A Systematic Review of the Effects of Equol (Soy Metabolite) on Breast Cancer
Abstract
1. Introduction
2. Results
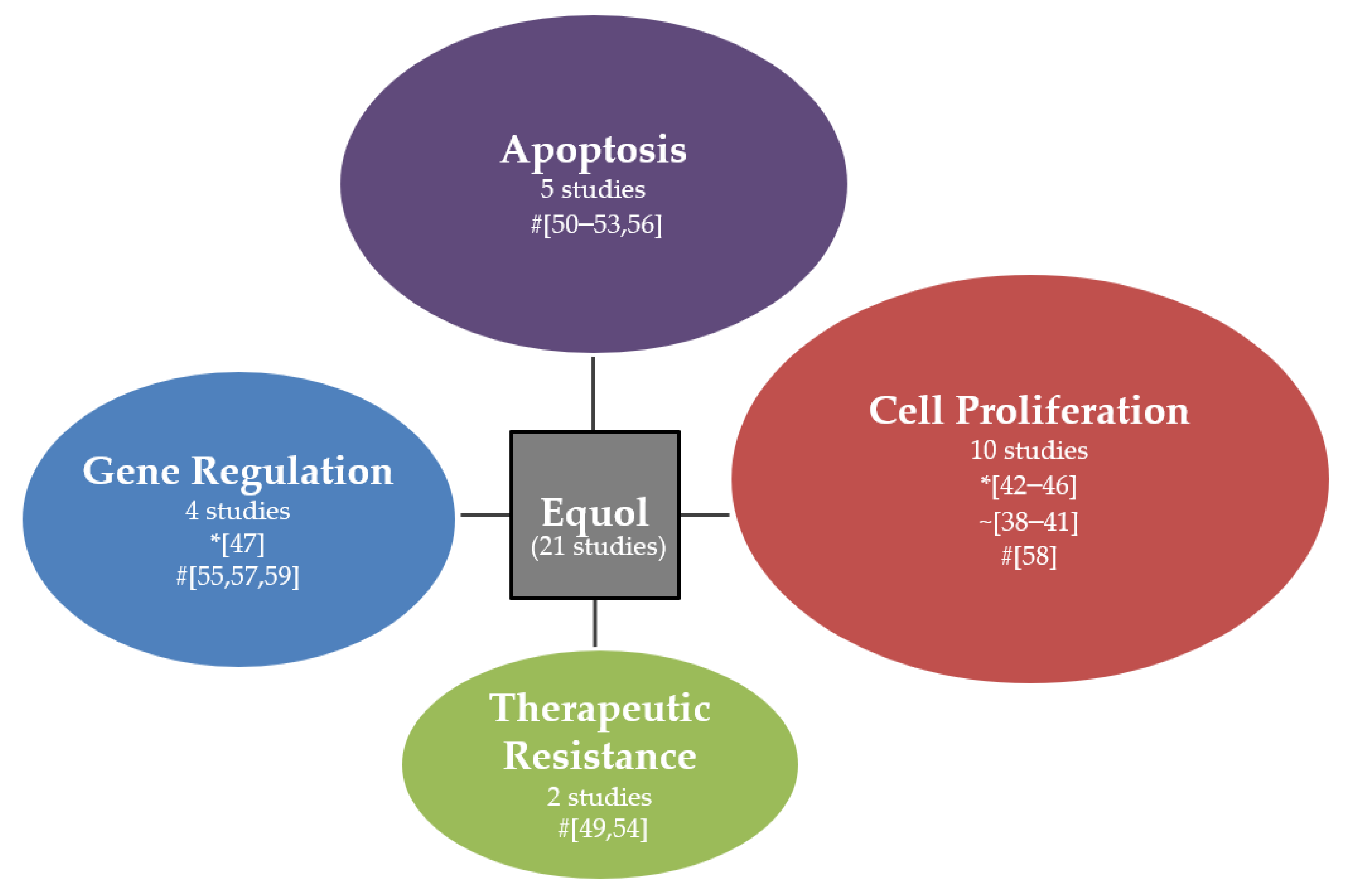
2.1. Cancer Properties of Equol
2.1.1. In Vitro Studies
2.1.2. MCF-7 Cells
2.1.3. MDA-MB-231 Cells
2.1.4. MDA-MB-453 Cells
2.1.5. In Vivo Studies
2.1.6. Mixed Studies
2.2. Concentration Variations of Equol
2.3. Oncogenic Pathways and Mechanism
3. Discussion
4. Methods
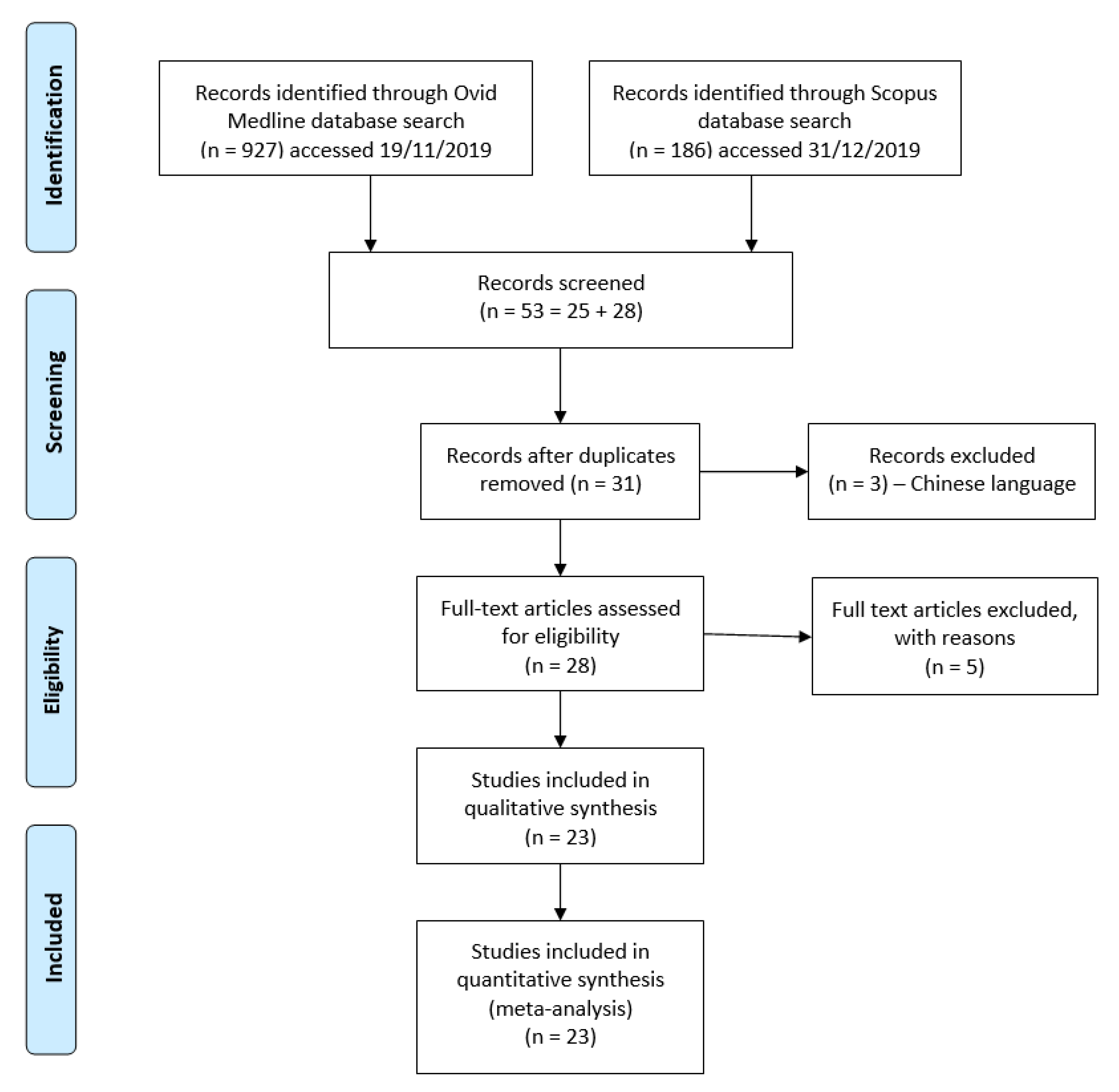
Search Strategy and Selection Criteria
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arai, Y.; Uehara, M.; Sato, Y.; Kimira, M.; Eboshida, A.; Adlercreutz, H.; Watanabe, S. Comparison of isoflavones among dietary intake, plasma concentration and urinary excretion for accurate estimation of phytoestrogen intake. J. Epidemiol. 2000, 10, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Frankenfeld, C.L.; Lampe, J.W. Gut bacterial metabolism of the soy isoflavone daidzein: Exploring the relevance to human health. Exp. Biol. Med. 2005, 230, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Haron, H.; Ismail, A.; Azlan, A.; Shahar, S.; Loh, S.P. Daidzein and genistein contents in tempeh and selected soy products. Food Chem. 2009, 115, 1350–1356. [Google Scholar] [CrossRef]
- Messina, M. Soyfoods and soybean phyto-oestrogens (isoflavones) as possible alternatives to hormone replacement therapy (HRT). Eur. J. Cancer 2000, 36 (Suppl. 4), S71–S72. [Google Scholar] [CrossRef]
- Valsta, L.M.; Kilkkinen, A.; Mazur, W.; Nurmi, T.; Lampi, A.M.; Ovaskainen, M.L.; Korhonen, T.; Adlercreutz, H.; Pietinen, P. Phyto-oestrogen database of foods and average intake in Finland. Br. J. Nutr. 2003, 89 (Suppl. 1), S31–S38. [Google Scholar] [CrossRef]
- Mulligan, A.A.; Welch, A.A.; McTaggart, A.A.; Bhaniani, A.; Bingham, S.A. Intakes and sources of soya foods and isoflavones in a UK population cohort study (EPIC-Norfolk). Eur. J. Clin. Nutr. 2007, 61, 248–254. [Google Scholar] [CrossRef]
- Tang, L.; Lee, A.H.; Xu, F.; Zhang, T.; Lei, J.; Binns, C.W. Soya and isoflavone intakes associated with reduced risk of oesophageal cancer in north-west China. Public Health Nutr. 2015, 18, 130–134. [Google Scholar] [CrossRef]
- Kimira, M.; Arai, Y.; Shimoi, K.; Watanabe, S. Japanese intake of flavonoids and isoflavonoids from foods. J. Epidemiol. 1998, 8, 168–175. [Google Scholar] [CrossRef]
- Frankenfeld, C.L. O-desmethylangolensin: The importance of equol’s lesser known cousin to human health. Adv. Nutr. 2011, 2, 317–324. [Google Scholar] [CrossRef]
- Yokoyama, S.; Suzuki, T. Isolation and characterization of a novel equol-producing bacterium from human feces. Biosci. Biotechnol. Biochem. 2008, 72, 2660–2666. [Google Scholar] [CrossRef]
- Tsuji, H.; Moriyama, K.; Nomoto, K.; Miyanaga, N.; Akaza, H. Isolation and characterization of the equol-producing bacterium Slackia sp. strain NATTS. Arch. Microbiol. 2010, 192, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Lampe, J.W. Is equol the key to the efficacy of soy foods? Am. J. Clin. Nutr. 2009, 89, 1664S–1667S. [Google Scholar] [CrossRef] [PubMed]
- Marrian, G.F.; Haslewood, G.A. Equol, a new inactive phenol isolated from the ketohydroxyoestrin fraction of mares’ urine. Biochem. J. 1932, 26, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Axelson, M.; Setchell, K.D. The excretion of lignans in rats-evidence for an intestinal bacterial source for this new group of compounds. FEBS Lett. 1981, 123, 337–342. [Google Scholar] [CrossRef]
- Nottle, M.C. Composition of some urinary calculi of ruminants in Western Australia. Res. Vet. Sci. 1976, 21, 309–317. [Google Scholar] [CrossRef]
- Saitoh, S.; Sato, T.; Harada, H.; Matsuda, T. Biotransformation of soy isoflavone-glycosides in laying hens: Intestinal absorption and preferential accumulation into egg yolk of equol, a more estrogenic metabolite of daidzein. Biochim. Biophys. Acta 2004, 1674, 122–130. [Google Scholar] [CrossRef]
- Juniewicz, P.E.; Pallante Morell, S.; Moser, A.; Ewing, L.L. Identification of phytoestrogens in the urine of male dogs. J. Steroid Biochem. 1988, 31, 987–994. [Google Scholar] [CrossRef]
- Setchell, K.D.; Clerici, C. Equol: History, chemistry, and formation. J. Nutr. 2010, 140, 1355S–1362S. [Google Scholar] [CrossRef]
- Chang, H.S.; Robinson, A.R.; Common, R.H. Lability of equol to acidic hydrolysis procedures. Anal. Biochem. 1975, 63, 290–292. [Google Scholar] [CrossRef]
- Setchell, K.D.; Borriello, S.P.; Hulme, P.; Kirk, D.N.; Axelson, M. Nonsteroidal estrogens of dietary origin: Possible roles in hormone-dependent disease. Am. J. Clin. Nutr. 1984, 40, 569–578. [Google Scholar] [CrossRef]
- Setchell, K.D.; Brown, N.M.; Lydeking-Olsen, E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar] [CrossRef]
- Daily, J.W.; Ko, B.S.; Ryuk, J.; Liu, M.; Zhang, W.; Park, S. Equol Decreases Hot Flashes in Postmenopausal Women: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Med. Food 2019, 22, 127–139. [Google Scholar] [CrossRef]
- Yoshikata, R.; Myint, K.Z.Y.; Ohta, H. Effects of Equol Supplement on Bone and Cardiovascular Parameters in Middle-Aged Japanese Women: A Prospective Observational Study. J. Altern. Complement. Med. 2018, 24, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Akaza, H.; Miyanaga, N.; Takashima, N.; Naito, S.; Hirao, Y.; Tsukamoto, T.; Fujioka, T.; Mori, M.; Kim, W.J.; Song, J.M.; et al. Comparisons of percent equol producers between prostate cancer patients and controls: Case-controlled studies of isoflavones in Japanese, Korean and American residents. Jpn. J. Clin. Oncol. 2004, 34, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.M.; Merz-Demlow, B.E.; Xu, X.; Phipps, W.R.; Kurzer, M.S. Premenopausal equol excretors show plasma hormone profiles associated with lowered risk of breast cancer. Cancer Epidemiol. Biomark. Prev. 2000, 9, 581–586. [Google Scholar]
- Lampe, J.W.; Karr, S.C.; Hutchins, A.M.; Slavin, J.L. Urinary equol excretion with a soy challenge: Influence of habitual diet. Proc. Soc. Exp. Biol. Med. 1998, 217, 335–339. [Google Scholar] [CrossRef]
- Newton, K.M.; Reed, S.D.; Uchiyama, S.; Qu, C.; Ueno, T.; Iwashita, S.; Gunderson, G.; Fuller, S.; Lampe, J.W. A cross-sectional study of equol producer status and self-reported vasomotor symptoms. Menopause 2015, 22, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.C.; O’Brien, B.; McCormack, T. Equol producer status, salivary estradiol profile and urinary excretion of isoflavones in Irish Caucasian women, following ingestion of soymilk. Steroids 2007, 72, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.W.; Ko, K.P.; Ahn, Y.; Kim, C.S.; Park, S.J.; Park, J.K.; Kim, S.S.; Kim, Y. Epidemiological profiles between equol producers and nonproducers: A genomewide association study of the equol-producing phenotype. Genes Nutr. 2012, 7, 567–574. [Google Scholar] [CrossRef]
- Abiru, Y.; Kumemura, M.; Ueno, T.; Uchiyama, S.; Masaki, K. Discovery of an S-equol rich food stinky tofu, a traditional fermented soy product in Taiwan. Int. J. Food Sci. Nutr. 2012, 63, 964–970. [Google Scholar] [CrossRef]
- Antignac, J.P.; Cariou, R.; Le Bizec, B.; Cravedi, J.P.; Andre, F. Identification of phytoestrogens in bovine milk using liquid chromatography/electrospray tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Kuhnle, G.G.; Dell’Aquila, C.; Aspinall, S.M.; Runswick, S.A.; Mulligan, A.A.; Bingham, S.A. Phytoestrogen content of foods of animal origin: Dairy products, eggs, meat, fish, and seafood. J. Agric. Food Chem. 2008, 56, 10099–10104. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Cole, S.J. Method of defining equol-producer status and its frequency among vegetarians. J. Nutr. 2006, 136, 2188–2193. [Google Scholar] [CrossRef] [PubMed]
- Muthyala, R.S.; Ju, Y.H.; Sheng, S.; Williams, L.D.; Doerge, D.R.; Katzenellenbogen, B.S.; Helferich, W.G.; Katzenellenbogen, J.A. Equol, a natural estrogenic metabolite from soy isoflavones: Convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg. Med. Chem. 2004, 12, 1559–1567. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Coley, H.M. Mechanisms and strategies to overcome chemotherapy resistance in metastatic breast cancer. Cancer Treat. Rev. 2008, 34, 378–390. [Google Scholar] [CrossRef]
- Foukakis, T.; Fornander, T.; Lekberg, T.; Hellborg, H.; Adolfsson, J.; Bergh, J. Age-specific trends of survival in metastatic breast cancer: 26 years longitudinal data from a population-based cancer registry in Stockholm, Sweden. Breast Cancer Res. Treat. 2011, 130, 553–560. [Google Scholar] [CrossRef]
- Tsuji, M.; Tanaka, T.; Nagashima, R.; Sagisaka, Y.; Tousen, Y.; Nishide, Y.; Ishimi, Y.; Ishimi, Y. Effect of daidzein and equol on DNA replication in MCF-7 cells. J. Biochem. 2018, 163, 371–380. [Google Scholar] [CrossRef]
- Ju, Y.H.; Fultz, J.; Allred, K.F.; Doerge, D.R.; Helferich, W.G. Effects of dietary daidzein and its metabolite, equol, at physiological concentrations on the growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in ovariectomized athymic mice. Carcinogenesis 2006, 27, 856–863. [Google Scholar] [CrossRef]
- Onoda, A.; Ueno, T.; Uchiyama, S.; Hayashi, S.; Kato, K.; Wake, N. Effects of S-equol and natural S-equol supplement (SE5-OH) on the growth of MCF-7 in vitro and as tumors implanted into ovariectomized athymic mice. Food Chem. Toxicol. 2011, 49, 2279–2284. [Google Scholar] [CrossRef]
- Song, H.; Hughes, J.R.; Turner, R.T.; Iwaniec, U.T.; Doerge, D.R.; Helferich, W.G. (+/−)-Equol does not interact with genistein on estrogen-dependent breast tumor growth. Food Chem. Toxicol. 2020, 136, 110979. [Google Scholar] [CrossRef] [PubMed]
- Carreau, C.; Flouriot, G.; Bennetau-Pelissero, C.; Potier, M. Respective contribution exerted by AF-1 and AF-2 transactivation functions in estrogen receptor alpha induced transcriptional activity by isoflavones and equol: Consequence on breast cancer cell proliferation. Mol. Nutr. Food Res. 2009, 53, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Welshons, W.V.; Murphy, C.S.; Koch, R.; Calaf, G.; Jordan, V.C. Stimulation of breast cancer cells in vitro by the environmental estrogen enterolactone and the phytoestrogen equol. Breast Cancer Res. Treat. 1987, 10, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Sathyamoorthy, N.; Wang, T.T. Differential effects of dietary phyto-oestrogens daidzein and equol on human breast cancer MCF-7 cells. Eur. J. Cancer 1997, 33, 2384–2389. [Google Scholar] [CrossRef]
- Liu, H.; Du, J.; Hu, C.; Qi, H.; Wang, X.; Wang, S.; Liu, Q.; Li, Z. Delayed activation of extracellular-signal-regulated kinase 1/2 is involved in genistein- and equol-induced cell proliferation and estrogen-receptor-alpha-mediated transcription in MCF-7 breast cancer cells. J. Nutr. Biochem. 2010, 21, 390–396. [Google Scholar] [CrossRef]
- Tonetti, D.A.; Zhang, Y.; Zhao, H.; Lim, S.B.; Constantinou, A.I. The effect of the phytoestrogens genistein, daidzein, and equol on the growth of tamoxifen-resistant T47D/PKC alpha. Nutr. Cancer 2007, 58, 222–229. [Google Scholar] [CrossRef]
- de la Parra, C.; Borrero-Garcia, L.D.; Cruz-Collazo, A.; Schneider, R.J.; Dharmawardhane, S. Equol, an isoflavone metabolite, regulates cancer cell viability and protein synthesis initiation via c-Myc and eIF4G. J. Biol. Chem. 2015, 290, 6047–6057. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.M.; Belles, C.A.; Lindley, S.L.; Zimmer-Nechemias, L.D.; Zhao, X.; Witte, D.P.; Kim, M.O.; Setchell, K.D. The chemopreventive action of equol enantiomers in a chemically induced animal model of breast cancer. Carcinogenesis 2010, 31, 886–893. [Google Scholar] [CrossRef]
- Rigalli, J.P.; Scholz, P.N.; Tocchetti, G.N.; Ruiz, M.L.; Weiss, J. The phytoestrogens daidzein and equol inhibit the drug transporter BCRP/ABCG2 in breast cancer cells: Potential chemosensitizing effect. Eur. J. Nutr. 2019, 58, 139–150. [Google Scholar] [CrossRef]
- Choi, E.J.; Ahn, W.S.; Bae, S.M. Equol induces apoptosis through cytochrome c-mediated caspases cascade in human breast cancer MDA-MB-453 cells. Chem. Biol. Interact. 2009, 177, 7–11. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, L.; Yu, M.; Liu, X.; Ma, W.; Huang, L.; Li, X.; Ye, X. S-equol inhibits proliferation and promotes apoptosis of human breast cancer MCF-7cells via regulating miR-10a-5p and PI3K/AKT pathway. Arch. Biochem. Biophys. 2019, 672, 108064. [Google Scholar] [CrossRef]
- Ono, M.; Ejima, K.; Higuchi, T.; Takeshima, M.; Wakimoto, R.; Nakano, S. Equol Enhances Apoptosis-inducing Activity of Genistein by Increasing Bax/Bcl-xL Expression Ratio in MCF-7 Human Breast Cancer Cells. Nutr. Cancer 2017, 69, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Kim, T. Equol induced apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 but not MCF-7 cells. Mol. Med. Rep. 2008, 1, 239–244. [Google Scholar] [PubMed]
- Taghizadeh, B.; Ghavami, L.; Nikoofar, A.; Goliaei, B. Equol as a potent radiosensitizer in estrogen receptor-positive and -negative human breast cancer cell lines. Breast Cancer 2015, 22, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Magee, P.J.; Allsopp, P.; Samaletdin, A.; Rowland, I.R. Daidzein, R-(+)equol and S-(-)equol inhibit the invasion of MDA-MB-231 breast cancer cells potentially via the down-regulation of matrix metalloproteinase-2. Eur. J. Nutr. 2014, 53, 345–350. [Google Scholar] [CrossRef]
- Charalambous, C.; Pitta, C.A.; Constantinou, A.I. Equol enhances tamoxifen’s anti-tumor activity by induction of caspase-mediated apoptosis in MCF-7 breast cancer cells. BMC Cancer 2013, 13, 238. [Google Scholar] [CrossRef]
- Bosviel, R.; Durif, J.; Dechelotte, P.; Bignon, Y.J.; Bernard-Gallon, D. Epigenetic modulation of BRCA1 and BRCA2 gene expression by equol in breast cancer cell lines. Br. J. Nutr. 2012, 108, 1187–1193. [Google Scholar] [CrossRef]
- Magee, P.J.; Raschke, M.; Steiner, C.; Duffin, J.G.; Pool-Zobel, B.L.; Jokela, T.; Wahala, K.; Rowland, I.R. Equol: A comparison of the effects of the racemic compound with that of the purified S-enantiomer on the growth, invasion, and DNA integrity of breast and prostate cells in vitro. Nutr. Cancer 2006, 54, 232–242. [Google Scholar] [CrossRef]
- Lechner, D.; Bajna, E.; Adlercreutz, H.; Cross, H.S. Genistein and 17beta-estradiol, but not equol, regulate vitamin D synthesis in human colon and breast cancer cells. Anticancer Res. 2006, 26, 2597–2603. [Google Scholar]
- Ma, D.; Zhang, Y.; Xue, Y.; Wang, P.; Yang, T.; Shao, X. Isoflavone and its metabolite equol inhibit the development of 7,12-dimethylbenz(a)anthracene(DMBA)-induced mammary tumors in normal and ovariectomized rats. J. Funct. Food 2014, 7, 580–589. [Google Scholar] [CrossRef]
- Karimi, B.; Ashrafi, M.; Shomali, T.; Yektaseresht, A. Therapeutic effect of simvastatin on DMBA-induced breast cancer in mice. Fundam. Clin. Pharmacol. 2019, 33, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Maruo, T.; Sakamoto, M.; Ito, C.; Toda, T.; Benno, Y. Adlercreutzia equolifaciens gen. nov., sp. nov., an equol-producing bacterium isolated from human faeces, and emended description of the genus Eggerthella. Int. J. Syst. Evol. Microbiol. 2008, 58, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Tsushida, T.; Shinohara, K. Isolation of an isoflavone-metabolizing, Clostridium-like bacterium, strain TM-40, from human faeces. Anaerobe 2007, 13, 32–35. [Google Scholar] [CrossRef]
- Wang, X.L.; Hur, H.G.; Lee, J.H.; Kim, K.T.; Kim, S.I. Enantioselective synthesis of S-equol from dihydrodaidzein by a newly isolated anaerobic human intestinal bacterium. Appl. Environ. Microbiol. 2005, 71, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Decroos, K.; Vanhemmens, S.; Cattoir, S.; Boon, N.; Verstraete, W. Isolation and characterisation of an equol-producing mixed microbial culture from a human faecal sample and its activity under gastrointestinal conditions. Arch. Microbiol. 2005, 183, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13, 215. [Google Scholar] [CrossRef]
- Subik, K.; Lee, J.F.; Baxter, L.; Strzepek, T.; Costello, D.; Crowley, P.; Xing, L.; Hung, M.C.; Bonfiglio, T.; Hicks, D.G.; et al. The Expression Patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by Immunohistochemical Analysis in Breast Cancer Cell Lines. Breast Cancer 2010, 4, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Holbro, T.; Civenni, G.; Hynes, N.E. The ErbB receptors and their role in cancer progression. Exp. Cell Res. 2003, 284, 99–110. [Google Scholar] [CrossRef]
- Tan, M.; Yu, D. Molecular mechanisms of erbB2-mediated breast cancer chemoresistance. Adv. Exp. Med. Biol. 2007, 608, 119–129. [Google Scholar]
- Denkert, C.; Liedtke, C.; Tutt, A.; von Minckwitz, G. Molecular alterations in triple-negative breast cancer-the road to new treatment strategies. Lancet 2017, 389, 2430–2442. [Google Scholar] [CrossRef]
- Prado-Vazquez, G.; Gamez-Pozo, A.; Trilla-Fuertes, L.; Arevalillo, J.M.; Zapater-Moros, A.; Ferrer-Gomez, M.; Diaz-Almiron, M.; Lopez-Vacas, R.; Navarro, H.; Main, P.; et al. A novel approach to triple-negative breast cancer molecular classification reveals a luminal immune-positive subgroup with good prognoses. Sci. Rep. 2019, 9, 1538. [Google Scholar] [CrossRef] [PubMed]
- Hubalek, M.; Czech, T.; Muller, H. Biological Subtypes of Triple-Negative Breast Cancer. Breast Care 2017, 12, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Peddi, P.F.; Ellis, M.J.; Ma, C. Molecular basis of triple negative breast cancer and implications for therapy. Int. J. Breast Cancer 2012, 2012, 217185. [Google Scholar] [CrossRef]
- Li, L.T.; Jiang, G.; Chen, Q.; Zheng, J.N. Ki67 is a promising molecular target in the diagnosis of cancer (review). Mol. Med. Rep. 2015, 11, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Dias, K.; Dvorkin-Gheva, A.; Hallett, R.M.; Wu, Y.; Hassell, J.; Pond, G.R.; Levine, M.; Whelan, T.; Bane, A.L. Claudin-Low Breast Cancer; Clinical & Pathological Characteristics. PLoS ONE 2017, 12, e0168669. [Google Scholar]
- Fougner, C.; Bergholtz, H.; Norum, J.H.; Sorlie, T. Re-definition of claudin-low as a breast cancer phenotype. Nat. Commun. 2020, 11, 1787. [Google Scholar] [CrossRef]
- Kariya, R.; Matsuda, K.; Gotoh, K.; Vaeteewoottacharn, K.; Hattori, S.; Okada, S. Establishment of nude mice with complete loss of lymphocytes and NK cells and application for in vivo bio-imaging. In Vivo 2014, 28, 779–784. [Google Scholar]
- Szadvari, I.; Krizanova, O.; Babula, P. Athymic nude mice as an experimental model for cancer treatment. Physiol. Res. 2016, 65 (Suppl. 4), S441–S453. [Google Scholar] [CrossRef]
- Jackson, R.L.; Greiwe, J.S.; Schwen, R.J. Emerging evidence of the health benefits of S-equol, an estrogen receptor beta agonist. Nutr. Rev. 2011, 69, 432–448. [Google Scholar] [CrossRef]
- Sekikawa, A.; Ihara, M.; Lopez, O.; Kakuta, C.; Lopresti, B.; Higashiyama, A.; Aizenstein, H.; Chang, Y.F.; Mathis, C.; Miyamoto, Y.; et al. Effect of S-equol and Soy Isoflavones on Heart and Brain. Curr. Cardiol. Rev. 2019, 15, 114–135. [Google Scholar] [CrossRef]
- Messina, M. Soy and Health Update: Evaluation of the Clinical and Epidemiologic Literature. Nutrients 2016, 8, 754. [Google Scholar] [CrossRef]
- Messina, M.; Rogero, M.M.; Fisberg, M.; Waitzberg, D. Health impact of childhood and adolescent soy consumption. Nutr. Rev. 2017, 75, 500–515. [Google Scholar] [CrossRef]
- Ariyani, W.; Miyazaki, W.; Koibuchi, N. A Novel Mechanism of S-equol Action in Neurons and Astrocytes: The Possible Involvement of GPR30/GPER1. Int. J. Mol. Sci. 2019, 20, 5178. [Google Scholar] [CrossRef]
- Blake, C.; Fabick, K.M.; Setchell, K.D.; Lund, T.D.; Lephart, E.D. Neuromodulation by soy diets or equol: Anti-depressive & anti-obesity-like influences, age- & hormone-dependent effects. BMC Neurosci. 2011, 12, 28. [Google Scholar]
- Johnson, S.L.; Park, H.Y.; Vattem, D.A.; Grammas, P.; Ma, H.; Seeram, N.P. Equol, a Blood-Brain Barrier Permeable Gut Microbial Metabolite of Dietary Isoflavone Daidzein, Exhibits Neuroprotective Effects against Neurotoxins Induced Toxicity in Human Neuroblastoma SH-SY5Y Cells and Caenorhabditis elegans. Plant. Foods Hum. Nutr. 2020, 75, 512–517. [Google Scholar] [CrossRef]
- Bonorden, M.J.; Greany, K.A.; Wangen, K.E.; Phipps, W.R.; Feirtag, J.; Adlercreutz, H.; Kurzer, M.S. Consumption of Lactobacillus acidophilus and Bifidobacterium longum do not alter urinary equol excretion and plasma reproductive hormones in premenopausal women. Eur. J. Clin. Nutr. 2004, 58, 1635–1642. [Google Scholar] [CrossRef]
- Miyanaga, N.; Akaza, H.; Takashima, N.; Nagata, Y.; Sonoda, T.; Mori, M.; Naito, S.; Hirao, Y.; Tsukamoto, T.; Fujioka, T. Higher consumption of green tea may enhance equol production. Asian Pac. J. Cancer Prev. 2003, 4, 297–301. [Google Scholar]
| Anticancer (13 Studies) | No Effect (4 Studies) | Oncogenic (6 Studies) | |||
|---|---|---|---|---|---|
| Ref, Year | Equol Concentration | Ref, Year | Equol Concentration | Ref, Year | Equol Concentration |
| [58] 2006 | 0–50 µM R-equol and S-equol | [40] 2011 | * 250 ppm–500 ppm (1031.9–2063.8 µM) S-equol | [43] 1987 | 0.1–1 µM |
| [55] 2014 | 0–50 µM R-equol and S-equol | [39] 2006 | * 0–100 µM | [44] 1997 | 0.001–1 µM |
| [53] 2008 | 0–100 µM R-equol and S-equol | [38] 2018 | 1 µM S-equol | [44] 1997 | 0.001–1 µM |
| [50] 2009 | 0–100 µM R-equol and S-equol | [41] 2020 | * 0–100 µM 1.72–4.42 µM | [46] 2007 | 0.1 µM R-equol and S-equol |
| [51] 2019 | 0–150 µg/mL (0–619.14 µM) S-equol | [42] 2009 | 10 µM | ||
| [48] 2010 | * 36.8 0 ± 8.4 ng/mL (0.15 µM ± 0.03 µM) of R-equol 13.9 ± 2.3 ng/mL (0.05 µM ± 0.002 µM) of S-equol | [45] 2010 | 10 µM | ||
| [59] 2006 | 1 µM | [47] 2015 | 25 µM | ||
| [57] 2012 | 1 µM (S-equol) | ||||
| [54] 2015 | 50 µM | ||||
| [52] 2017 | 50 µM | ||||
| [56] 2013 | 100 µM | ||||
| [60] 2014 | * 100–400 mg/kg | ||||
| [49] 2019 | 10 µM (S-equol) 10 µM (R-equol) | ||||
| Ref | Study Population | Study Design | Type of Intervention | Conclusion | Cancer Properties |
|---|---|---|---|---|---|
| [38] | In vitro | MCF-7 | S-equol Daidzein | Equol does not stimulate the generation of cancer cells. | No effect |
| [48] | In vivo | Tumour-induced Sprague-Dawley rats | Group 1: AIN93G diet Group 2: 250 mg/kg R-(−)equol Group 1: 250 mg/kg S-(−)equol | R-(+) is chemopreventive and increases tumour latency in the animal model. S-(−) equol has no chemopreventive or stimulatory effect. | Anticancer |
| [42] | In vitro | HeLa HepG2 MCF-7 | Genistein Daidzein Equol | Erα transcriptional activation by isoflavones (genistein and daidzein) and equol is mediated through AF-1 and have similar properties to oestradiol on Erα expression and MCF-7 cell proliferation. | Oncogenic |
| [50] | In vitro | MDA-MB-453 | Equol | Equol induced intrinsic apoptotic pathway via cytochrome c release and caspase 9 in human breast cancer MDA-MB-453 cells. | Anticancer |
| [39] | In vitro | MCF-7 | In vitro (±)-equol Daidzein | (±)-equol stimulates ER-regulated MCF-7 cells growth, but daidzein and (±)-equol is not the active isoflavones that affect breast cancer growth in athymic mouse-MCF-7 xenograft preclinical model for postmenopausal oestrogen-dependent breast cancer. | No effect |
| In vivo | Athymic BALB/c mice | In vivo (±)-equol 250 ppm Group 7 (±)-equol 500 ppm Group 8 (±)-equol 1000 ppm | |||
| [51] | In vitro | MCF-7 Control- MCF-10A Others: MDA-MB-231 SKBr-3 | S-equol | S-equol upregulates the expression of miR-10a-5p in MCF-7 cells to inhibit the activation of the PI3K/Akt pathway and subsequently regulate the downstream apoptotic cascade. | Anticancer |
| [49] | In vitro | MCF-7 MDA-MB-231 | Daidzein R-equol S-equol | Daidzein, S-equol, and R-equol treated cells showed a significant decrease in doxorubicin and metoxantrone efflux by potent inhibition of breast cancer resistance protein (BCRP). | Anticancer |
| [43] | In vitro | MCF-7 T47D | Enterolactone Equol | Equol stimulates in vitro breast cancer cell proliferation. | Oncogenic |
| [44] | In vitro | MCF-7 | Equol Daidzein | Equol has a higher affinity for the ER in breast cancer cells compared to daidzein. | Oncogenic |
| [52] | In vitro | MCF-7 Others: MDA-MB-468 SKBr-3 | Genistein Equol Genistein + equol | The synergistic growth-inhibitory mechanism of genistein in a combination of equol is postulated to be mediated by modulation of Bax and Bcl-xL expressions. | Anticancer |
| [53] | In vitro | MCF-7 MDA-MB-453 | Equol | Equol significantly inhibited cell proliferation and induced cell cycle arrest and apoptosis in ER-negative MDA-MB-435 cells. | Anticancer |
| [54] | In vitro | MDA-MB-231 T47D | Equol Radiation + equol | Equol exhibits anticancer properties: To inhibit proliferation, induce apoptosis, and enhanced radiosensitivity on both oestrogen-positive and -negative breast cancer cells. | Anticancer |
| [55] | In vitro | MDA-MB-231 | Daidzein R-equol S-equol | Daidzein, R-equol, and S-equol inhibited the invasion of MDA-MB-231 human breast cancer cells, in part, via down-regulation of MMP-2. | Anticancer |
| [56] | In vitro | MCF-7 | Equol Tamoxifen Equol + tamoxifen | Equol induces MCF-7 cell apoptosis and enhances tamoxifen’s pro-apoptotic effect via activation of the intrinsic apoptotic pathway. | Anticancer |
| [57] | In vitro | MDA-MB-231 MCF-7 MCF-10A | S-equol | S-equol has a demethylating effect on cytosine phosphate guanine islands in the promoters of BRCA1 and BRCA2 genes. | Anticancer |
| [40] | In vitro | MCF-7 MCF-7-E10 | In vitro S-equol Daidzein Glycitein Genistein Isoflavonoids mixture | Purified S-equol, SE5-OH, and genistein do not stimulate oestrogen-dependent or-independent MCF-7 cell proliferation at a concentration less than or equal to 500 ppm. | No effect |
| In vivo | Athymic BALB/c mice | Group 1 AIN-93G Group 2 S-equol 250 ppm Group 3 S-equol 500 ppm Group 4 Genistein 250 ppm Group 5 Genistein 500 ppm Group 4 Natural S-equol supplement (SE5-OH) 250 ppm Group 4 Natural S-equol supplement (SE5-OH) 500 ppm | |||
| [45] | In vitro | MCF-7 MDA-MB-231 | Genistein (±)-equol | Low concentration of genistein and equol stimulate cell growth and cell cycle progression via ER-mediated transcription and activation of ERK1/2. | Oncogenic |
| [46] | In vitro | T47D:A18/PKC (tamoxifen resistant) T47D/A18 (hormone-dependent) | (R,S)-equol Daidzein Genistein | Simultaneous consumption of isoflavone supplements with tamoxifen may not be safe. | Oncogenic |
| In vivo | Athymic BALB/c mice | Group 1 Control Group 2 17β-oestradiol Group 3 Tamoxifen Group 4 Genistein Group 5 Genistein + tamoxifen Group 6 Daidzein Group 7 Daidzein + tamoxifen | |||
| [58] | In vitro | MCF-7 MDA-MB-231, MCF-10A LNCap LAPC-4 PC-3 | Daidzein S-equol R-equol | Racemic and S-equol showed equipotent biological effects on breast and prostate cancer cell proliferation and invasion in vitro. | Anticancer |
| [59] | In vitro | MCF-7 Caco-2 | Genistein Equol | 17β-oestradiol and genistein at physiological doses upregulate CYP27B1 (vitamin D activation) and downregulate CYP24 (vitamin D hydroxylase catabolism). | Anticancer |
| [60] | In vivo | Ovariectomised Sprague Dawley | Group 1 AIN-93G Group 2 AIN-93G + 100 mg/kg isoflavones Group 3 AIN-93G + 500 mg/kg isoflavones Group 4 AIN-93G + 1000 mg/kg isoflavones Group 5 AIN-93G + 100 mg/kg equol Group 6 AIN-93G + 200 mg/kg equol Group 7 AIN-93G + 400 mg/kg equol Group 8 AIN-93G + 2.5 mg/kg stilboestrol | Isoflavones and equol intake significantly inhibited the development of postmenopausal mammary tumours by antioxidant and oestrogenic activities. | Anticancer |
| [49] | In vitro | MCF-7 T47D | Genistein (±)-Equol | Dietary equol intake did not alter the oestrogen-dependent tumour growth in either T47D or MCF-7 models in long term studies. | No effect |
| In vivo | Ovariectomised athymic BALB/c nude mice | Mice injected with T47D Group 1 Negative Control (AIN-93G) Group 2 Positive control (17B-oestradiol) Group 3 AIN-93G + 250 ppm equol Group 4 AIN-93G + 750 ppm equol Mice injected with MCF-7 Group 1 Negative Control (AIN-93G) Group 2 Positive control (17B-oestradiol) Group 3 AIN-93G + 250 ppm equol Group 4 AIN-93G + 750 ppm equol Group 5 500 ppm genistein Group 6 500 ppm genistein + 250 ppm equol Group 7 500 ppm genistein + 500 ppm equol Group 8 500 ppm genistein + 1000 ppm equol | |||
| [47] | In vitro | MDA-MB-435 (ER-) Hs578t (ER-) MCF-10A | (R,S)-equol | Equol possesses pro-cancer properties and may influence cancer potential via the upregulation of c-Myc transcription, leading to c-Myc dependent and -independent eIF4G-mediated translation initiation of oncogenes increased cancer cell survival. | Oncogenic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hod, R.; Maniam, S.; Mohd Nor, N.H. A Systematic Review of the Effects of Equol (Soy Metabolite) on Breast Cancer. Molecules 2021, 26, 1105. https://doi.org/10.3390/molecules26041105
Hod R, Maniam S, Mohd Nor NH. A Systematic Review of the Effects of Equol (Soy Metabolite) on Breast Cancer. Molecules. 2021; 26(4):1105. https://doi.org/10.3390/molecules26041105
Chicago/Turabian StyleHod, Rafidah, Sandra Maniam, and Nurul Huda Mohd Nor. 2021. "A Systematic Review of the Effects of Equol (Soy Metabolite) on Breast Cancer" Molecules 26, no. 4: 1105. https://doi.org/10.3390/molecules26041105
APA StyleHod, R., Maniam, S., & Mohd Nor, N. H. (2021). A Systematic Review of the Effects of Equol (Soy Metabolite) on Breast Cancer. Molecules, 26(4), 1105. https://doi.org/10.3390/molecules26041105





