Extracts of Digested Berries Increase the Survival of Saccharomyces cerevisiae during H2O2 Induced Oxidative Stress
Abstract
1. Introduction
2. Results
2.1. Anthocyanin Profiles of Nondigested and Digested Berries
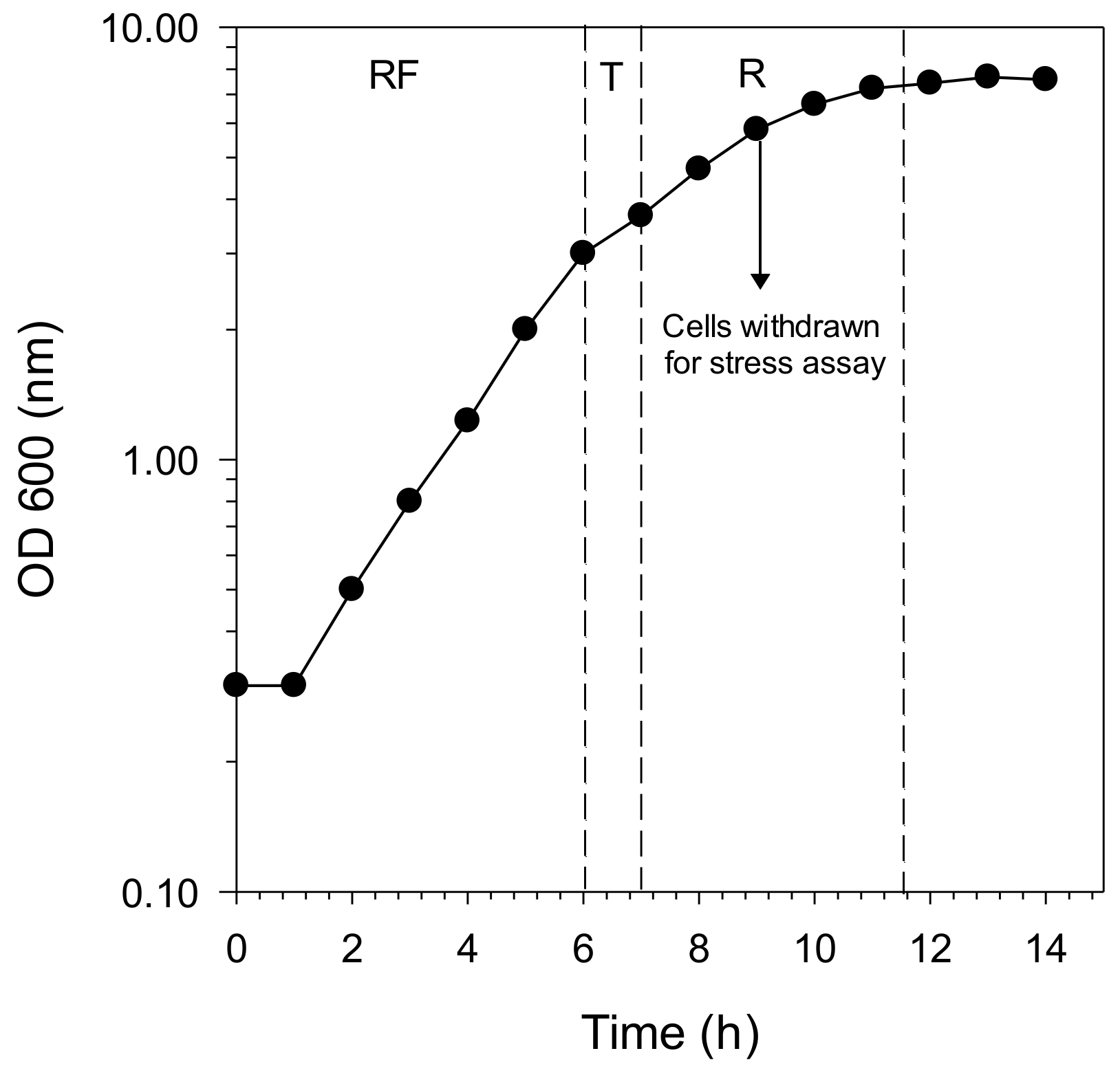
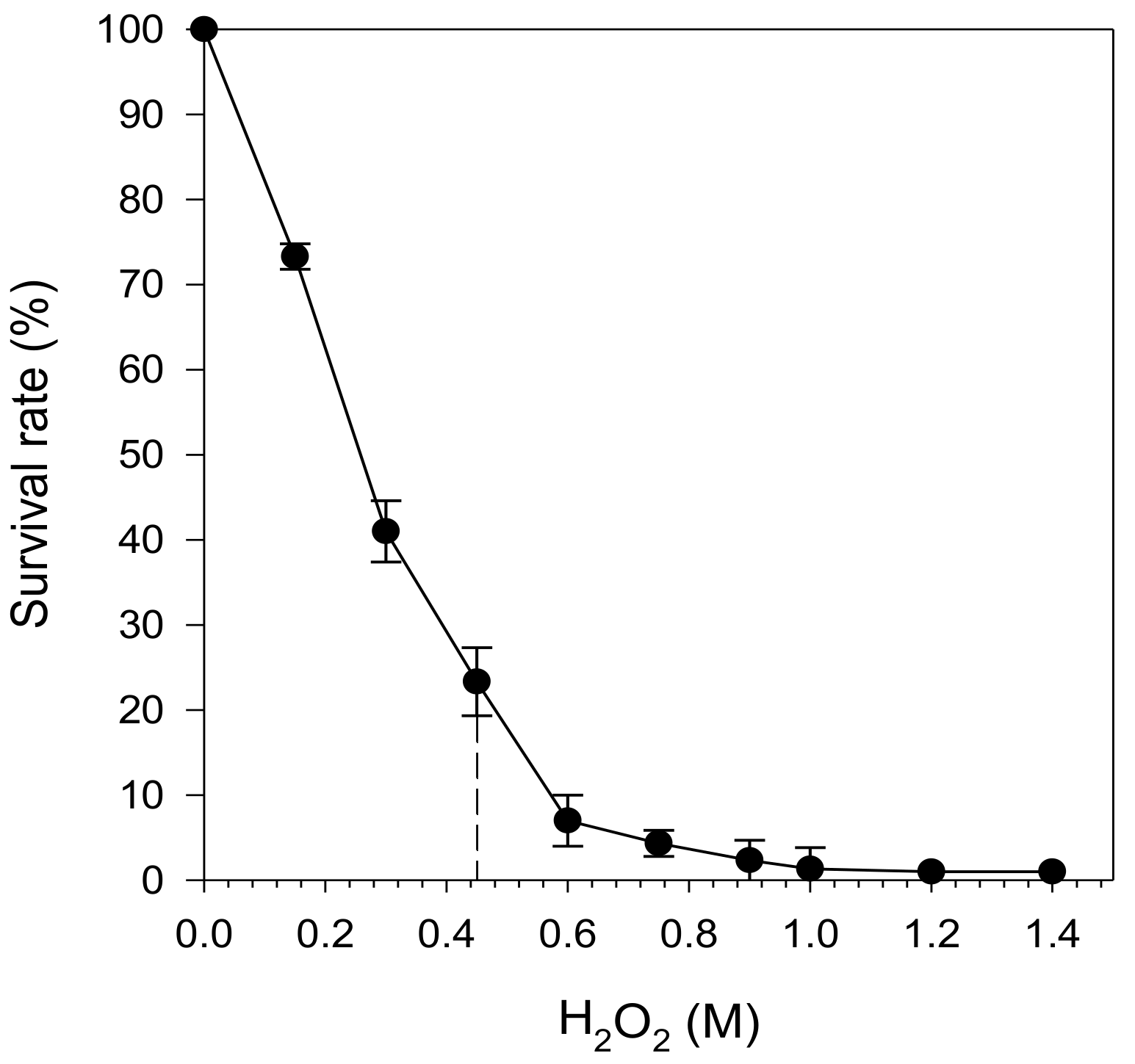
2.2. Determination of Respiratory State and Optimal H2O2 Concentration for Oxidative Stress Assay
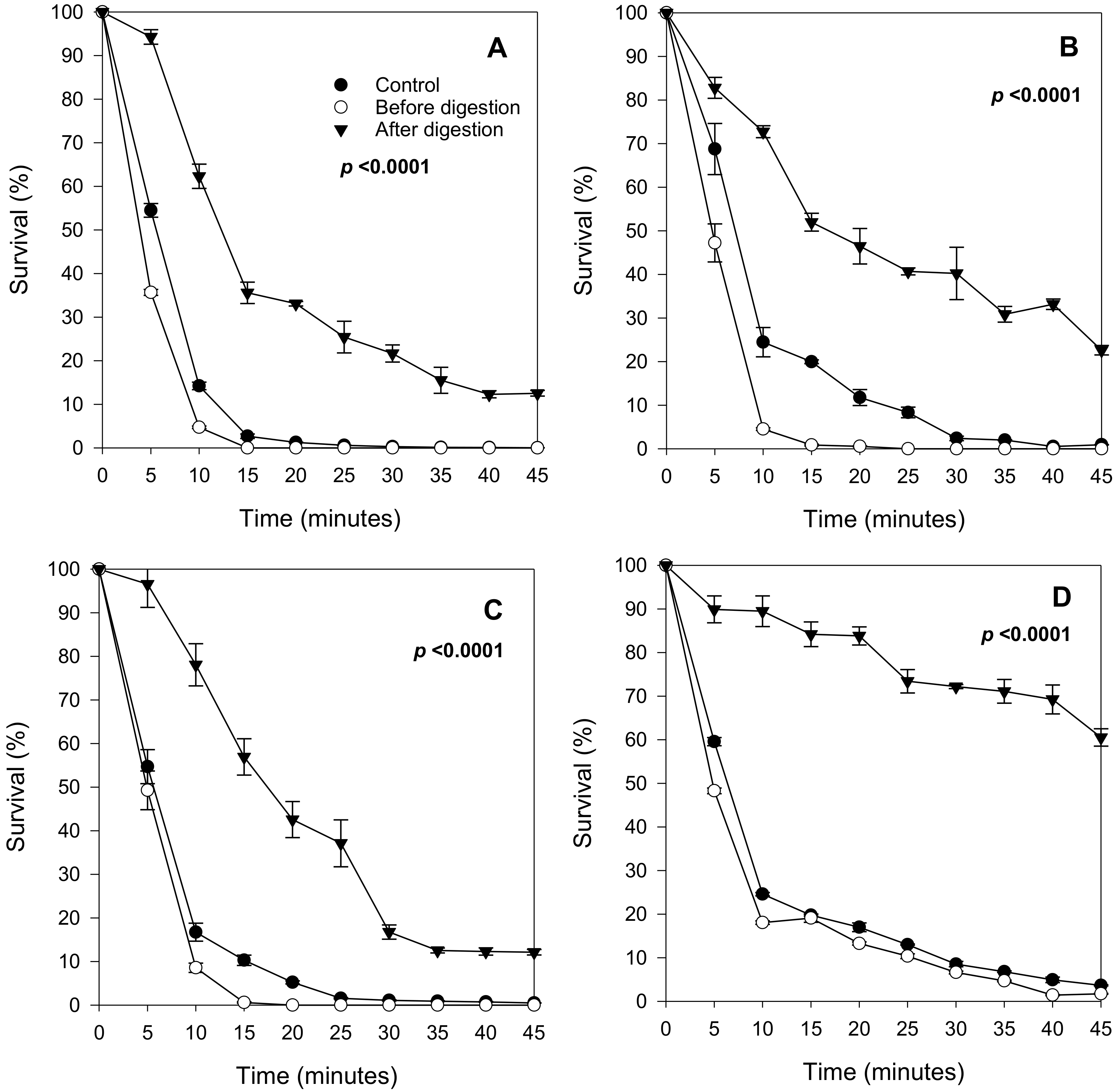
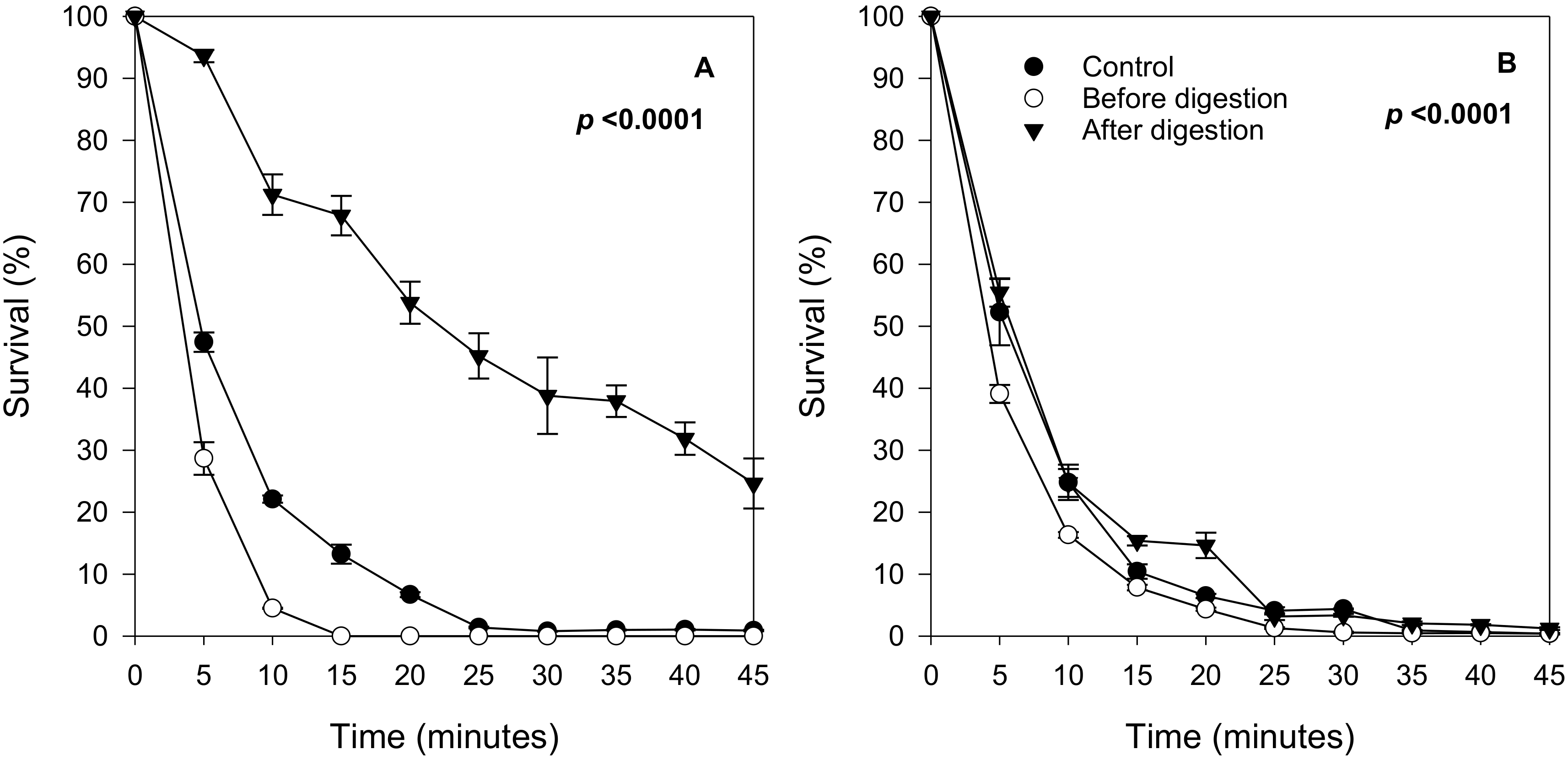
2.3. Effect of In Vitro Digested Berry Extracts on Yeast Survival
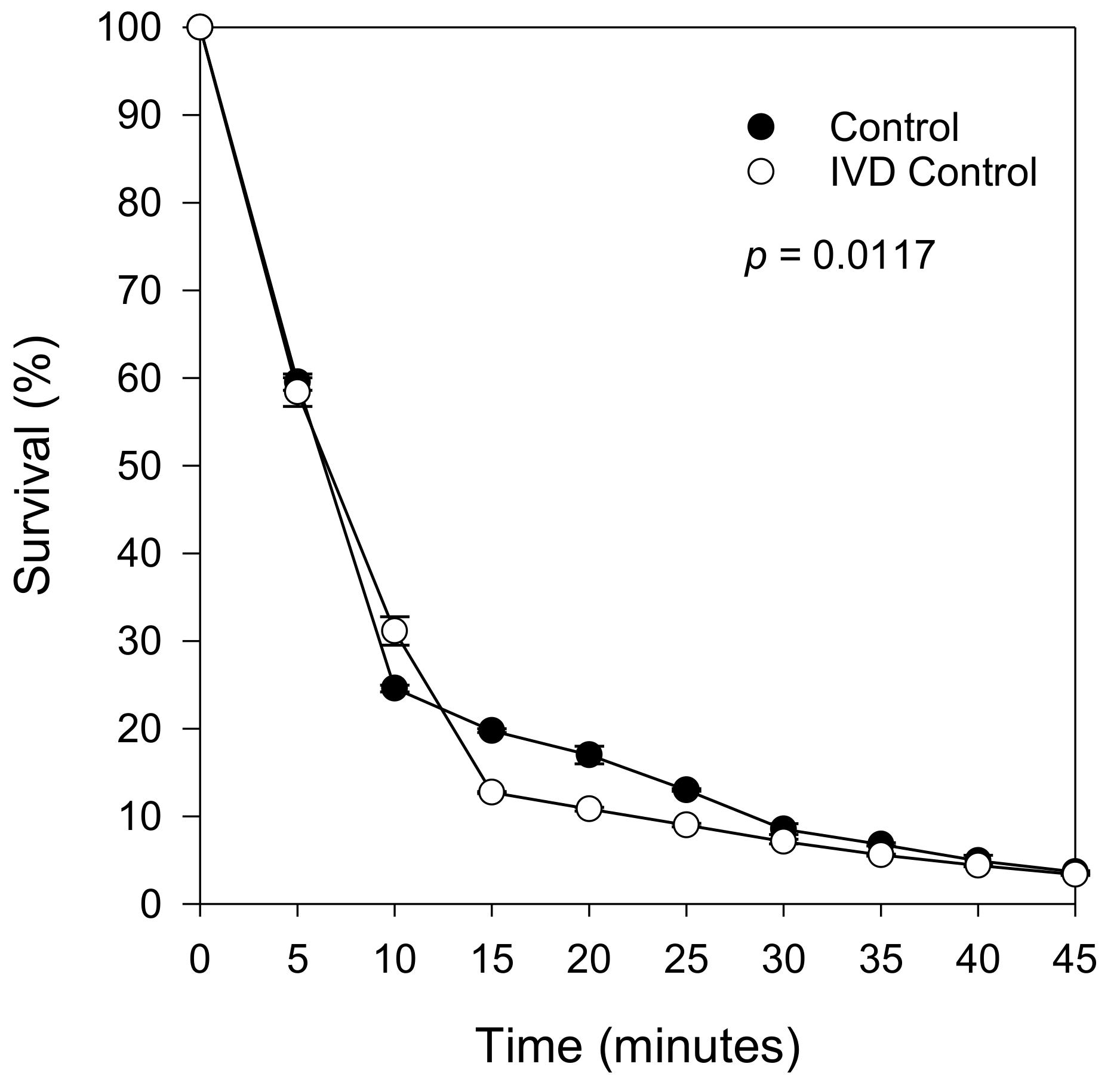
2.4. Protective Effect Remains after the Removal of Digested Extracts
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. In Vitro Digestion of Berry Samples
4.3. Extraction of Anthocyanins
4.4. HPLC-UV/VIS Analysis of Anthocyanins
4.5. Yeast Strain and Growth Conditions
4.6. Assessment of the Optimal Concentration of H2O2 for Oxidative Stress
4.7. Assessment of the Protective Effect of Berry Extracts against Oxidative Stress
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Szajdek, A.; Borowska, E.J. Bioactive compounds and health-promoting properties of berry fruits: A review. Plant. Foods Hum. Nutr. 2008, 63, 147–156. [Google Scholar] [CrossRef]
- Veberic, R.; Slatnar, A.; Bizjak, J.; Stampar, F.; Mikulic-Petkovsek, M. Anthocyanin composition of different wild and cultivated berry species. LWT Food Sci. Technol. 2015, 60, 509–517. [Google Scholar] [CrossRef]
- Chanoca, A.; Kovinich, N.; Burkel., B.; Stecha, S.; Bohorquez-Restrepo, A.; Ueda, T.; Eliceiri, K.W.; Grotewold, E.; Otegui, M.S. Anthocyanin vacuolar inclusion form by a microautophagy mechanism. Plant. Cell 2015, 27, 2545–2559. [Google Scholar] [CrossRef] [PubMed]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Mejia, E.G. Natural Pigments: Stabilization methods of anthocyanins for food applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 180–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Tao, C.; Liu, M.; Pan, Y.; Lv, Z. Effect of temperature and pH on stability of anthocyanin obtained from blueberry. J. Food Meas Charac. 2018, 12, 1744–1753. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Oliveira, H.; Wu, N.; Zhang, Q.; Wang, J.; Oliveira, V.F.; Mateus, N.; He, J.; Fernandes, I. Bioavailability studies and anticancer properties of malvidin based anthocyanins, pyranoanthocyanins and non-oxonium derivatives. Food Funct. 2016, 7, 2462–2468. [Google Scholar] [CrossRef]
- Burton-Freeman, B.; Brzeziński, M.; Park, E.; Sandhu, A.; Xiao, D.; Edirisinghe, I. A selective role of dietary anthocyanins and flavan-3-ols in reducing the risk of type 2 diabetes mellitus. A review of recent evidence. Nutrients 2019, 11, 841. [Google Scholar] [CrossRef]
- Mink, P.J.; Scrafford, C.G.; Barraj, L.M.; Harnack, L.; Hong, C.P.; Nettleton, J.A.; Jacobs, D.R. Flavonoid intake and cardiovascular disease mortality: A prospective study in postmenopausal women. Am. J. Clin. Nutr. 2007, 85, 895–909. [Google Scholar] [CrossRef] [PubMed]
- Bunea, A.; Ruginǎ, D.; Sconţa, Z.; Pop, R.M.; Pintea, A.; Socaciu, C.; Tǎbǎrǎn, F.; Grootaert, C.; Struijs, K.; VanCamp, J. Anthocyanin determination in blueberry extracts from various cultivars and their antiproliferative and apoptotic properties in B16-F10 metastatic murine melanoma cells. Phytochemistry 2013, 95, 436–444. [Google Scholar] [CrossRef]
- Guo, X.; Yang, B.; Tan, J.; Jiang, J.; Li, D. Associations of dietary intakes of anthocyanins and berry fruits with risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2016, 70, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Lin, F.; Qu, D.; Liang, X.; Wang, L. Characterization of purified red cabbage anthocyanins: Improvement in HPLC separation and protective effect against H2O2-induced oxidative stress in HepG2 cells. Molecules 2019, 24, 124. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, H.; Zhang, G.; Meng, J.; Deng, K.; Zhou, W.; Wang, H.; Wang, Z.; Hu, N.; Suo, Y. Anthocyanins from Lycium ruthenicum Murr. Ameliorated D-Galactose-induced memory impairment, oxidative stress, and neuroinflammation in adult rats. J. Agric. Food Chem. 2019, 67, 3140–3149. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Zheng, X. Anthocyanin-rich mulberry fruit improves insulin resistance and protects hepatocytes against oxidative stress during hyperglycemia by regulating AMPK/ACC/mTOR pathway. J. Funct. Foods 2017, 30, 270–281. [Google Scholar] [CrossRef]
- Song, Y.; Huang, L.; Yu, J. Effects of blueberry anthocyanins on retinal oxidative stress and inflammation in diabetes through Nrf2/HO-1 signaling. J. Neuroimmunol. 2016, 301, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Menezes, R.; Foito, A.C.; Costa, I.; Garcia, G.; Rosado-Ramos, R.; Freitag, S.; Alexandre, C.J.; Outeiro, T.F.; Stewart, D.; Santos, C.N. Bioprospection of natural sources of polyphenols with therapeutic potential for redox-related diseases. Antioxidants 2020, 9, 789. [Google Scholar] [CrossRef]
- Correa-Betanzo, J.; Allen-Vercoe, E.; McDonald, J.; Schroeter, K.; Corredig, M.; Paliyath, G. Stability and biological activity of wild blueberry (Vaccinium angustifolium) polyphenols during simulated in vitro gastrointestinal digestion. Food Chem. 2014, 165, 522–531. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Conte, A. The first tract of alimentary canal as an extractor. Release of phytochemicals from solid food matrices during simulated digestion. J. Food Biochem. 2011, 36, 555–568. [Google Scholar] [CrossRef]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Lamaison, J.L.; Rémésy, C. Anthocyanins are efficiently absorbed from the stomach in anesthetized rats. J. Nutr. 2003, 133, 4178–4182. [Google Scholar] [CrossRef]
- Röhrig, T.; Kirsch, V.; Schipp, D.; Galan, J.; Richling, E. Absorption of anthocyanin rutinoside after consumption of blackcurrant (Ribes nugrum L.) extract. J. Agric. Food Chem. 2019, 67, 6792–6797. [Google Scholar] [CrossRef]
- Garcia, G.; Nanni, S.; Figueira, I.; Ivanov, I.; McDougall, G.J.; Stewart, D.; Ferreira, R.B.; Pinto, P.; Silva, R.F.M.; Brites, D.; et al. Bioaccessible (poly)phenol metabolites from raspberry protect neural cells from oxidative stress and attenuate microglia activation. Food Chem. 2017, 215, 274–283. [Google Scholar] [CrossRef]
- Garcia, G.; Pais, T.F.; Pinto, P.; Dobson, G.; McDougall, G.J.; Stewart, D.; Santos, C.N. Bioaccessible raspberry extracts enriched in ellagitannins and ellagic acid derivatives have anti-neuroinflammatory properties. Antioxidants 2020, 9, 970. [Google Scholar] [CrossRef] [PubMed]
- Eleutherio, E.; Brasil, A.A.; França, M.B.; Almeida, D.S.G.; Rona, B.; Magalhães, R.S.S. Oxidative stress and aging: Learning from yeast lessons. Fungal Biol. 2018, 122, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Tenreiro, S.; Franssens, V.; Winderikx, J.; Outeiro, T.F. Yeast models of Parkinson’s disease-associated molecular pathologies. Curr. Opin. Genet. Dev. 2017, 44, 74–83. [Google Scholar] [CrossRef]
- Karlsen, A.; Retterstøl, L.; Laake, P.; Paur, I.; Kjølsrud-Bøhn, S.; Sandvik, L.; Blomhoff, R. Anthocyanins inhibit nuclear factor-κB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J. Nutr. 2007, 137, 1951–1954. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Heiss, C.; Borges, G.; Crozier, A. Berry (poly)phenols and cardiovascular health. J. Agric. Food Chem. 2014, 62, 3842. [Google Scholar] [CrossRef]
- Del Bo, C.; Riso, P.; Campolo, J.; Møller, P.; Loft, S.; Klimis-Zacas, D.; Brambilla, A.; Rizzolo, A.; Porrini, M. A single portion of blueberry (Vaccinum corymbosum L) improves protection against DNA damage but not vascular function in healthy male volunteers. Nutr. Res. 2013, 33, 220–227. [Google Scholar] [CrossRef]
- Chan, S.W.; Tomlinson, B. Effects of Bilberry supplementation on metabolic and cardiovascular disease risk. Molecules 2020, 25, 1653. [Google Scholar] [CrossRef]
- Arevstrom, L.; Bergh, C.; Landberg, R.; Wu, H.; Rodriguez-Mateos, A.; Waldenborg, M.; Magnuson, A.; Blanc, S.; Frobert, O. Freeze-dried bilberry (Vaccinum myrtillus) dietary supplement improves walking distance and lipids after myocardial infarction: An open-label randomized clinical trial. Nutr. Res. 2019, 62, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, T.; Akpinar-Bayizil, A.; Yilmaz-Ersan, L.; Delikanli, B. Phenolics in human health. Int. J. Chem. Eng. 2014, 5, 393–396. [Google Scholar] [CrossRef]
- Dani, C.; Pasquali, M.A.; Oliveira, M.R.; Umezu, F.M.; Salvador, M.; Henriques, J.A.; Moreira, J.C. Protective effects of purple grape juice on carbon tetrachloride-induced oxidative stress in brains of adults Wistar rats. J. Med. Food 2008, 11, 55–61. [Google Scholar] [CrossRef]
- Jiménez, A.; Lisa-Santamaría, P.; García-Mariano, M.; Escribano-Bailón, M.T.; Rivas-Gonzalo, J.C.; Revuelta, J. The biological activity of the wine anthocyanins delphinidin and petunidin is mediated through Msn2 and Msn4 in Saccharomyces cerevisiae. FEMS Yeast Res. 2010, 10, 858–869. [Google Scholar] [CrossRef]
- Winter, A.N.; Bickford, P.C. Anthocyanins and their metabolites as therapeutic agents for neurodegenerative disease. Antioxidants 2019, 8, 333. [Google Scholar] [CrossRef]
- Wu, T.; Gao, Y.; Guo, X.; Zhang, M.; Gong, L. Blackberry and blueberry anthocyanin supplementation counteract high-fat-diet-induced obesity by alleviating oxidative stress and inflammation and accelerating energy expenditure. Oxid. Med. Cell Longev 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Busch, C.; Burkard, M.; Leischner, C.; Lauer, U.M.; Frank, J.; Venturelli, S. Epigenetic activities of flavonoids in the prevention and treatment of cancer. Clin. Epigenetics 2015, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Huang, C.; Chang, Y. Propyl gallate inhibits hepatocellular carcinoma cell growth through the induction of ROS and the activation of autophagy. PLoS ONE 2019, 14, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Macedo, D.; Jardim, C.; Figueira, I.; Almeida, F.; McDougall, G.J.; Stewart, D.; Yuste, J.E.; Tomás-Barberán, F.A.; Terneiro, S.; Outeiro, T.F.; et al. (Poly)phenol-digested metabolites modulated alpha-synuclein toxicity by regulating proteostasis. Sci. Rep. 2018, 8, 6965. [Google Scholar] [CrossRef]
- Tavares, L.; Figueira, I.; Macedo, D.; McDougall, G.J.; Leitão, M.C.; Vieira, H.L.A.; Stewart, D.; Alves, P.M.; Ferreira, R.B.; Santos, C.N. Neuroprotective effect of blackberry (Rubus sp.) polyphenols is potentiated after simulated gastrointestinal digestion. Food Chem. 2012, 131, 1443–1452. [Google Scholar] [CrossRef]
- Jardim, C.; Macedo, D.; Figueira, I.; Dobson, G.; McDougall, G.J.; Stewart, D.; Ferreira, R.B.; Menezes, R.; Santos, C.N. (Poly)phenols metabolites from Arbutus unedo leaves protect yeast from oxidative injury by activation of antioxidant protein clearance pathways. J. Funct. Foods 2017, 32, 333–346. [Google Scholar] [CrossRef]
- Luo, H.; Li, W.; Zhang, X.; Deng, S.; Xu, Q.; Hou, T.; Pang, X.; Zhang, Z.; Zhang, X. In plant high levels of hydrolysable tannins inhibit peroxidase mediated anthocyanin degradation and maintain abaxially red leaves of Excoecaria cochinchinesis. BMC Plant Biol. 2019, 19, 315. [Google Scholar] [CrossRef] [PubMed]
- Horváth, E.; Szalai, G.; Pál, M.; Páldi, E.; Janda, T. Differences between the catalase isozymes of maize (Zea mays L.) in respect of inhibition by various phenolic compounds. Acta. Biol. Szeged 2002, 46, 33–34. [Google Scholar]
- Bendokas, V.; Šarkinas, A.; Jasinauskienè, D.; Anisiomovienè, N.; Morkunaitè-Haimi, Š.; Stanya, V.; Šikšnianas, T. Antimicrobial activity of berries extracts of four Ribes species their phenolic content and anthocyanin composition. Foila. Hort. 2018, 30, 249–257. [Google Scholar] [CrossRef]
- Brown, E.; Nitecki, S.; Pereira-Caro, G.; McDougall, G.J.; Stewart, D.; Rowland, I.; Crozier, A.; Gill, C.I.R. Comparison of in vivo and in vitro digestion on polyphenol composition in lingonberries: Potential impact on colonic health. Biofactors 2014, 40, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.B.; Tylewicz, U.; Dalla Rosa, M.; Andlid, T.; Alminger, M. Effects of pulsed electric field-assisted osmotic dehydration and edible coating on the recovery of anthocyanins from in vitro digested berries. Foods 2019, 8, 505. [Google Scholar] [CrossRef]
- Riihinen, K.; Jaakola, L.; Kärenlampi, S.; Hohtola, A. Organ-specific distribution of phenolic compounds in bilberry (Vaccinium myrtillus) and “northblue” blueberry (Vaccinium corymbosum x V. angustifolium). Food Chem. 2008, 110, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Edirisinghe, I.; Banaszewski, K.; Cappozzo, J.; Sandhya, K.; Ellis, C.L.; Tadapaneni, R.; Kappagoda, C.T.; Burton-Freeman, B.M. Strawberry anthocyanin and its association with postprandial inflammation and insulin. Br. J. Nutr. 2011, 106, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Nour, V.; Stampar, F.; Veberic, R.; Jakopic, J. Anthocyanins profile, total phenolics and antioxidant activity of black currant ethanolic extracts as influenced by genotype and ethanol concentration. Food Chem. 2013, 141, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, I.A.; Mena, P.; Calani, L.; Borges, G.; Pereira-Caro, G.; Bresciani, L.; Del Rio, D.; Lean, M.E.J.; Crozier, A. New insights into the bioavailability of red raspberry anthocyanins and ellagitannins. Free Rad. Biol. Mod. 2015, 89, 758–769. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, C.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardized static in vitro digestion method suitable for food – an international consensus. Food Nutr. 2014, 5, 1113–1124. [Google Scholar]
- You, Q.; Wang, B.; Chen, F.; Huang, Z.; Wang, X.; Luo, P.G. Comparison of anthocyanins and phenolics in organically and conventionally grown blueberries in selected cultivars. Food Chem. 2011, 125, 201–208. [Google Scholar] [CrossRef]
- Andlid, T.; Blomberg, L.; Gustafsson, L.; Blomberg, A. Characterization of Saccharomyces cerevisiae CBS7764 Isolated from Rainbow trout intestine. System Appl. Microbiol. 1999, 22, 145–155. [Google Scholar] [CrossRef]
- Norbeck, J.; Blomberg, A. The level of cAMP-dependent protein kinase a activity strongly affects osmotolerance and osmo-instigated gene expression changes in Saccharomyces cerevisiae. Yeast 2000, 16, 121–137. [Google Scholar] [CrossRef]
- Maslanka, R.; Zadrag-Tecza, R.; Kwolek, K.; Kwolek-Mirek, M. The effect of berry juices on the level of oxidative stress in yeast cells exposed to acrylamide. J. Food Chem. 2016, 40, 686–695. [Google Scholar] [CrossRef]
- Oprea, E.; Ruta, L.L.; Nicolau, I.; Popa, C.V.; Neagoe, A.D.; Farcasanu, I.C. Vaccinium corymbosum L. (blueberry) extracts exhibit protective action against cadmium toxicity in Saccharomyces cerevisiae. Food Chem. 2014, 152, 516–521. [Google Scholar] [CrossRef] [PubMed]
| Nondigested (mg g−1 DW d) | SE a | Digested (mg g−1 DW) | SE | p Value (α = 0.05) b | |
|---|---|---|---|---|---|
| Bilberry | |||||
| Cyanidin-3-O-arabinose | 1.43 | 0.07 | 0.07 | 0.01 | 0.003 |
| Cyanidin-3-O-galactoside | 0.78 | 0.05 | 0.06 | 0.00 | 0.005 |
| Cyanidin-3-O-glucoside | 1.29 | 0.06 | 0.10 | 0.01 | 0.003 |
| Cyanidin-3-O-rutinoside | 0.51 | 0.03 | 0.04 | 0.00 | 0.004 |
| Delphinidin-3-O-galactoside | 1.07 | 0.05 | 0.05 | 0.01 | 0.003 |
| Delphinidin-3-O-glucoside | 1.91 | 0.10 | 0.04 | 0.00 | 0.003 |
| Delphinidin-3-O-rutinoside | 1.29 | 0.07 | 0.03 | 0.00 | 0.003 |
| Malvidin-3-O-galactoside | 0.43 | 0.02 | 0.05 | 0.00 | 0.005 |
| Malvidin-3-O-glucoside | 1.32 | 0.06 | 0.14 | 0.01 | 0.003 |
| Pelargonidin-3-O-glucoside | 0.15 | 0.02 | 0.02 | 0.00 | 0.022 |
| Cyanidin aglycone | 0.23 | 0.01 | ND c | ||
| Delphinidin aglycone | 0.19 | 0.01 | 0.01 | 0.00 | 0.001 |
| Malvidin aglycone | 0.08 | 0.00 | ND | ||
| Pelargonidin aglycone | 1.04 | 0.05 | 0.11 | 0.01 | 0.003 |
| Peonidin aglycone | 0.07 | 0.00 | ND | ||
| Sum of anthocyanins | 11.78 | 0.71 | |||
| Blackcurrant | |||||
| Cyanidin-3-O-arabinose | 0.067 | 0.004 | ND | ||
| Cyanidin-3-O-galactoside | 2.505 | 0.250 | 0.261 | 0.021 | 0.012 |
| Cyanidin-3-O-glucoside | 0.322 | 0.036 | 0.045 | 0.004 | 0.016 |
| Cyanidin-3-O-rutinoside | 1.258 | 0.128 | 0.182 | 0.013 | 0.013 |
| Delphinidin-3-O-glucoside | 0.739 | 0.074 | 0.074 | 0.007 | 0.012 |
| Malvidin-3-O-galactoside | 0.028 | 0.016 | 0.005 | 0.002 | 0.221 |
| Malvidin-3-O-glucoside | 0.018 | 0.018 | ND | ||
| Pelargonidin-3-O-glucoside | ND | 0.003 | 0.003 | ||
| Delphinidin aglycone | ND | 0.002 | |||
| Malvidin aglycone | ND | 0.001 | 0.001 | ||
| Pelargonidin aglycone | 0.026 | 0.013 | 0.004 | 0.000 | 0.237 |
| Peonidin aglycone | 0.063 | 0.001 | ND | ||
| Sum of anthocyanins | 5.03 | 0.58 | |||
| Raspberry | |||||
| Cyanidin-3-O-galactoside | 0.114 | 0.007 | 0.009 | 0.001 | 0.005 |
| Cyanidin-3-O-glucoside | 0.129 | 0.003 | 0.014 | 0.001 | 0.001 |
| Cyanidin-O-rutinoside | 0.026 | 0.001 | 0.003 | 0.000 | 0.003 |
| Delphinidin-3-O-glucoside | 0.623 | 0.020 | 0.050 | 0.005 | 0.002 |
| Delphinidin-3-O-rutinoside | ND | 0.002 | 0.000 | ||
| Sum of anthocyanins | 0.89 | 0.08 | |||
| Strawberry | |||||
| Cyanidin-3-O-glucoside | 0.034 | 0.001 | 0.003 | 0.001 | 0.001 |
| Cyanidin-3-O-rutinoside | 0.656 | 0.003 | 0.087 | 0.015 | 0.001 |
| Cyanidin aglycone | ND | 0.005 | 0.001 | ||
| Pelargonidin aglycone | 0.049 | 0.000 | 0.007 | 0.001 | 0.001 |
| Sum of anthocyanins | 0.74 | 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, G.; Radovanovic, N.; Nunes, M.C.d.N.; Fristedt, R.; Alminger, M.; Andlid, T. Extracts of Digested Berries Increase the Survival of Saccharomyces cerevisiae during H2O2 Induced Oxidative Stress. Molecules 2021, 26, 1057. https://doi.org/10.3390/molecules26041057
Oliveira G, Radovanovic N, Nunes MCdN, Fristedt R, Alminger M, Andlid T. Extracts of Digested Berries Increase the Survival of Saccharomyces cerevisiae during H2O2 Induced Oxidative Stress. Molecules. 2021; 26(4):1057. https://doi.org/10.3390/molecules26041057
Chicago/Turabian StyleOliveira, Gabriel, Nataša Radovanovic, Maria Cecilia do Nascimento Nunes, Rikard Fristedt, Marie Alminger, and Thomas Andlid. 2021. "Extracts of Digested Berries Increase the Survival of Saccharomyces cerevisiae during H2O2 Induced Oxidative Stress" Molecules 26, no. 4: 1057. https://doi.org/10.3390/molecules26041057
APA StyleOliveira, G., Radovanovic, N., Nunes, M. C. d. N., Fristedt, R., Alminger, M., & Andlid, T. (2021). Extracts of Digested Berries Increase the Survival of Saccharomyces cerevisiae during H2O2 Induced Oxidative Stress. Molecules, 26(4), 1057. https://doi.org/10.3390/molecules26041057








