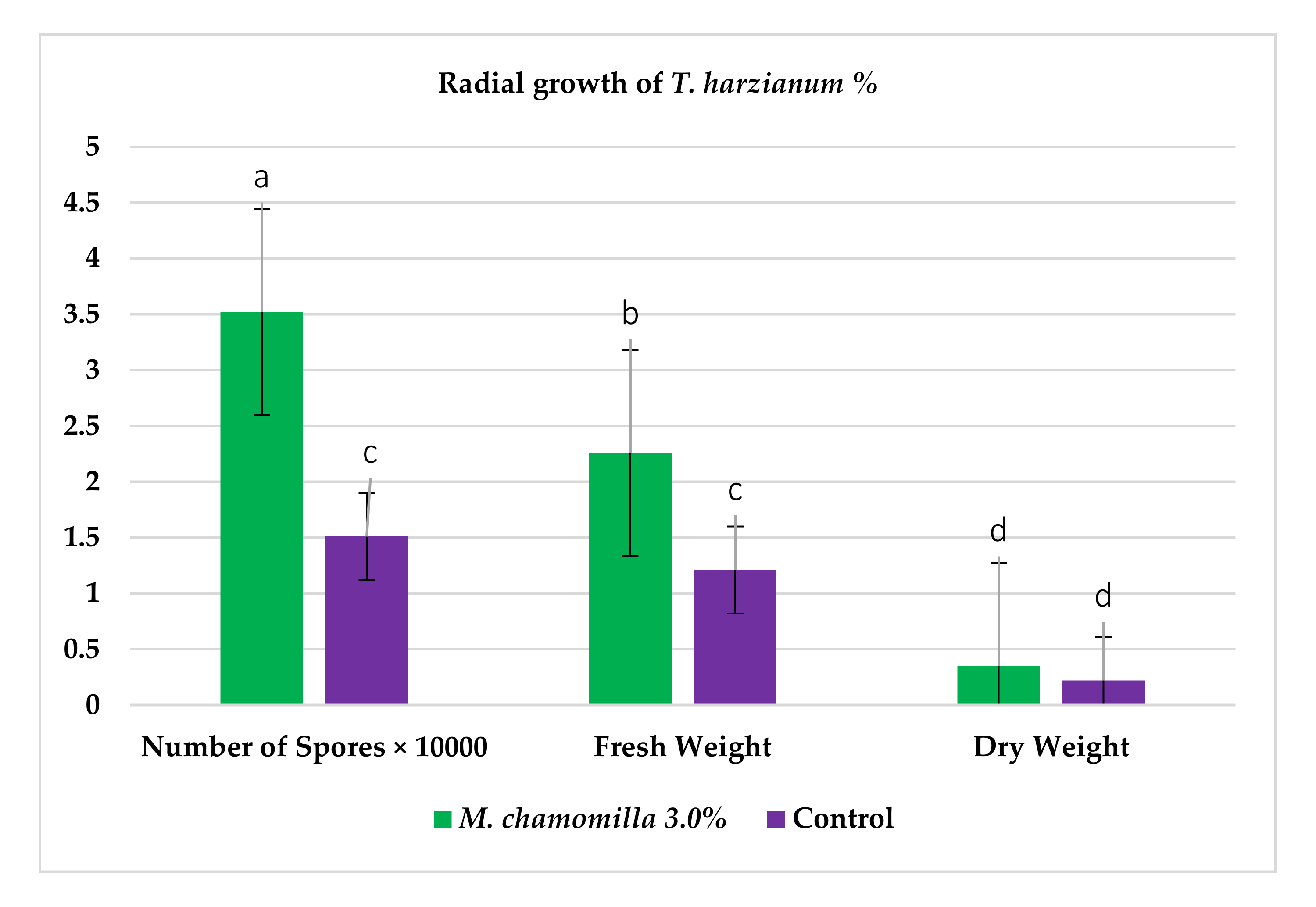
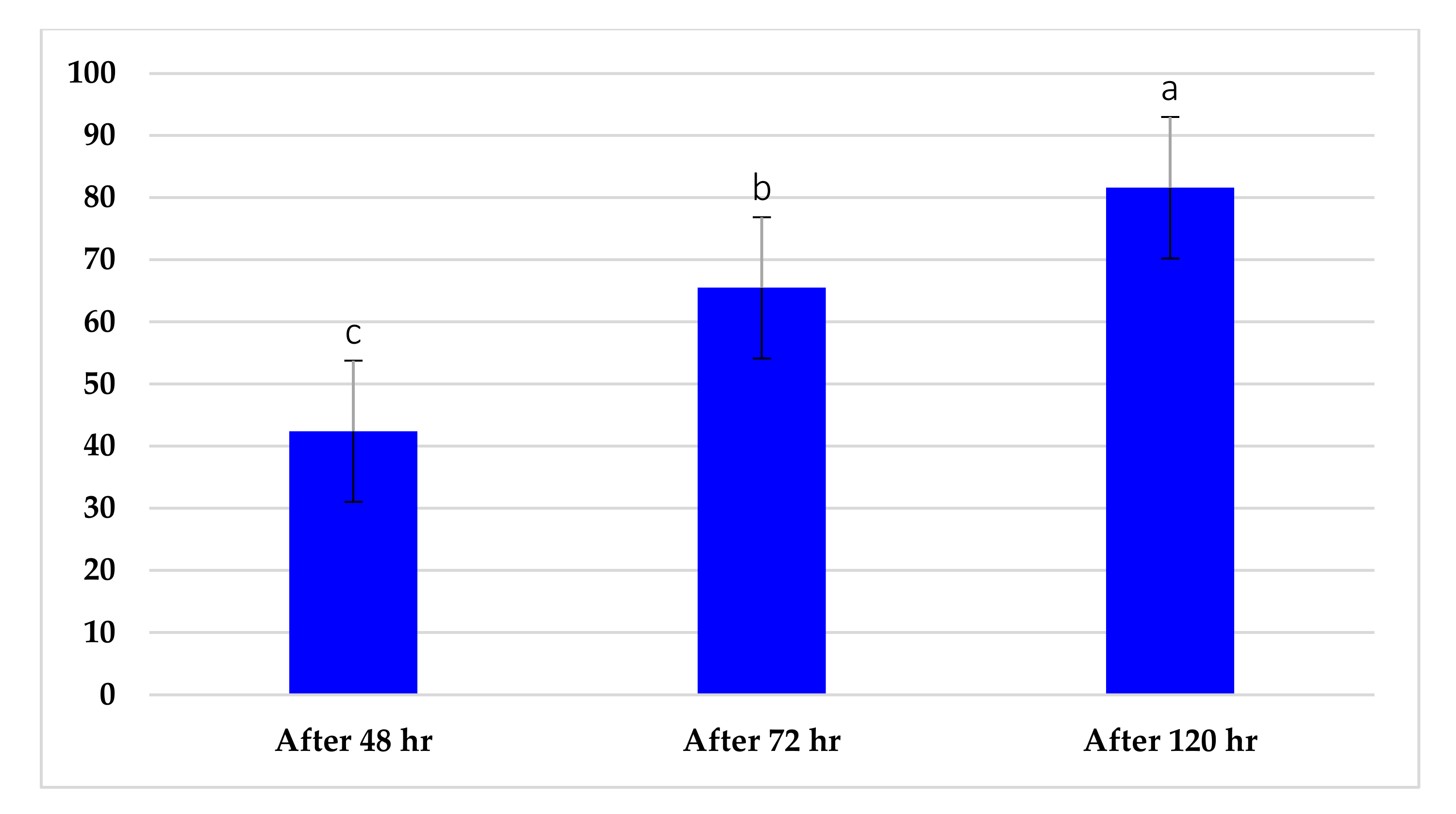
2.1. In Vitro, the Comparative Response of P. ultimum to Aqueous Extract of Chamomile Flower and T. harzianum
2.2. In Vivo, Investigation of the Potentiality of Chamomile Flower Extract and Culture of T. Harzianum Against Pythium Pathogen of Bean
2.2.1. Disease Assessment
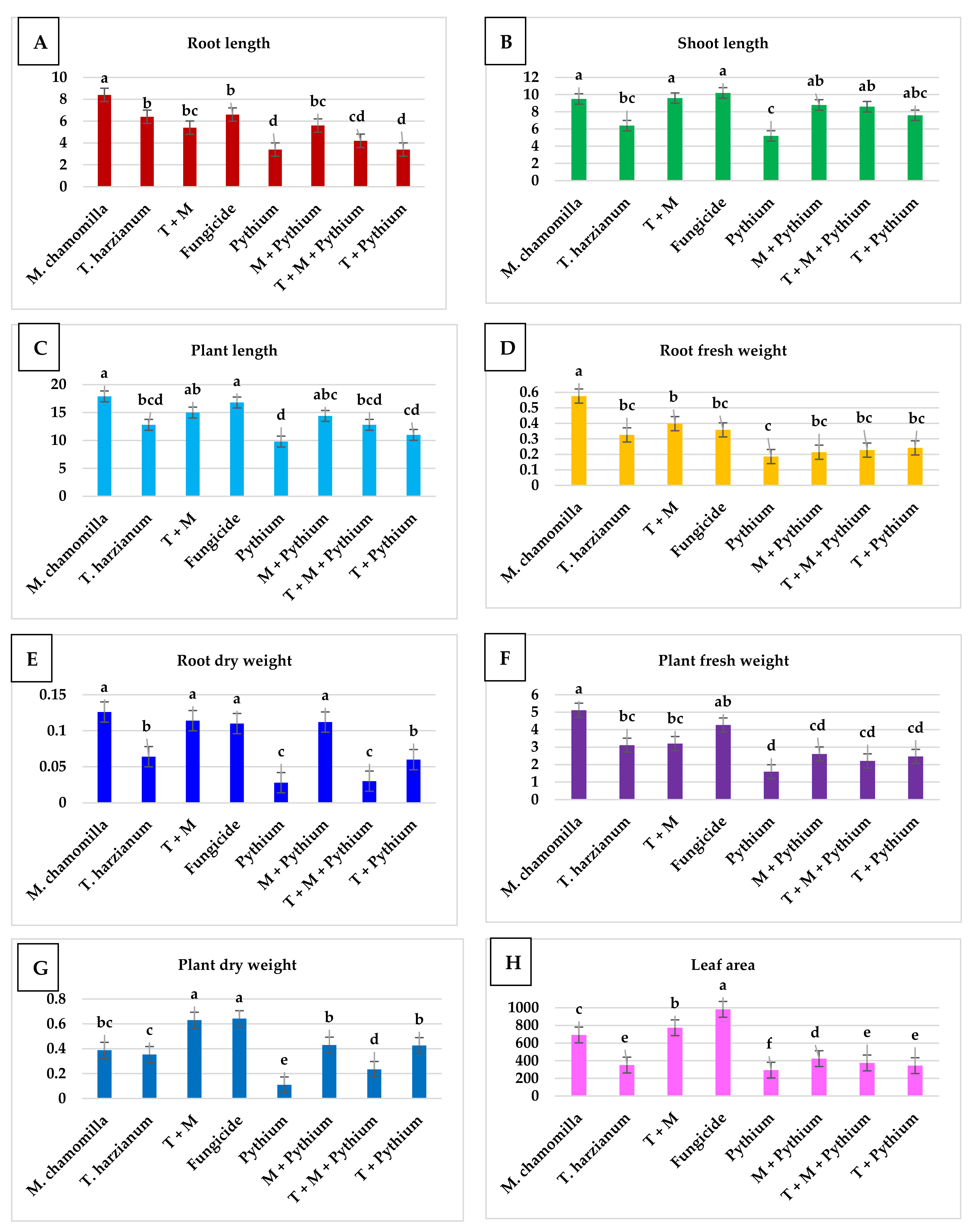
2.2.2. Morphological Features of Bean Plants
2.2.3. Physiological Characters
- Total phenols, total flavonoids, defense related enzymes and antioxidant capacity
- Photosynthesis pigments
2.2.4. Disease Symptoms of Bean (Phaseolus vulgaris, L.) as Pythium Pathogen Infection
3. Materials and Methods
3.1. Preparation of Matricaria Chamomilla Flower Extract
3.2. Fractionation and Identification of Phenolic Compounds
3.3. Evaluation of Chamomile Flower Extracts Concentrations on Pythium Ultimum Growth
3.4. Antifungal activity of Trichoderma Harzianum
- Dual Culture Assay
3.5. Evaluation of M. Chamomilla Flower Extract Concentration on T. harzianum Linear Growth
3.6. Evaluation of Chamomile Flower Extract on T. harzianum Sporulation
3.7. Greenhouse Experiment
3.7.1. Inoculum Preparation
- The inoculum of Pythium
- T. harzianum inoculum
3.7.2. Greenhouse Evaluation of Chamomile Flower Extract and/or T. harzianum on Phaseolus vulgaris Pathogen
- Disease assessment
- Vegetative growth parameters
- Chlorophylls and carotenoids content of investigated leaves
- Total polyphenols content
- Total flavonoids content
- Determination of antioxidant activity by the DPPH and ABTS radicals scavenging methods
- Polyphenol oxidase and peroxidase activities
3.8. SEM Analysis
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Binagwa, P.H.; Bonsi, C.K.; Msolla, S.N. Evaluation of common bean (Phaseolus vulgaris) genotypes for resistance to root rot disease caused by Pythium aphanidermatum and Pythium splendens under screen house conditions. J. Nat. Sci. Res. 2016, 6, 36–43. [Google Scholar]
- Kiptoo, G.J.; Kinyua, M.G.; Kiplagat, O.K. Evaluation of phenolic content of common bean (Phaseolus vulgaris L.) in association to bean fly (Ophiomyia spp.) infestation. Int. J. Agron. Agric. Res. 2019, 14, 9–13. [Google Scholar]
- Devi, M.; Dhanalakshmi, S.; Govindarajan, G.T.; Tanisha, B.; Sonalika, T.; Ruth, J.; Avinash, T.; Sri, C.J.; Logeswaran, K.; Ramasamy, M.N. A Review on Phaseolus vulgaris Linn. Pharmacogn. J. 2020, 12. [Google Scholar] [CrossRef]
- Schwartz, H.F.; Steadman, J.R.; Hall, R.; Forster, R.L. Compendium of Bean Diseases; American Phytopathological Society (APS Press): Sherman, CT, USA, 2005. [Google Scholar]
- Al-Mahmooli, I.; Al-Fahdi, A.; Al-Sadi, A.; Deadman, M. First report of root rot and crown necrosis caused by Pythium aphanidermatum on Phaseolus vulgaris in Oman. Dis. Notes 2015, 99, 419. [Google Scholar] [CrossRef]
- Haritha, V.; Gopal, K.; Madhnsudhan, P.; Viswanath, K.; Rao, S. Integrated Management of Damping-off Disease Incited by Pythium aphanidermatum (Edson) Fitzp. in Tobacco Nursery. Plant Dis. Sci. 2010, 5, 41–47. [Google Scholar]
- Kilany, M.; Ibrahim, E.H.; Al Amry, S.; Al Roman, S.; Siddiqi, S. Microbial suppressiveness of Pythium damping-Off diseases. In Organic Amendments and Soil Suppressiveness in Plant Disease Management; Springer: Zurich, Switzerland, 2015; pp. 187–206. [Google Scholar]
- Mukuma, C.; Godoy-Lutz, G.; Eskridge, K.; Steadman, J.; Urrea, C.; Muimui, K. Use of culture and molecular methods for identification and characterization of dry bean fungal root rot pathogens in Zambia. Trop. Plant Pathol. 2020, 45, 385–396. [Google Scholar] [CrossRef]
- Lucas, B.; Griffiths, P.D. Evaluation of common bean accessions for resistance to Pythium ultimum. HortScience 2004, 39, 1193–1195. [Google Scholar] [CrossRef]
- Abeysinghe, S. Systemic resistance induced by Trichoderma harzianum RU01 against Uromyces appendiculatus on Phaseolus vulgaris. J. Natl. Sci. Found. Sri Lanka 2009, 37. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.N. Biological control of soil borne plant pathogens by Trichoderma spp. Indian J. Mycol. Pathol. 1987, 17, 1–10. [Google Scholar]
- Boughalleb-M’Hamdi, N.; Salem, I.B.; M’Hamdi, M. Evaluation of the efficiency of Trichoderma, Penicillium, and Aspergillus species as biological control agents against four soil-borne fungi of melon and watermelon. Egypt. J. Biol. Pest Control 2018, 28, 1–12. [Google Scholar]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, D.; Wilson, C. The process of antagonism of Sclerotium cepivorum in white rot affected onion roots by Trichoderma koningii. Plant Pathol. 2001, 50, 249–257. [Google Scholar] [CrossRef]
- Negi, S.; Bharat, N.K.; Kaushal, R.; Rohiwala, P. Screening of bioagents for seed biopriming in French bean (Phaseolus vulgaris L.) under Laboratory condition. IJCS 2020, 8, 790–793. [Google Scholar] [CrossRef]
- Howell, C. Cotton seedling preemergence damping-off incited by Rhizopus oryzae and Pythium spp. and its biological control with Trichoderma spp. Phytopathology 2002, 92, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Maheshwary, N.; Gangadhara Naik, B.; Amoghavarsha Chittaragi, M.; Naik, S.K.; Nandish, M. Compatibility of Trichoderma asperellum with fungicides. Pharma Innov. J. 2020, 9, 136–140. [Google Scholar]
- Ghisalberti, E.; Sivasithamparam, K. Biochemistry, Antifungal antibiotics produced by Trichoderma spp. Soil Biol. Biochem. 1991, 23, 1011–1020. [Google Scholar] [CrossRef]
- Ahmed, M.; El-Fiki, I. Effect of biological control of root rot diseases of strawberry using Trichoderma spp. Middle East J. Appl. Sci. 2017, 7, 482–492. [Google Scholar]
- Hasan, M.F.; Islam, M.A.; Sikdar, B. Evaluation of possible biological control of Fusarium sp. using plant extracts and antagonistic species of microbes in vitro. Eur. PMC Plus 2020, 9, 1394. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Orlikowski, L.; Skrzypczak, C. Biocides in the control of soil-borne and leaf pathogens. Ital. J. Agron. 2003, 22, 426–433. [Google Scholar]
- Putnik, P.; Bursać Kovačević, D.; Režek Jambrak, A.; Barba, F.J.; Cravotto, G.; Binello, A.; Lorenzo, J.M.; Shpigelman, A. Innovative “green” and novel strategies for the extraction of bioactive added value compounds from citrus wastes—A review. Molecules 2017, 22, 680. [Google Scholar] [CrossRef]
- Akkari, H.; B’chir, F.; Hajaji, S.; Rekik, M.; Sebai, E.; Hamza, H.; Darghouth, M.; Gharbi, M. Potential anthelmintic effect of Capparis spinosa (Capparidaceae) as related to its polyphenolic content and antioxidant activity. Vet. Med. 2016, 61, 308–316. [Google Scholar] [CrossRef]
- Najjaa, H.; Abdelkarim, B.A.; Doria, E.; Boubakri, A.; Trabelsi, N.; Falleh, H.; Tlili, H.; Neffati, M. Phenolic composition of some Tunisian medicinal plants associated with anti-proliferative effect on human breast cancer MCF-7 cells. EuroBiotech. J. 2020, 4, 104–112. [Google Scholar] [CrossRef]
- Žlabur, J.Š.; Žutić, I.; Radman, S.; Pleša, M.; Brnčić, M.; Barba, F.J.; Rocchetti, G.; Lucini, L.; Lorenzo, J.M.; Domínguez, R. Effect of different green extraction methods and solvents on bioactive components of chamomile (Matricaria chamomilla L.) flowers. Molecules 2020, 25, 810. [Google Scholar] [CrossRef]
- Raal, A.; Orav, A.; Püssa, T.; Valner, C.; Malmiste, B.; Arak, E. Content of essential oil, terpenoids and polyphenols in commercial chamomile (Chamomilla recutita L. Rauschert) teas from different countries. Food Chem. 2012, 131, 632–638. [Google Scholar] [CrossRef]
- Sotiropoulou, N.S.; Megremi, S.F.; Tarantilis, P. Evaluation of antioxidant activity, toxicity, and phenolic profile of aqueous extracts of chamomile (Matricaria chamomilla L.) and sage (Salvia officinalis L.) prepared at different temperatures. Appl. Sci. 2020, 10, 2270. [Google Scholar] [CrossRef]
- Da Silva Pinto, M. Tea: A new perspective on health benefits. Food Res. Int. 2013, 53, 558–567. [Google Scholar] [CrossRef]
- Jiang, T.; Zhan, S.; Li, S.; Zhu, Z.; He, J.; Lorenzo, J.M.; Barba, F.J. From ‘green’technologies to ‘red’antioxidant compounds extraction of purple corn: A combined ultrasound–ultrafiltration–purification approach. J. Sci. Food Agric. 2018, 98, 4919–4927. [Google Scholar] [CrossRef] [PubMed]
- Landeka Jurčević, I.; Dora, M.; Guberović, I.; Petras, M.; Rimac Brnčić, S.; Đikić, D. Polyphenols from wine lees as a novel functional bioactive compound in the protection against oxidative stress and hyperlipidaemia. Food Technol. Biotechnol. 2017, 55, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Agregan, R.; Munekata, P.E.; Franco, D.; Dominguez, R.; Carballo, J.; Lorenzo, J.M. Phenolic compounds from three brown seaweed species using LC-DAD–ESI-MS/MS. Food Res. Int. 2017, 99, 979–985. [Google Scholar] [CrossRef]
- Meziadi, C.; Richard, M.M.; Derquennes, A.; Thareau, V.; Blanchet, S.; Gratias, A.; Pflieger, S.; Geffroy, V. Development of molecular markers linked to disease resistance genes in common bean based on whole genome sequence. Plant Sci. 2016, 242, 351–357. [Google Scholar] [CrossRef]
- Sendi, Y.; Pfeiffer, T.; Koch, E.; Mhadhbi, H.; Mrabet, M. Potential of common bean (Phaseolus vulgaris L.) root microbiome in the biocontrol of root rot disease and traits of performance. J. Plant Dis. Prot. 2020, 127, 453–462. [Google Scholar] [CrossRef]
- Martins, S.A.; Schurt, D.A.; Seabra, S.S.; Martins, S.J.; Ramalho, M.A.P.; de Souza Moreira, F.M.; da Silva, J.C.P.; da Silva, J.A.G.; de Medeiros, F.H.V. Common bean (Phaseolus vulgaris L.) growth promotion and biocontrol by rhizobacteria under Rhizoctonia solani suppressive and conducive soils. Appl. Soil Ecol. 2018, 127, 129–135. [Google Scholar] [CrossRef]
- Ellis, C.; Karafyllidis, I.; Turner, J.G. Constitutive activation of jasmonate signaling in an Arabidopsis mutant correlates with enhanced resistance to Erysiphe cichoracearum, Pseudomonas syringae, and Myzus persicae. Mol. Plant Microbe Interact. 2002, 15, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Lingk, W. Health risk evaluation of pesticide contaminations in drinking water. Occup. Dis. Environ. Med. 1991, 6, 119–129. [Google Scholar] [CrossRef][Green Version]
- Surekha, C.; Neelapu, N.; Kamala, G.; Prasad, B.S.; Ganesh, P.S. Efficacy of Trichoderma viride to induce disease resistance and antioxidant responses in legume Vigna mungo infested by Fusarium oxysporum and Alternaria alternata. Int. J. Agric. Sci. Res. 2013, 3, 285–294. [Google Scholar]
- Gajera, H.P.; Bambharolia, R.P.; Patel, S.V.; Khatrani, T.J.; Goalkiya, B.A. Antagonism of Trichoderma spp. against Macrophomina phaseolina: Evaluation of coiling and cell wall degrading enzymatic activities. J. Plant Pathol. Microb. 2012, 3–7. [Google Scholar] [CrossRef]
- Nasir Hussein, A.; Abbasi, S.; Sharifi, R.; Jamali, S. The effect of biocontrol agents consortia against Rhizoctonia root rot of common bean Phaseolus vulgaris. J. Crop Prot. 2018, 7, 73–85. [Google Scholar]
- Rajkonda, J.; Sawant, V.; Ambuse, M.; Bhale, U. Inimical potential of Trichoderma species against pathogenic fungi. Plant Sci. Feed. 2011, 1, 10–13. [Google Scholar]
- El-Khateeb, N.M.; Nehela, Y. The dual inoculation with Rhizobium sp. and cyanobacterial extracts enhances the common bean (Phaseolus vulgaris L.) responses to white rot disease caused by Sclerotinia sclerotiorum. Middle East J. Appl. Sci. 2019, 872–887. [Google Scholar]
- Jones, E.; Stewart, A. Biological control of Sclerotinia minor in lettuce using Trichoderma species. In Proceedings of the New Zealand Plant Protection Conference, Canterbury, New Zealand, 19 August 1997; pp. 154–158. [Google Scholar] [CrossRef]
- McLean, K.; Hunt, J.; Stewart, A. Compatibility of the biocontrol agent Trichoderma harzianum C52 with selected fungicides. N. Z. Plant Prot. 2001, 54, 84–88. [Google Scholar] [CrossRef]
- Ghoniem, A.A.; Rashad, E.M.; El-Khateeb, A.Y.; Saber, W.I. Bacteriological Therapeutic-Based Strategy for Management of Fusarium Wilt Disease in Tomato Plants. Available online: https://www.semanticscholar.org/paper/Bacteriological-Therapeutic-Based-Strategy-for-of-Ghoniem-Rashad/517b4554902636dda3f4bdcb4e3f42b15a93f986 (accessed on 22 February 2021).
- Agamy, R.; Alamri, S.; Moustafa, M.F.; Hashem, M. Management of tomato leaf spot caused by Alternaria tenuissima Wiltshire using salicylic acid and agrileen. Int. J. Agric. Biol. 2013, 15, 266–272. [Google Scholar]
- Al-Askar, A.; Ezzat, A.; Ghoneem, K.; Saber, W.J.E. Trichoderma harzianum WKY5 and its Gibberellic Acid Control of Rhizoctonia solani, Improve Sprouting, Growth and Productivity of Potato. Egypt. J. Biol. 2016, 26, 787–796. [Google Scholar]
- Hoyos-Carvajal, L.; Orduz, S.; Bissett, J. Growth stimulation in bean (Phaseolus vulgaris L.) by Trichoderma. Biol. Control 2009, 51, 409–416. [Google Scholar] [CrossRef]
- Yu, T.; Zheng, X.D. Salicylic acid enhances biocontrol efficacy of the antagonist Cryptococcus laurentii in apple fruit. J. Plant Growth Regul. 2006, 25, 166–174. [Google Scholar] [CrossRef]
- Raats, P. Effect of Trichoderma species on damping off diseases incidence, some plant enzymes activity and nutritional status of bean plants. J. Am. Sci. 2012, 2, 13–25. [Google Scholar]
- Gajera, H.; Domadiya, R.; Patel, S.; Kapopara, M.; Golakiya, B. Molecular mechanism of Trichoderma as bio-control agents against phytopathogen system–a review. Curr. Res. Microbiol. Biotechnol. 2013, 1, 133–142. [Google Scholar]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Woo, S.L.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Ruocco, M.; Lanzuise, S. Trichoderma secondary metabolites active on plants and fungal pathogens. Open Mycol. J. 2014, 8, 127–139. [Google Scholar] [CrossRef]
- Kredics, L.; Manczinger, L.; Antal, Z.; Pénzes, Z.; Szekeres, A.; Kevei, F.; Nagy, E.J. In vitro water activity and pH dependence of mycelial growth and extracellular enzyme activities of Trichoderma strains with biocontrol potential. J. Appl. Microbiol. 2004, 96, 491–498. [Google Scholar] [CrossRef]
- Beck, C.B. An Introduction to Plant Structure and Development: Plant Anatomy for the Twenty-First Century; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- El-Samra, I.; Noman, K.; Fayed, M.; El-Farnawany, M.J. Studies on some histopathological and enzymatic activity aspects during the Rhizoctonia damping-off disease of beans. J. Agric. Sci. Mansoura Univ. Egypt 1994, 10, 2209–2225. [Google Scholar]
- El-Hai, K.; El-Metwally, M.; El-Baz, S. Reduction of soybean root and stalk rots by growth substances under salt stress conditions. Plant. Pathol. J. 2010, 9, 149–161. [Google Scholar] [CrossRef]
- El-Hai, A.; Ali, A.A. Amelioration of the Structural and Biochemical Features of Kidney Bean against Root Rot and Rust Diseases. J. Plant Prot. Pathol. 2018, 9, 237–245. [Google Scholar]
- Yadeta, K.; Thomma, B. The xylem as battleground for plant hosts and vascular wilt pathogens. Front Plant Sci. 2013, 4, 97. [Google Scholar] [CrossRef]
- Ploetz, R.C.; Kendra, P.E.; Choudhury, R.A.; Rollins, J.A.; Campbell, A.; Garrett, K.; Hughes, M.; Dreaden, T. Laurel wilt in natural and agricultural ecosystems: Understanding the drivers and scales of complex pathosystems. Forests 2017, 8, 48. [Google Scholar] [CrossRef]
- Dent, M.; Dragović-Uzelac, V.; Penić, M.; Bosiljkov, T.; Levaj, B. The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in Dalmatian wild sage (Salvia officinalis L.) extracts. Food Technol. Biotechnol. 2013, 51, 84–91. [Google Scholar]
- Määttä, K.R.; Kamal-Eldin, A.; Törrönen, A.R. High-performance liquid chromatography (HPLC) analysis of phenolic compounds in berries with diode array and electrospray ionization mass spectrometric (MS) detection: Ribes species. J. Agric. Food Chem. 2003, 51, 6736–6744. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, O.; Sinclair, J. Basic Plant Pathology Methods, 2nd ed.; Lewis Publishers: Boca Raton, FL, USA, 1995. [Google Scholar]
- Bell, D.; Wells, H.; Markham, C. In vitro antagonism of Trichoderma species against six fungal plant pathogens. Phytopathology 1982, 72, 379–382. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Mackinney, G. Absorption of light by chlorophyll solutions. J. Biol. Chem. 1941, 140, 315–322. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; De Mello, J.C. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Das, S.; Ray, A.; Nasim, N.; Nayak, S.; Mohanty, S. Effect of different extraction techniques on total phenolic and flavonoid contents, and antioxidant activity of betelvine and quantification of its phenolic constituents by validated HPTLC method. 3 Biotech 2019, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Christodouleas, D.C.; Fotakis, C.; Nikokavoura, A.; Papadopoulos, K.; Calokerinos, A.C. Modified DPPH and ABTS assays to assess the antioxidant profile of untreated oils. Food Anal. Methods 2015, 8, 1294–1302. [Google Scholar] [CrossRef]
- Seleim, M.; Abo-Elyousr, K.; Mohamed, A.; Al-Marzoky, H. Peroxidase and polyphenoloxidase activities as biochemical markers for biocontrol efficacy in the control of tomato bacterial wilt. J. Plant Physiol. Pathol. 2014, 2, 2–8. [Google Scholar] [CrossRef]
- Caldwell, D.; Iyer-Pascuzzi, A.S. A Scanning Electron Microscopy Technique for Viewing Plant-Microbe Interactions at Tissue and Cell-Type Resolution. Phytopathology 2019, 109, 1302–1311. [Google Scholar] [CrossRef] [PubMed]


| Compound/Structure | Retention Time (min) | Concentration (ppm) | Compound/Structure | Retention Time (min) | Concentration (ppm) |
|---|---|---|---|---|---|
 Syringic acid | 3.431 | 137.446 |  Myricetin | 12.850 | 1587.823 |
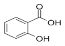 Salicylic acid | 3.733 | 66.672 | 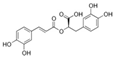 Rosmarinic acid | 13.624 | 370.598 |
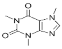 Caffeine | 6.215 | 79.975 | 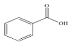 Benzoic acid | 13.797 | 414.887 |
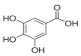 Gallic acid | 6.813 | 77.146 | 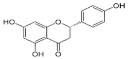 Naringenin | 13.977 | 400.997 |
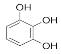 Pyrogallol | 7.245 | 324.612 | 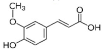 Ferulic acid | 15.243 | 47.982 |
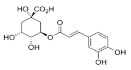 Chlorogenic acid | 8.493 | 38.02 | 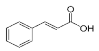 Cinnamic acid | 15.410 | 9.992 |
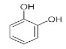 Catechol | 9.621 | 11.289 |  Kaempferol | 16.766 | 118.772 |
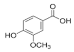 Vanillic acid | 10.312 | 112.577 |  Vanillin | 19.026 | 17.298 |
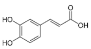 Caffeic acid | 10.483 | 99.691 |  p-Hydroxybenzoic acid | 20.451 | 30.563 |
 Quercetin | 11.305 | 927.727 | 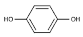 Hydroquinone | 20.779 | 20.133 |
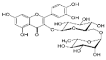 Rutin | 11.569 | 127.074 | 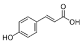 p-Coumaric acid | 22.467 | 23.591 |
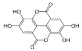 Ellagic acid | 12.507 | 17.016 |  o-Coumaric acid | 24.345 | 20.999 |
| Treatments | Rotted Seeds | Infected Seedling | Survival% |
|---|---|---|---|
| M | 30 b | 12 a | 58 bc |
| T. harzianum | 38 b | 8 ab | 54 bc |
| T + M | 28 b | 8 ab | 64 b |
| Fungcide | 12 c | 4 b | 84 a |
| Pythium | 56 a | 14 a | 30 d |
| Ex + Pythium | 32 b | 12 a | 64 b |
| T + M + Pythium | 38 b | 12 a | 50 bc |
| T + Pythium | 40 b | 16 a | 44 cd |
| Treatment | Total Polyphenols | Total Flavonoids | Polyphenol Oxidase | Peroxidase | ABTS Inhibition% | DPPH Inhibition% |
|---|---|---|---|---|---|---|
| M. chamomilla | 28.699 d | 15.056 d | 9.776 d | 0.619 d | 29.936 d | 13.677 e |
| T. harzianum | 27.755 d | 14.189 d | 9.414 d | 0.599 d | 21.726 e | 11.495 f |
| T + M | 43.222 a | 28.469 a | 15.353 a | 0.948 a | 58.234 a | 26.151 a |
| Fungcide | 40.960 b | 26.381 b | 11.152 c | 0.897 b | 55.647 b | 25.036 b |
| Pythium | 26.071 e | 12.632 e | 8.766 e | 0.561 e | 14.129 g | 7.147 h |
| M + Pythium | 32.945 c | 18.980 c | 11.408 c | 0.716 c | 33.658 c | 17.919 d |
| T + M + Pythium | 40.079 b | 25.575 b | 14.150 b | 0.877 b | 34.495 c | 19.557 c |
| Treatment | Chlorophyll A | Chlorophyll B | Total Chlorophyll | Carotenoids |
|---|---|---|---|---|
| M. chamomilla | 2.814 a | 1.651 b | 4.466 b | 0.679 c |
| T. harzianum | 2.123 b | 1.418 c | 3.542 d | 0.578 d |
| T + M | 2.908 a | 1.875 a | 4.784 a | 0.934 a |
| Fungcide | 1.592 d | 1.180 e | 2.771 f | 0.529 e |
| Pythium | 1.526 d | 1.113 e | 2.639 f | 0.286 f |
| M + Pythium | 2.136 b | 1.430 c | 3.566 d | 0.894 b |
| T + M + Pythium | 2.248 b | 1.598 b | 3.846 c | 0.597 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghoniem, A.A.; Abd El-Hai, K.M.; El-khateeb, A.Y.; Eldadamony, N.M.; Mahmoud, S.F.; Elsayed, A. Enhancing the Potentiality of Trichoderma harzianum against Pythium Pathogen of Beans Using Chamomile (Matricaria chamomilla, L.) Flower Extract. Molecules 2021, 26, 1178. https://doi.org/10.3390/molecules26041178
Ghoniem AA, Abd El-Hai KM, El-khateeb AY, Eldadamony NM, Mahmoud SF, Elsayed A. Enhancing the Potentiality of Trichoderma harzianum against Pythium Pathogen of Beans Using Chamomile (Matricaria chamomilla, L.) Flower Extract. Molecules. 2021; 26(4):1178. https://doi.org/10.3390/molecules26041178
Chicago/Turabian StyleGhoniem, Abeer Abdulkhalek, Kamar M. Abd El-Hai, Ayman Y. El-khateeb, Noha M. Eldadamony, Samy F. Mahmoud, and Ashraf Elsayed. 2021. "Enhancing the Potentiality of Trichoderma harzianum against Pythium Pathogen of Beans Using Chamomile (Matricaria chamomilla, L.) Flower Extract" Molecules 26, no. 4: 1178. https://doi.org/10.3390/molecules26041178
APA StyleGhoniem, A. A., Abd El-Hai, K. M., El-khateeb, A. Y., Eldadamony, N. M., Mahmoud, S. F., & Elsayed, A. (2021). Enhancing the Potentiality of Trichoderma harzianum against Pythium Pathogen of Beans Using Chamomile (Matricaria chamomilla, L.) Flower Extract. Molecules, 26(4), 1178. https://doi.org/10.3390/molecules26041178







