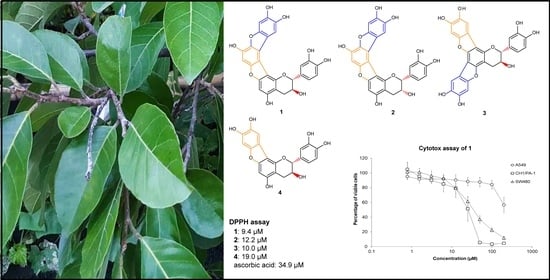
Highly Aromatic Flavan-3-ol Derivatives from Palaeotropical Artocarpus lacucha Buch.-Ham Possess Radical Scavenging and Antiproliferative Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure Elucidation
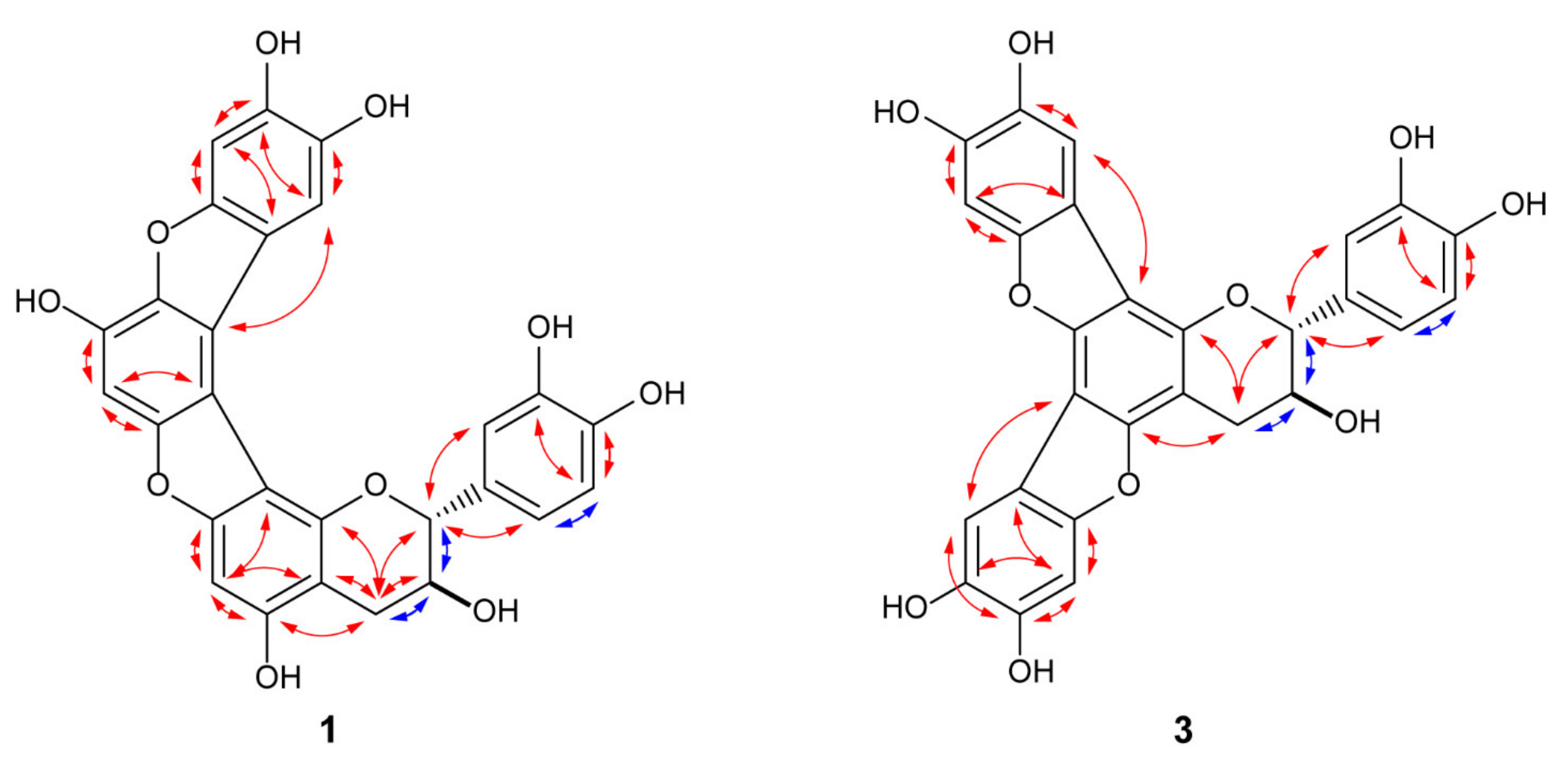
2.2. Stereochemistry of 1–3
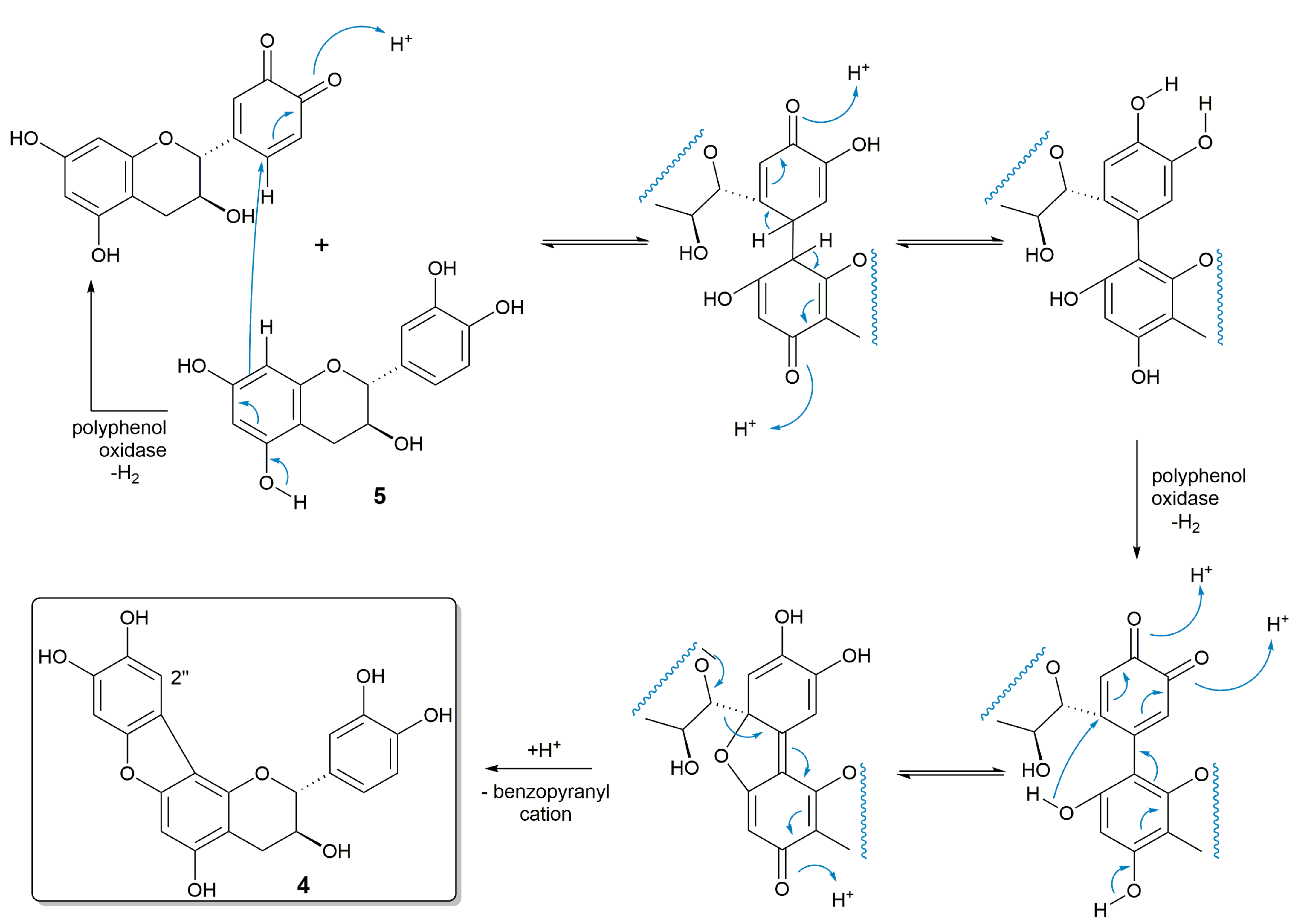
2.3. Proposed Biosynthesis of 1–3
2.4. Oxidation of 1–4 to Ortho-Quinone Structures
2.5. Radical Scavenging Activities of 1–4
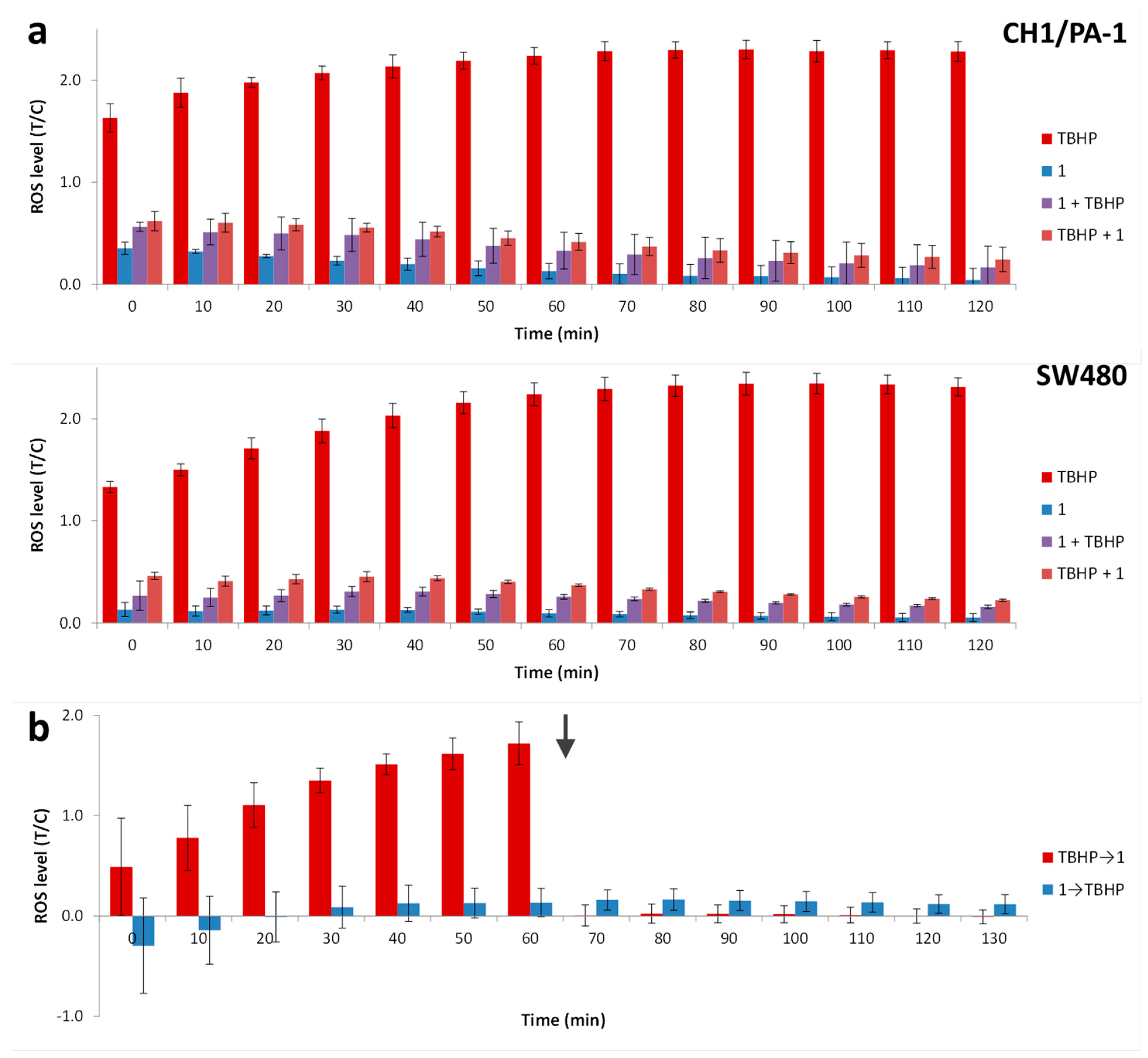
2.6. Antioxidative Effects of Artocarpinol A (1) in Cancer Cells
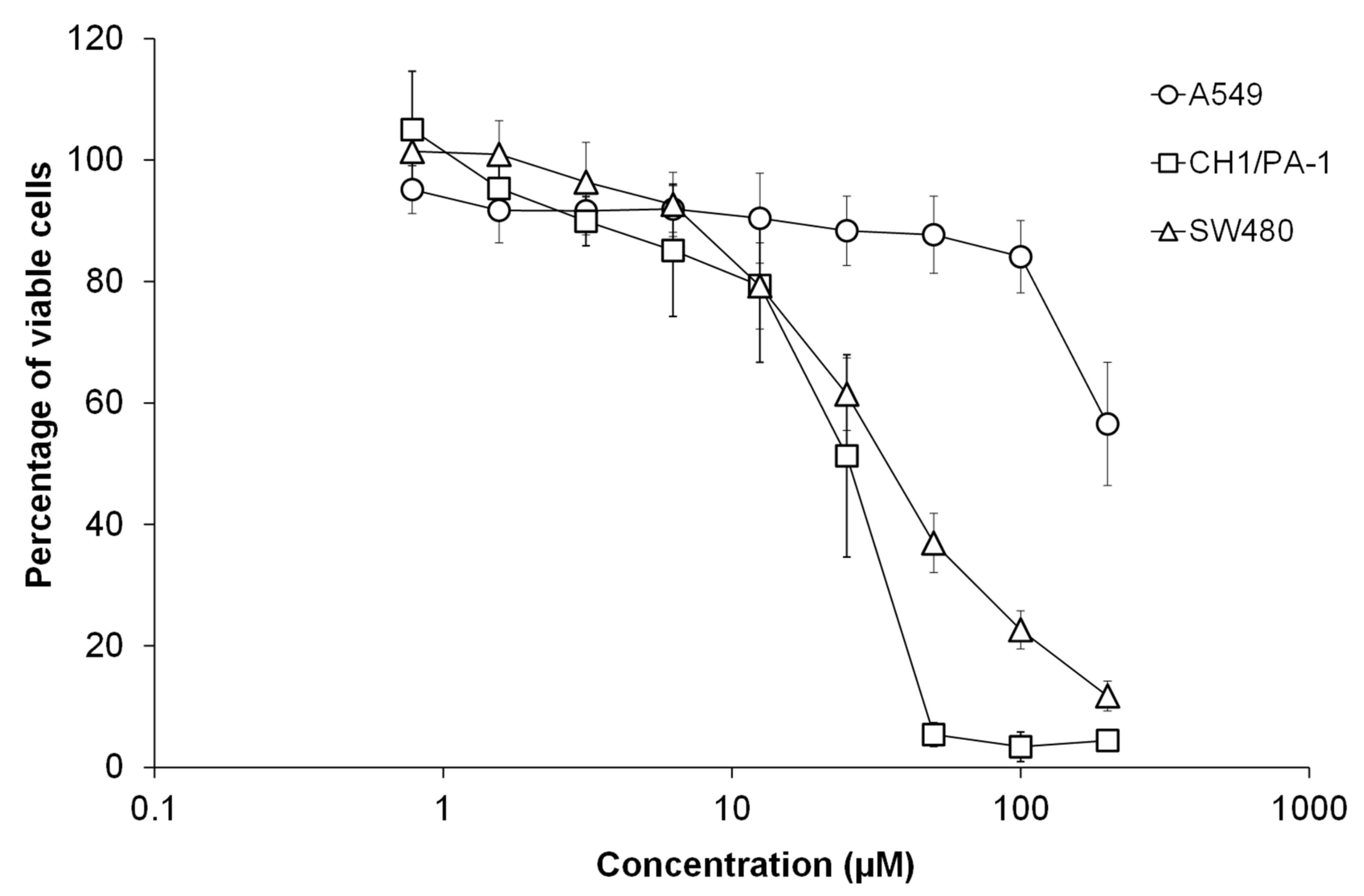
2.7. Cytotoxic Properties of Artocarpinol A (1)
3. Experimental
3.1. Plant Material and Extraction Procedure
3.2. General Experimental Procedures
3.3. Isolation Procedure
3.4. DPPH Assay
3.5. Cytotoxicity Assay
3.6. DCFH-DA Assay
3.7. Isolated Compounds
3.7.1. Artocarpinol A (1)
3.7.2. 3-epi-Artocarpinol A (2)
3.7.3. Artocarpinol B (3)
3.7.4. Gambircatechol (4)
3.7.5. (+)- Catechin (5)
3.7.6. (+)- Afzelechin (6)
3.7.7. Oxyresveratrol (7)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Berg, C.C.; Corner, E.J.H.; Jarrett, F.M. Moraceae genera other than Ficus. Flora Males. Ser. 1 Spermatophyta 2006, 17, 1–146. [Google Scholar]
- Zerega, N.J.C.; Supardi, M.N.N.; Motley, T.J. Phylogeny and Recircumscription of Artocarpeae (Moraceae) with a Focus on Artocarpus. Syst. Bot. 2010, 35, 766–782. [Google Scholar]
- Aneklaphakij, C.; Bunsupa, S.; Sirichamorn, Y.; Bongcheewin, B.; Satitpatipan, V. Taxonomic Notes on the ‘Mahat’ (Artocarpus lacucha and A. thailandicus, Moraceae) Species Complex in Thailand. Plants 2020, 9, 391. [Google Scholar] [CrossRef]
- Jagtap, U.B.; Bapat, V.A. Artocarpus: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2010, 129, 142–166. [Google Scholar] [CrossRef] [PubMed]
- Saowakon, N.; Soonklang, N.; Wanichanon, C.; Sobhon, P. The anthelmintic effects of Artocarpus lakoocha stem extract on Fasciola gigantica. Planta Med. 2019, 85, 1429. [Google Scholar]
- Wongon, M.; Limpeanchob, N. Inhibitory effect of Artocarpus lakoocha Roxb and oxyresveratrol on alpha-glucosidase and sugar digestion in Caco-2 cells. Heliyon 2020, 6, e03458. [Google Scholar] [CrossRef]
- Boonyaketgoson, S.; Rukachaisirikul, V.; Phongpaichit, S.; Trisuwan, K. Deoxybenzoin and flavan derivatives from the twigs of Artocarpus lakoocha. Phytochem. Lett. 2019, 31, 96–100. [Google Scholar] [CrossRef]
- Sritularak, B.; Tantrakarnsakul, K.; Lipipun, V.; Likhitwitayawuid, K. Flavonoids with Anti-HSV Activity from the Root Bark of Artocarpus lakoocha. Nat. Prod. Commun. 2013, 8, 1079–1080. [Google Scholar] [CrossRef]
- Puntumchai, A.; Kittakoop, P.; Rajviroongit, S.; Vimuttipong, S.; Likhitwitayawuid, K.; Thebtaranonth, Y. Lakoochins A and B, new antimycobacterial stilbene derivatives from Artocarpus lakoocha. J. Nat. Prod. 2004, 67, 485–486. [Google Scholar] [CrossRef] [PubMed]
- Sritularak, B.; Tantrakarnsakul, K.; Likhitwitayawuid, K.; Lipipun, V. New 2-Arylbenzofurans from the Root Bark of Artocarpus lakoocha. Molecules 2010, 15, 6548–6558. [Google Scholar] [CrossRef] [PubMed]
- Chatsumpun, N.; Chuanasa, T.; Sritularak, B.; Lipipun, V.; Jongbunprasert, V.; Ruchirawat, S.; Ploypradith, P.; Likhitwitayawuid, K. Oxyresveratrol: Structural Modification and Evaluation of Biological Activities. Molecules 2016, 21, 489. [Google Scholar] [CrossRef] [PubMed]
- Joung, D.K.; Mun, S.H.; Choi, S.H.; Kang, O.H.; Kim, S.B.; Lee, Y.S.; Zhou, T.; Kong, R.; Choi, J.G.; Shin, D.W.; et al. Antibacterial activity of oxyresveratrol against methicillin-resistant Staphylococcus aureus and its mechanism. Exp. Ther. Med. 2016, 12, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Povichit, N.; Phrutivorapongkul, A.; Suttajit, M.; Leelapornpisid, P. Antiglycation and antioxidant activities of oxyresveratrol extracted from the heartwood of Artocarpus lakoocha Roxb. Maejo Int. J. Sci. Tech. 2010, 4, 454–461. [Google Scholar]
- Maneechai, S.; Likhitwitayawuid, K.; Sritularak, B.; Palanuvej, C.; Ruangrungsi, N.; Sirisa-Ard, P. Quantitative Analysis of Oxyresveratrol Content in Artocarpus lakoocha and ‘Puag-Haad’. Med. Prin. Pract. 2009, 18, 223–227. [Google Scholar] [CrossRef]
- Wongkham, S.; Wongkham, C.; Boonsiri, P.; Simasathiansophon, S.; Trisonthi, C.; Atisook, K. Isolectins from Seeds of Artocarpus lakoocha. Phytochemistry 1995, 40, 1331–1334. [Google Scholar] [CrossRef]
- Hakim, E.H.; Achmad, S.A.; Juliawaty, L.D.; Makmur, L.; Syah, Y.M.; Aimi, N.; Kitajima, M.; Takayama, H.; Ghisalberti, E.L. Prenylated flavonoids and related compounds of the Indonesian Artocarpus (Moraceae). J. Nat. Med. 2006, 60, 161–184. [Google Scholar] [CrossRef]
- Yoshikado, N.; Taniguchi, S.; Kasajima, N.; Ohashi, F.; Doi, K.I.; Shibata, T.; Yoshida, T.; Hatano, T. Uncariagambiriine and gambircatechol: Novel constituents of Uncaria gambir leaves. Heterocycles 2009, 77, 793–800. [Google Scholar]
- Lorenz, P.; Roychowdhury, S.; Engelmann, M.; Wolf, G.; Horn, T.F.W. Oxyresveratrol and resveratrol are potent antioxidants and free radical scavengers: Effect on nitrosative and oxidative stress derived from microglial cells. Nitric Oxide-Biol. CH 2003, 9, 64–76. [Google Scholar] [CrossRef]
- Grzesik, M.; Naparlo, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Saijyo, J.; Suzuki, Y.; Okuno, Y.; Yamaki, H.; Suzuki, T.; Miyazawa, M. α-glucosidase inhibitor from Bergenia ligulata. J. Oleo Sci. 2008, 57, 431–435. [Google Scholar] [CrossRef]
- Namdaung, U.; Athipornchai, A.; Khammee, T.; Kuno, M.; Suksamrarn, S. 2-Arylbenzofurans from Artocarpus lakoocha and methyl ether analogs with potent cholinesterase inhibitory activity. Eur. J. Med. Chem. 2018, 143, 1301–1311. [Google Scholar] [CrossRef]
- Sasaki, K.; Tsurumaru, Y.; Yazaki, K. Prenylation of flavonoids by biotransformation of yeast expressing plant membrane-bound prenyltransferase SfN8DT-1. Biosci. Biotechnol. Biochem. 2009, 73, 759–761. [Google Scholar] [CrossRef]
- Yazaki, K.; Sasaki, K.; Tsurumaru, Y. Prenylation of aromatic compounds, a key diversification of plant secondary metabolites. Phytochemistry 2009, 70, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Khadem, S.; Marles, R.J. Chromone and flavonoid alkaloids: Occurrence and bioactivity. Molecules 2012, 17, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Li, B.; Qiao, M.; Li, J.; Xu, H.; Zhang, L.; Zhang, X. Advances on the in vivo and in vitro glycosylations of flavonoids. Appl. Microbiol. Biotechnol. 2020, 104, 6587–6600. [Google Scholar] [CrossRef]
- Guyot, S.; Vercauteren, J.; Cheynier, V. Structural determination of colourless and yellow dimers resulting from (+)-catechin coupling catalysed by grape polyphenoloxidase. Phytochemistry 1996, 42, 1279–1288. [Google Scholar] [CrossRef]
- Van Rensburg, W.J.; Ferreira, D.; Malan, E.; Steenkamp, J.A. Tyrosinase catalyzed biphenyl construction from flavan-3-ol substrates. Phytochemistry 2000, 53, 285–292. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Constabel, P.C. Tannins in plant-herbivore interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef] [PubMed]
- Sudžuković, N.; Schinnerl, J.; Brecker, L. Phytochemical meanings of tetrahydro-beta-carboline moiety in strictosidine derivatives. Bioorg. Med. Chem. 2016, 24, 588–595. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Structure-Activity Relationships of Flavonoids in the Cellular Antioxidant Activity Assay. J. Agric. Food Chem. 2008, 56, 8404–8411. [Google Scholar] [CrossRef] [PubMed]


| Position | Artocarpinol A (1) | 3-epi-Artocarpinol A (2) | Artocarpinol B (3) | |||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | |
| Catechin moiety | ||||||
| 2 | 5.70 (1H, d, 3.1) | 80.4, d | 5.65 (1H, d, 3.3) | 79.7, d | 4.96 (1H, d, 7.6) | 83.7, d |
| 3 | 4.51 (1H, q, 3.9) | 67.4, d | 4.47 (1H, ddd, 8.1, 5.0, 3.3) | 67.3, d | 4.28 (1H, td, 8.0, 5.3) | 68.1, d |
| 4 | 2.89 (1H, ddd, 16.8, 3.7, 1.7) 2.68 (1H, dd, 16.7, 4.3) | 25.5, t | 3.11 (1H, ddd, 15.9, 5.0, 1.2) 2.72 (1H, dd, 15.9, 7.7) | 27.9, t | 3.34 (1H, dd, 15.8, 5.3) 3.05 (1H, dd, 15.8, 8.2) | 28.6, t |
| 5 | - | 156.0, s | - | 155.8, s | - | 155.3, s |
| 6 | 6.63 (1H, s) | 91.2, d | 6.65 (1H, s) | 91.3, d | - | 109.8, s |
| 7 | - | 158.0, s | - | 158.2, s | - | 149.4, s |
| 8 | - | 107.4, s | - | 107.4, s | - | 104.6, s |
| 9 | - | 148.7, s | - | 149.4, s | - | 148.4, s |
| 10 | - | 102.9, s | - | 103.6, s | - | 100.5, s |
| 1′ | - | 132.1, s | - | 130.7, s | - | 131.9, s |
| 2′ | 6.82 (1H, d, 2.0) | 113.9, d | 7.01 (1H, d, 2.1) | 116.0, d | 7.01 (1H, d, 2.1) | 115.2, d |
| 3′ | - | 145.9, s | - | 145.8, s | - | 146.5, s |
| 4′ | - | 146.3, s | - | 146.1, s | - | 146.4, s |
| 5′ | 6.68 (1H, d, 8.3) | 116.4, d | 6.68 (1H, d, 8.2) | 115.8, d | 6.82 (1H, d, 8.1) | 116.2, d |
| 6′ | 6.73 (1H, dd, 8.3, 2.1) | 120.3, d | 6.87 (1H, dd, 8.5, 2.0) | 120.4, d | 6.89 (1H, dd, 8.2, 2.1) | 120.2, d |
| D-ring | ||||||
| 1″ | - | 108.7, s | - | 108.6, s | - | 115.1, s |
| 2″ | 119.7, s | - | 119.7, s | 7.47 (1H, s) | 107.2, d | |
| 3″ | - | 141.7, s | - | 141.9, s | - | 143.5, s |
| 4″ | - | 143.6, s | - | 143.6, s | - | 146.0, s |
| 5″ | 7.00 (1H, s) | 98.2, d | 6.98 (1H, s) | 98.1, d | 7.05 (1H, s) | 99.1, d |
| 6″ | - | 153.8, s | 153.8, s | - | 151.7, s | |
| E-ring | ||||||
| 1‴ | - | 117.7, s | - | 117.5, s | - | 116.3, s |
| 2‴ | 8.45 (1H, s) | 111.7, d | 8.34 (1H, s) | 111.9, d | 7.38 (1H, s) | 108.1, d |
| 3‴ | - | 142.5, s | - | 142.4, s | - | 143.2, s |
| 4‴ | - | 147.1, s | - | 147.1, s | - | 145.7, s |
| 5‴ | 7.00 (1H, s) | 96.3, d | 6.97 (1H, s) | 96.3, d | 7.09 (1H, s) | 99.3, d |
| 6‴ | - | 152.8, s | - | 152.5, s | - | 151.5, s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Songoen, W.; Phanchai, W.; Brecker, L.; Wenisch, D.; Jakupec, M.A.; Pluempanupat, W.; Schinnerl, J. Highly Aromatic Flavan-3-ol Derivatives from Palaeotropical Artocarpus lacucha Buch.-Ham Possess Radical Scavenging and Antiproliferative Properties. Molecules 2021, 26, 1078. https://doi.org/10.3390/molecules26041078
Songoen W, Phanchai W, Brecker L, Wenisch D, Jakupec MA, Pluempanupat W, Schinnerl J. Highly Aromatic Flavan-3-ol Derivatives from Palaeotropical Artocarpus lacucha Buch.-Ham Possess Radical Scavenging and Antiproliferative Properties. Molecules. 2021; 26(4):1078. https://doi.org/10.3390/molecules26041078
Chicago/Turabian StyleSongoen, Weerasak, Witthawat Phanchai, Lothar Brecker, Dominik Wenisch, Michael A. Jakupec, Wanchai Pluempanupat, and Johann Schinnerl. 2021. "Highly Aromatic Flavan-3-ol Derivatives from Palaeotropical Artocarpus lacucha Buch.-Ham Possess Radical Scavenging and Antiproliferative Properties" Molecules 26, no. 4: 1078. https://doi.org/10.3390/molecules26041078
APA StyleSongoen, W., Phanchai, W., Brecker, L., Wenisch, D., Jakupec, M. A., Pluempanupat, W., & Schinnerl, J. (2021). Highly Aromatic Flavan-3-ol Derivatives from Palaeotropical Artocarpus lacucha Buch.-Ham Possess Radical Scavenging and Antiproliferative Properties. Molecules, 26(4), 1078. https://doi.org/10.3390/molecules26041078