Novel Synthetic Routes to Prepare Biologically Active Quinoxalines and Their Derivatives: A Synthetic Review for the Last Two Decades
Abstract
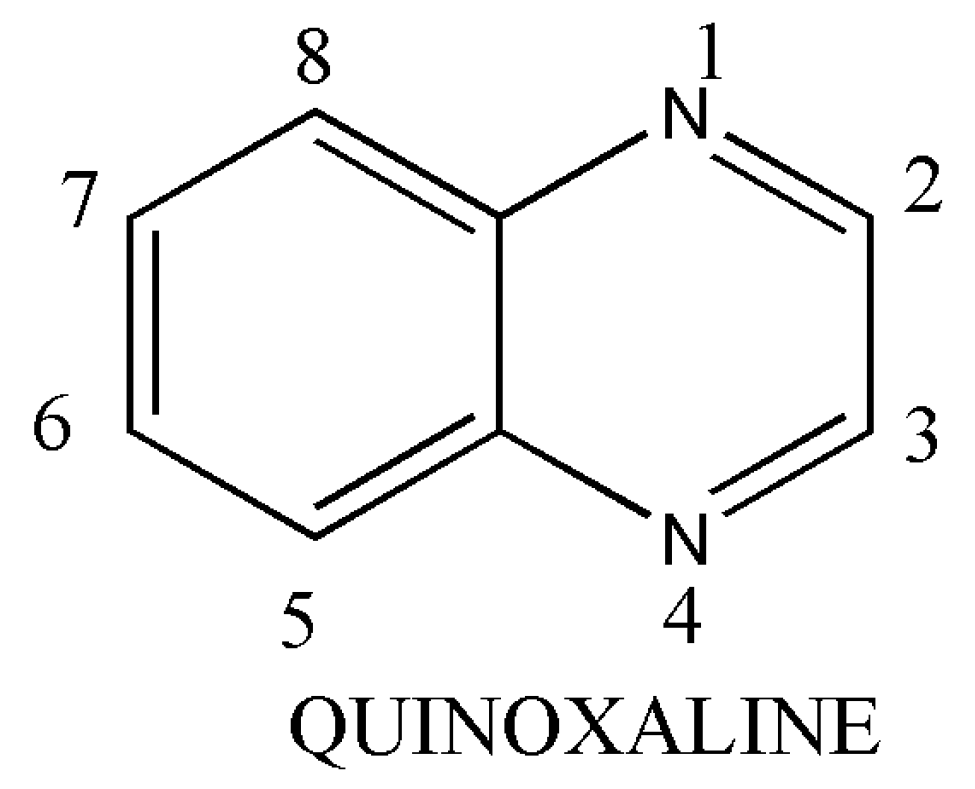
1. Introduction
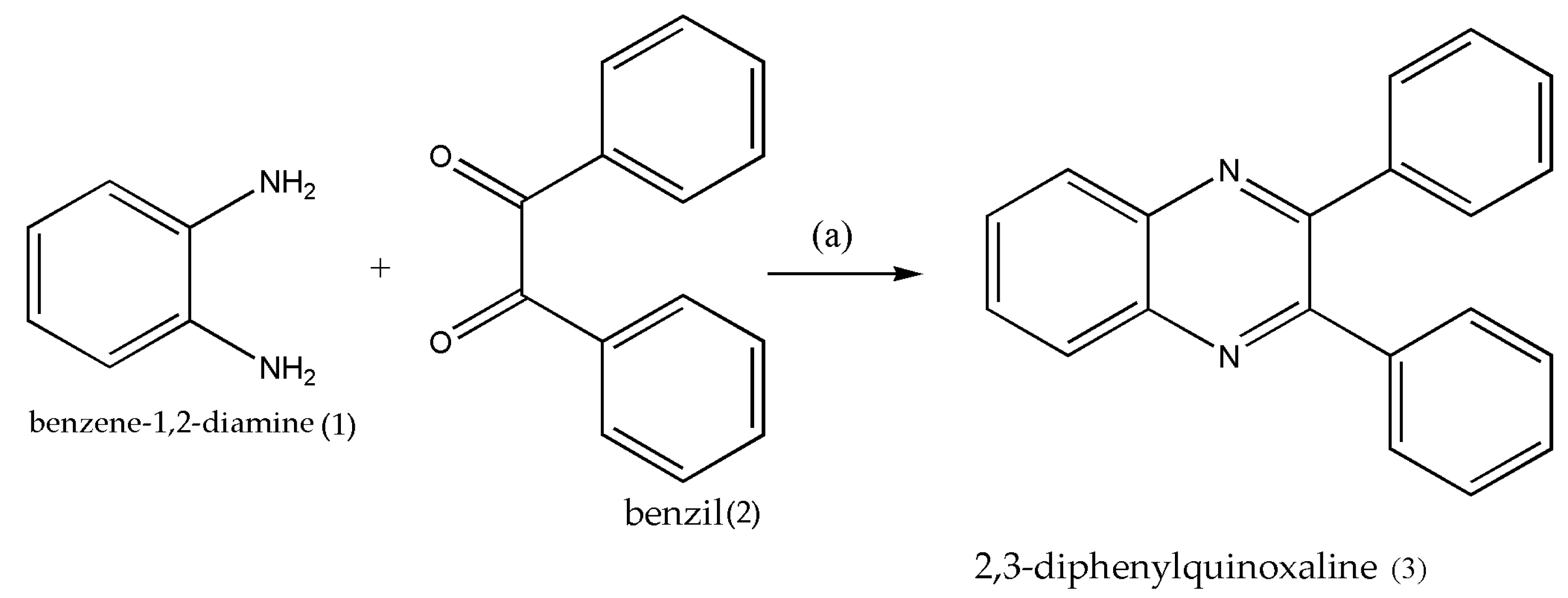
2. Synthetic Pathways to Prepare Quinoxalines via Cost-Effective and Green Synthetic Approach
2.1. Using Bentonite Clay K-10
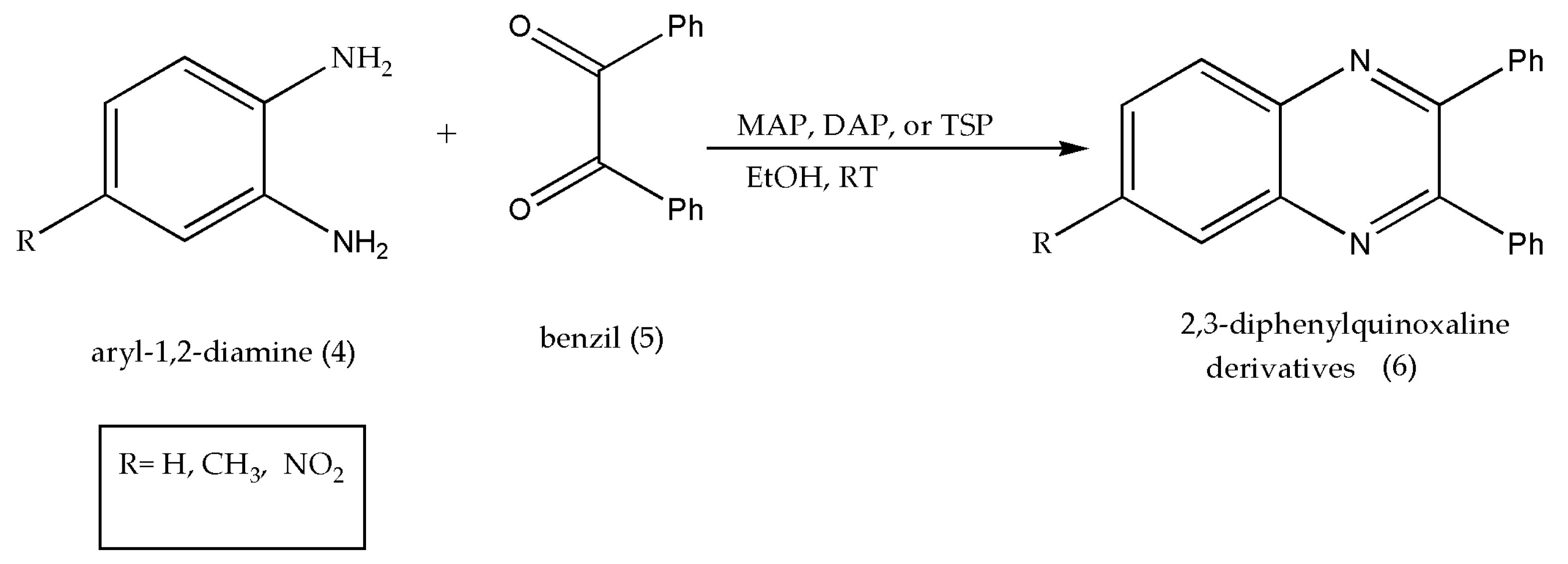
2.2. Using Phosphate Based Heterogeneous Catalyst (MAP, DAP, TSP)
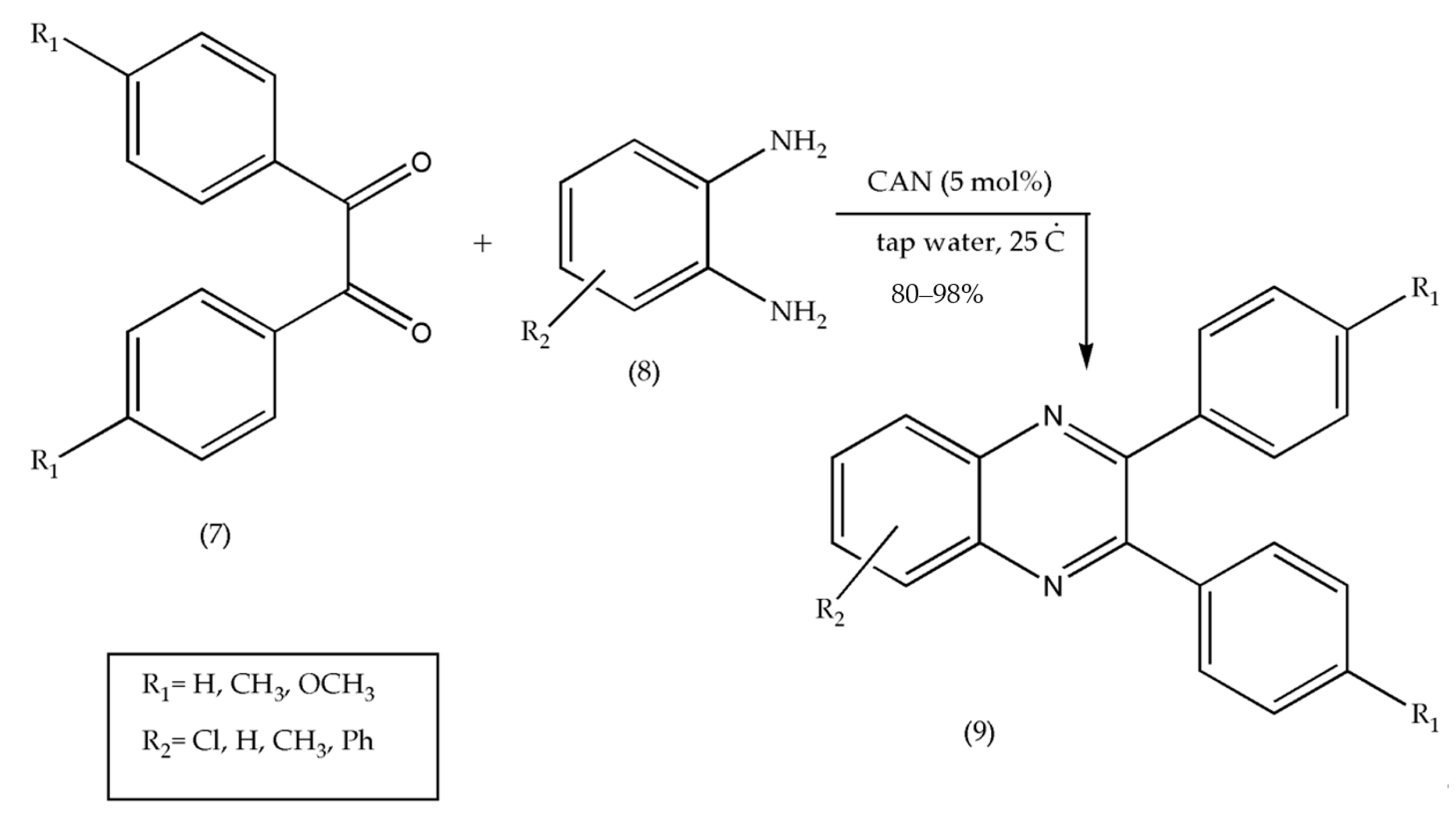
2.3. Using Lanthanide Reagent (CAN)
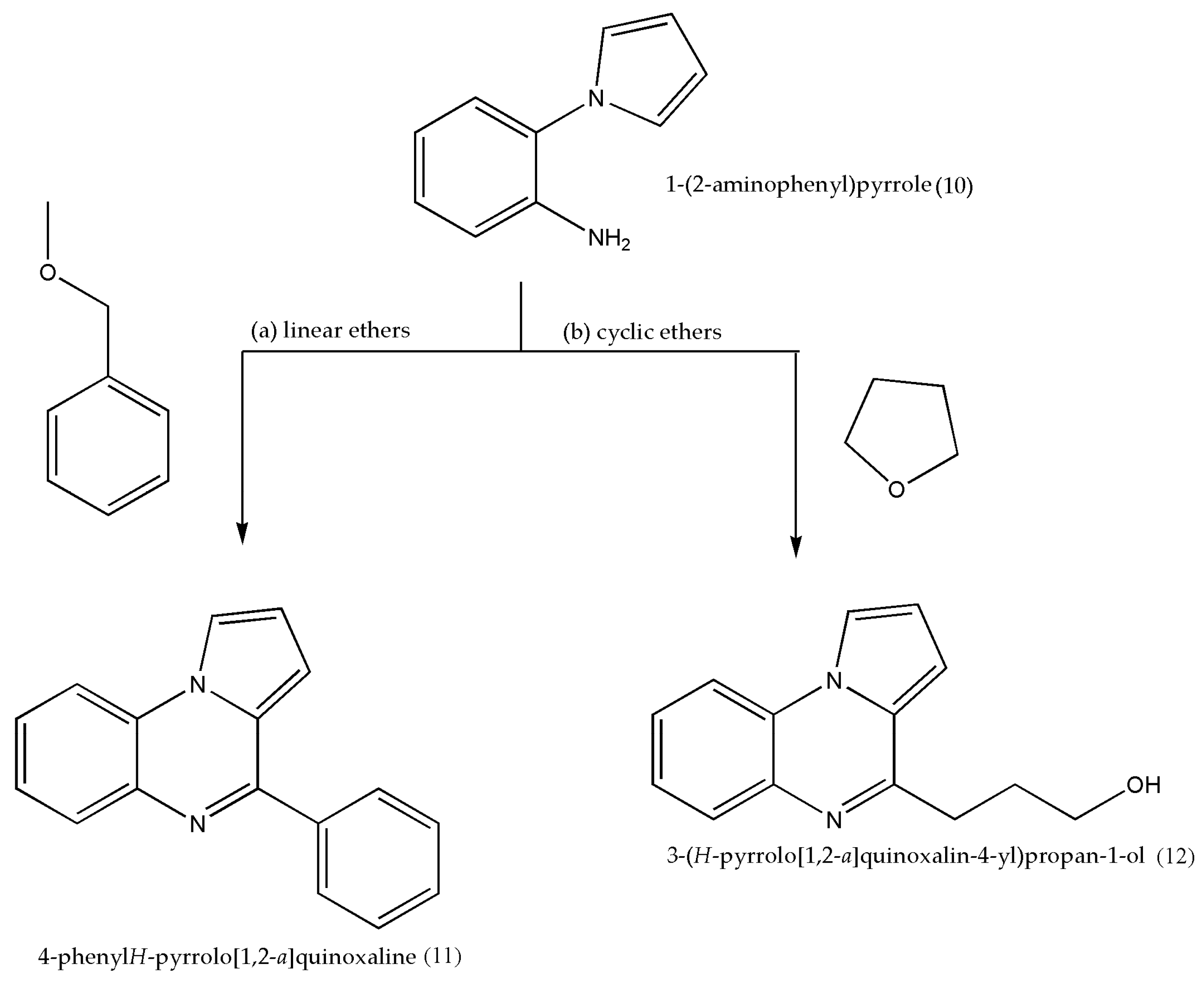
2.4. Using Fe as a Catalyst
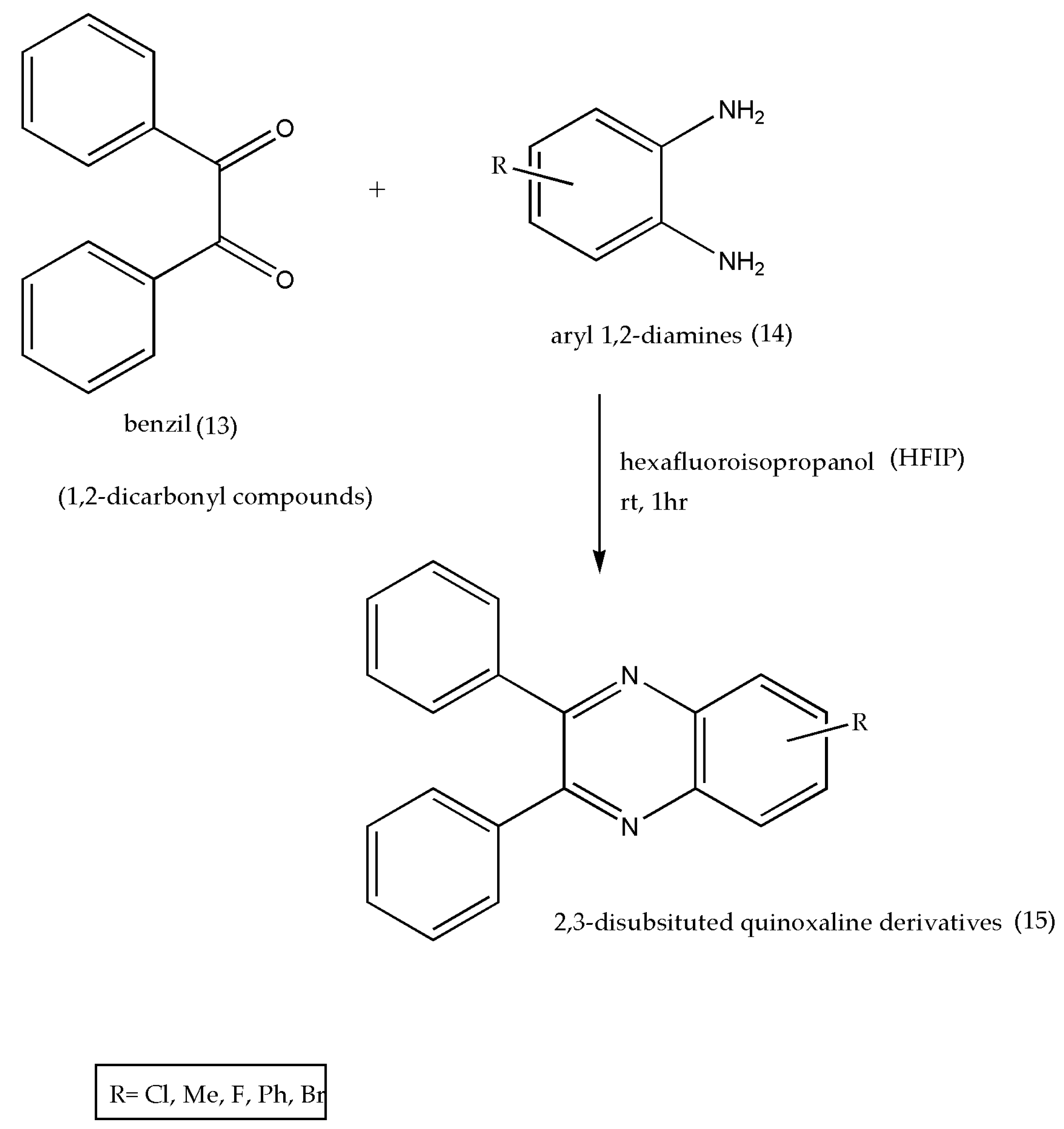
2.5. Using Fluorinated Alcohols (HFIP)
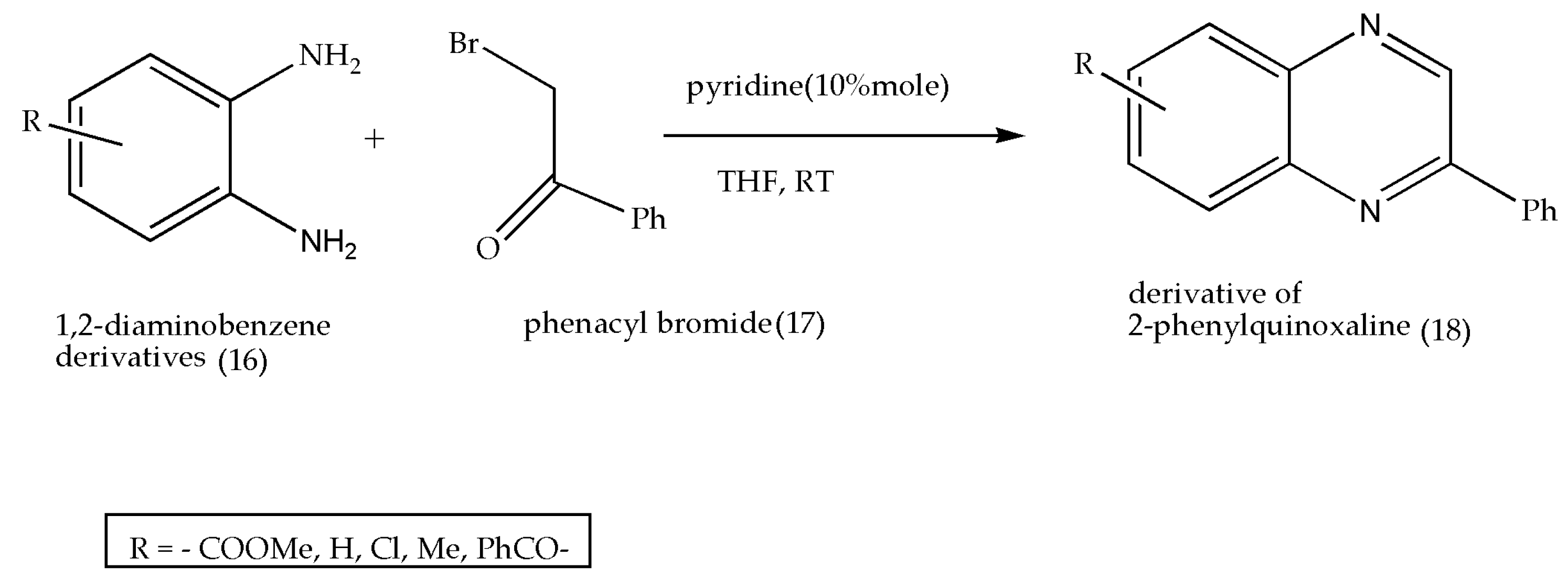
2.6. Using Pyridine as a Catalyst
2.7. Using a Solid Acid Catalyst, TiO2-Pr-SO3H
2.8. Using the Catalytic Amount of Acetic Acid and Aldehydes
2.9. Using an Excess of Secondary Amines in Boiling Benzene
2.10. Using 6-chloro-7-fluoro-1,2-Diaminobenzene with Diketones
3. Synthetic Pathways to Prepare Biologically Active Quinoxaline Derivatives
3.1. Synthesis of Quinoxalin-2-Mercaptoacetyl Urea as Antiviral Agents
3.2. Synthesis of Quinoxaline Nucleosides as Anti-HIV Agents
3.3. Synthesis of Penta-1,4-dien-3-one Oxime Containing a Quinoxaline Nucleus as Antiviral Agents
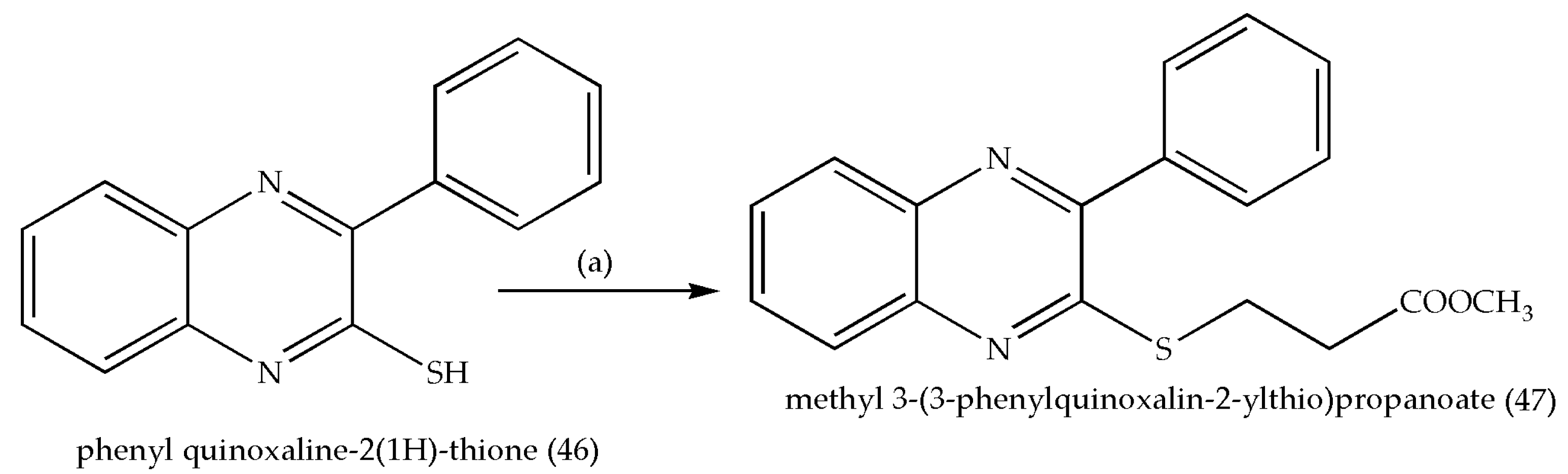
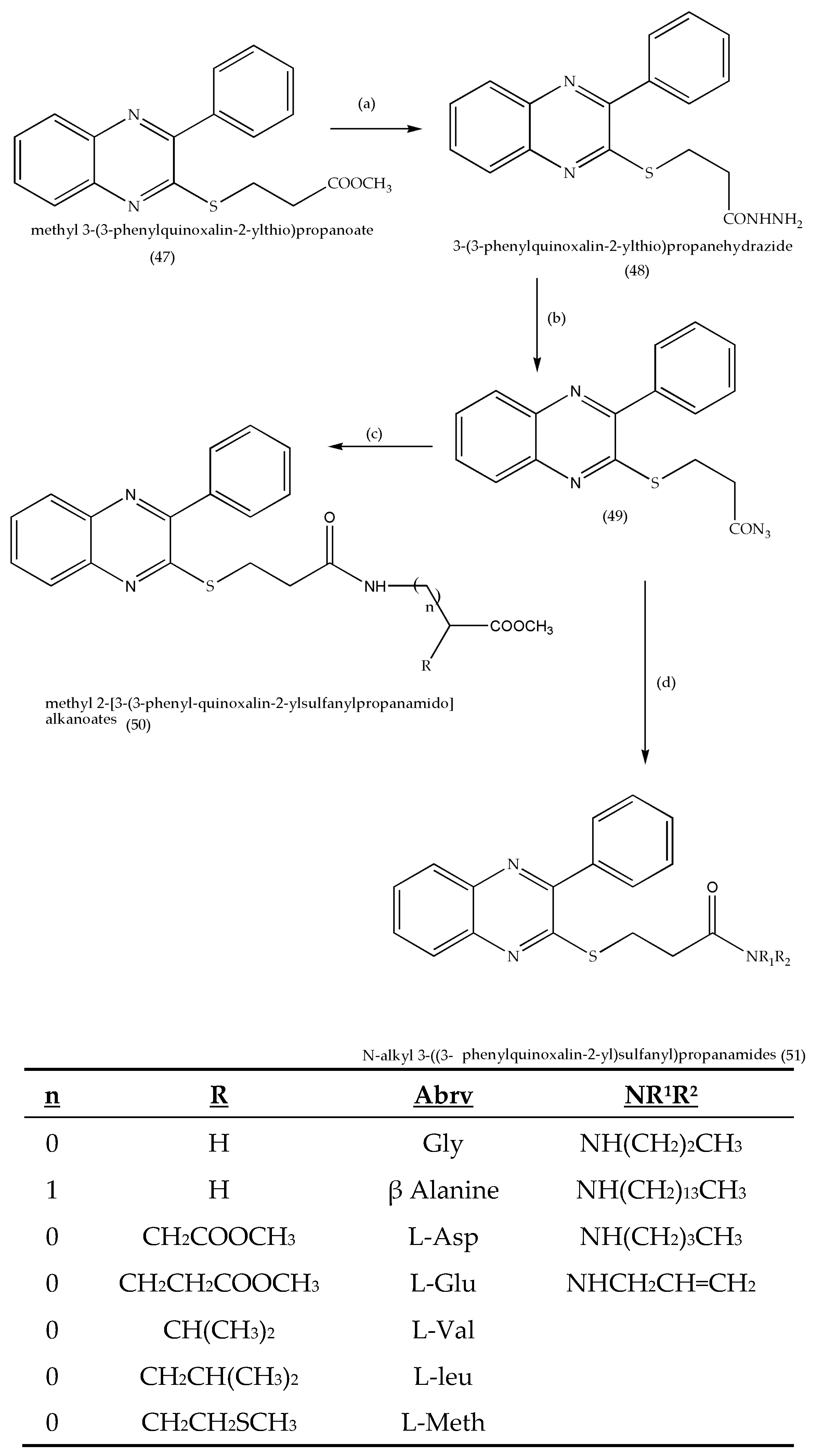
3.4. Synthesis of Methyl-2-[3-(3-phenylquinoxalin-2-ylsulfanyl)propanamidoalkanoates and N-Alkyl-3-((3-phenyl quinoxalin-2ylsulfanyl)propanamides as Antitumor Agents
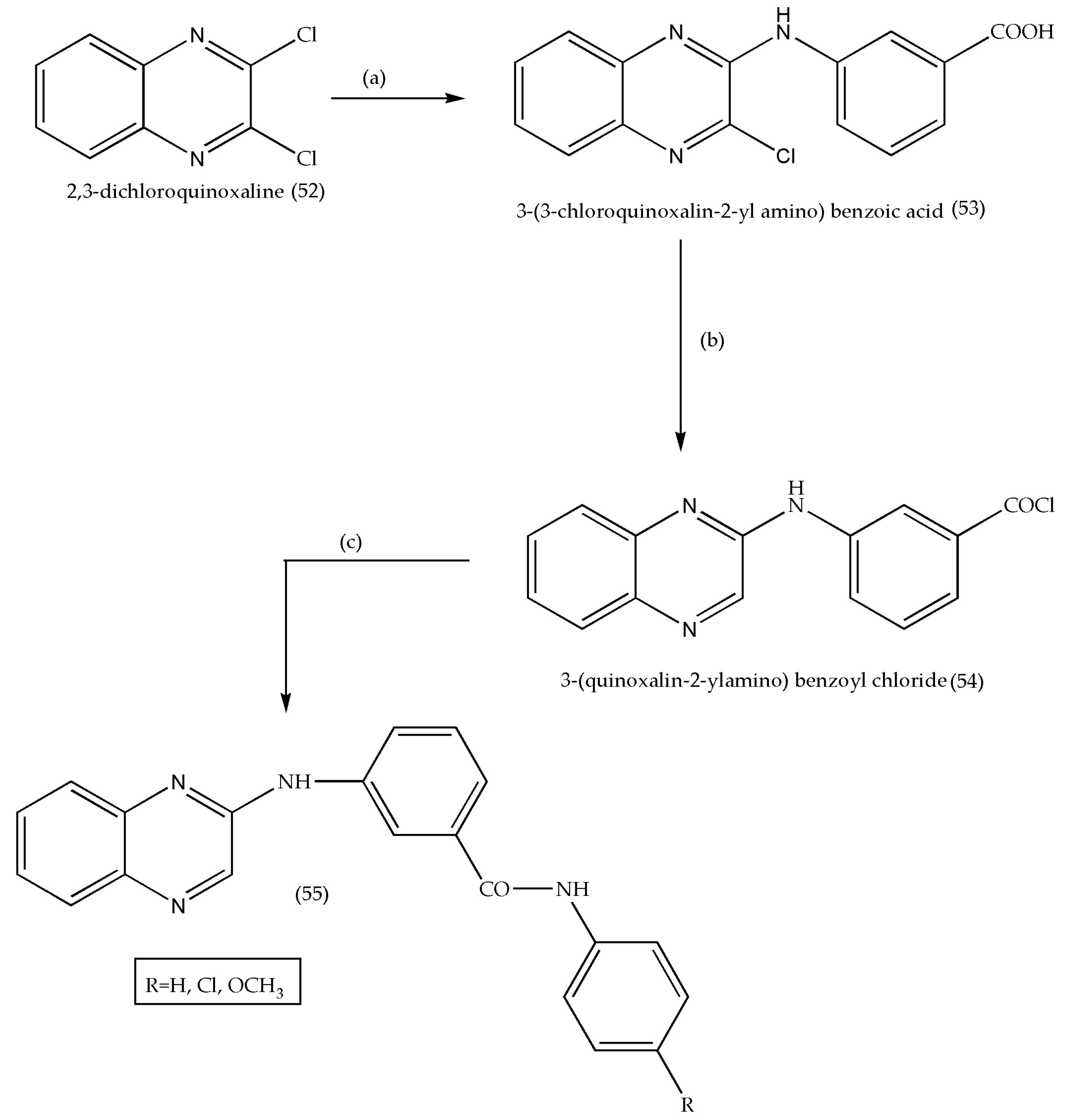
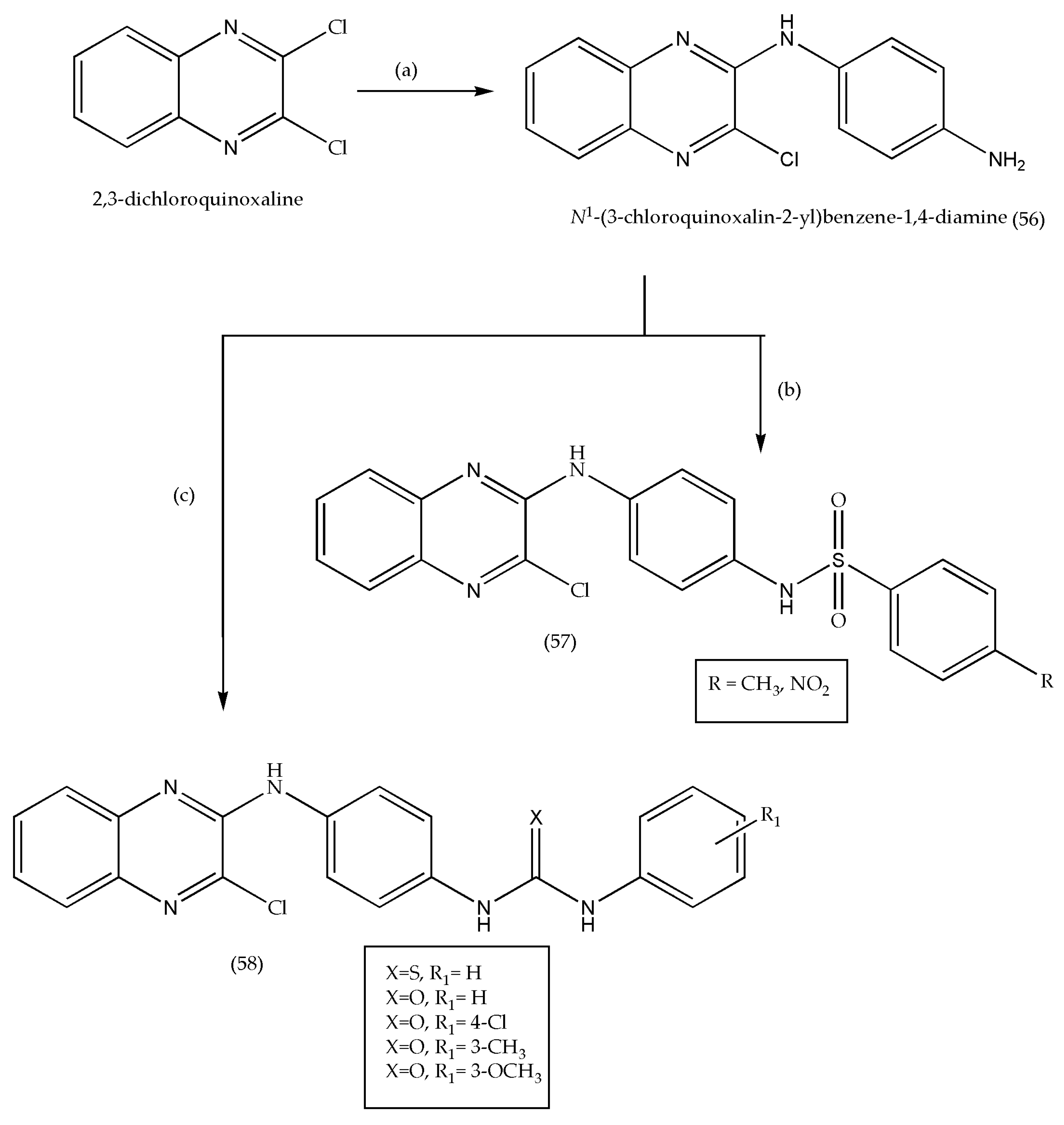
3.5. Synthesis of Quinoxaline Derivatives as Anticancer Agents
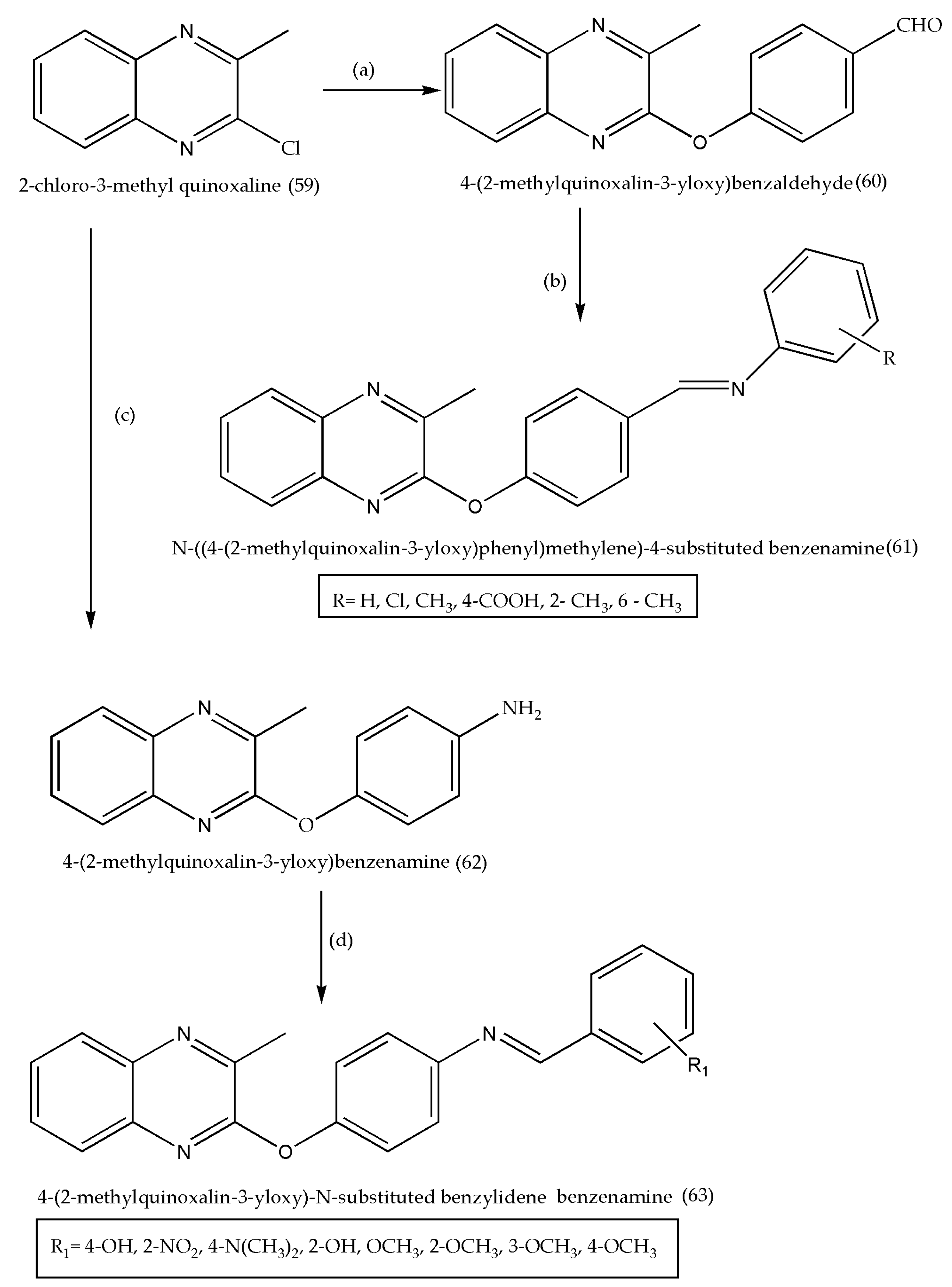
3.6. Synthesis of 4-(2-Methyl quinoxaline-3-yloxy)benzaldehyde and N-((4-(2-methylquinoxaline-3-yloxy)phenyl)methylene)-4-Substituted Benzenamine as Antibacterial/Antifungal Agents
3.7. Synthesis of 2-(5-Arylthiazolo[2,3-c][1,2,4]triazol-3-yl)quinoxaline Derivatives as TP Enzyme Inhibitor
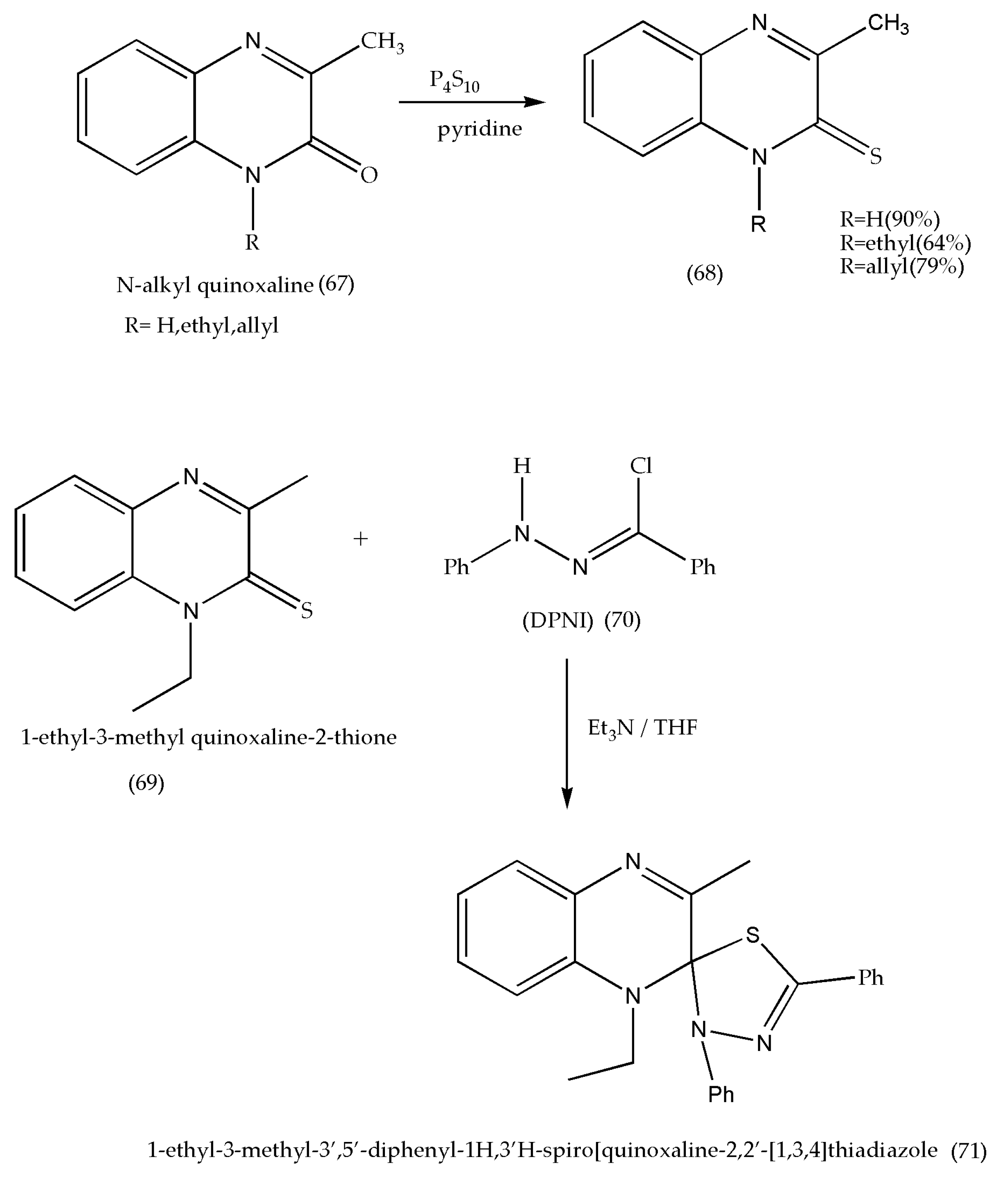
3.8. Synthesis of Spiro[thiadozoline-quinoxaline] Derivatives as Antibacterial Agents
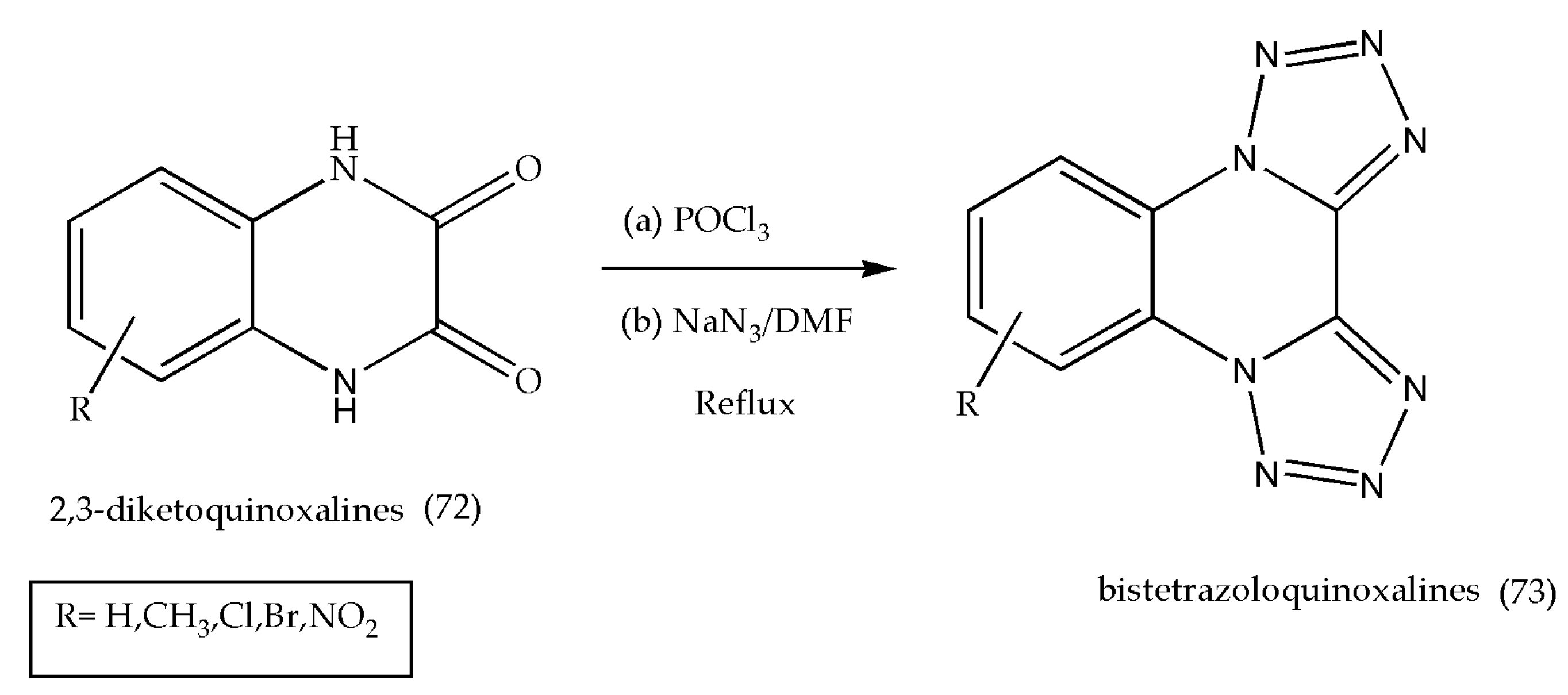
3.9. Synthesis of Bistetrazoloquinoxalines as Antiallergic Agents
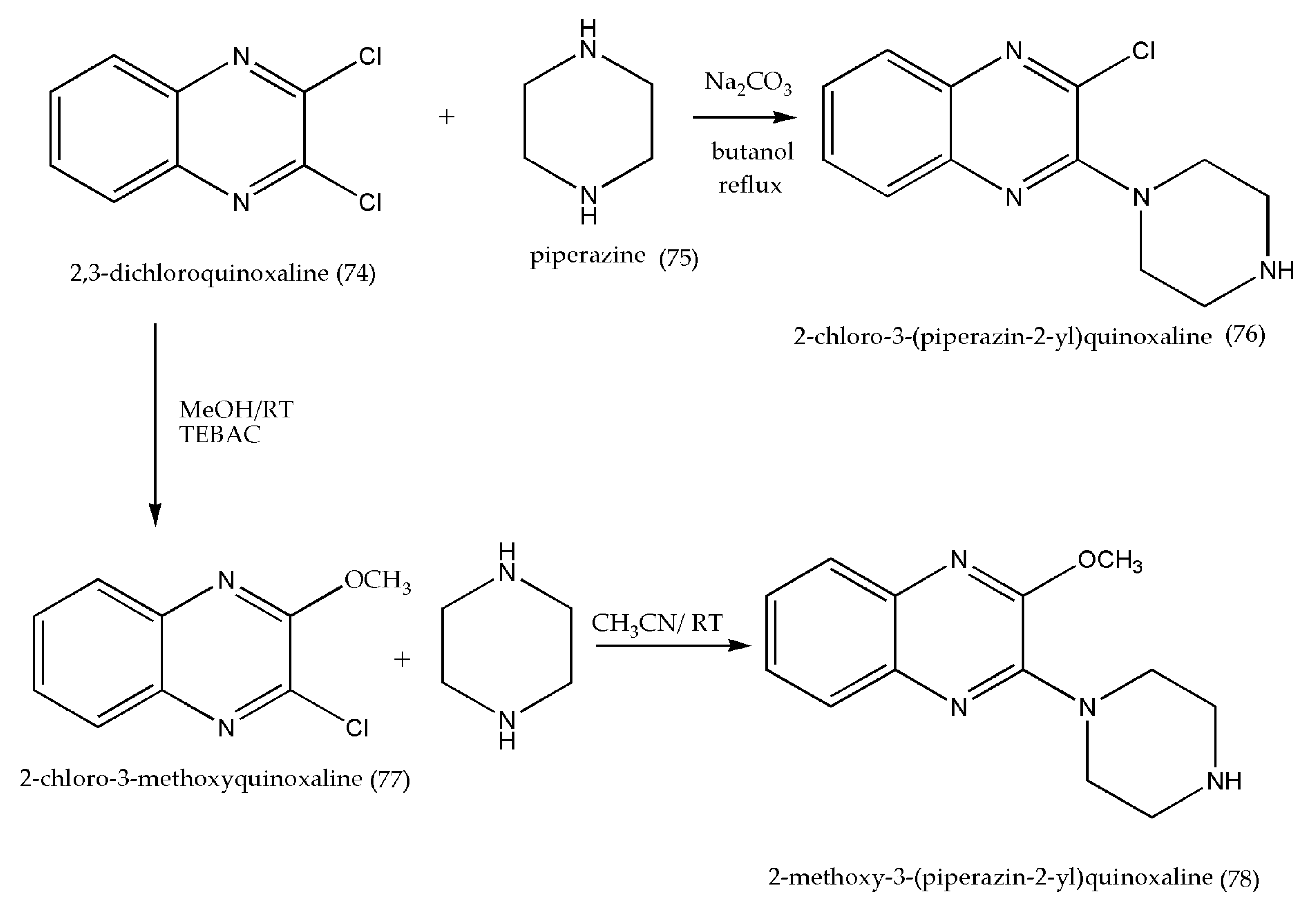
3.10. Synthesis of 4-{4-[2-(4-(2-Substituted quinoxaline-3-yl)piperazin-1-yl)ethyl] phenyl} Thiazoles Antipsychotic Agents
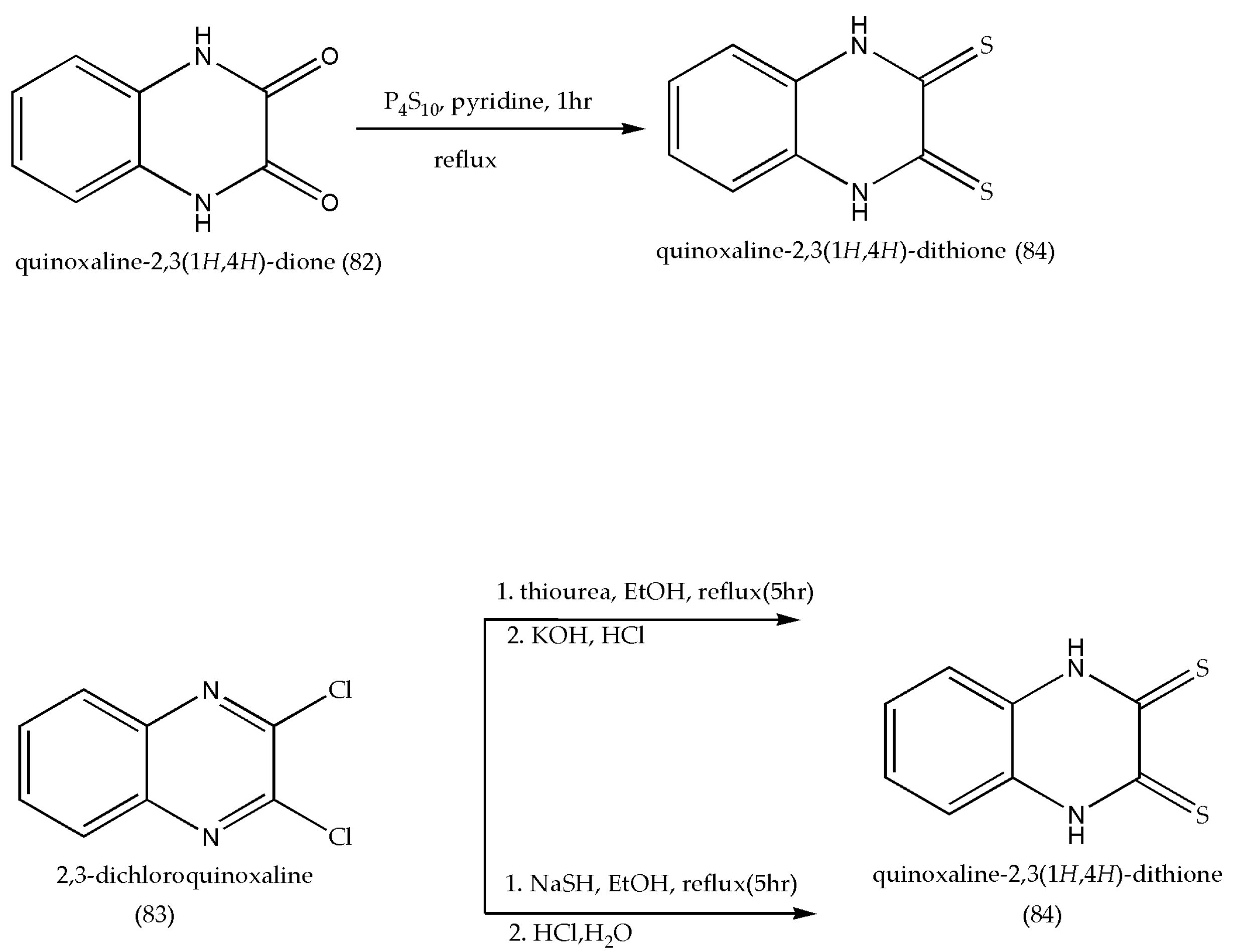
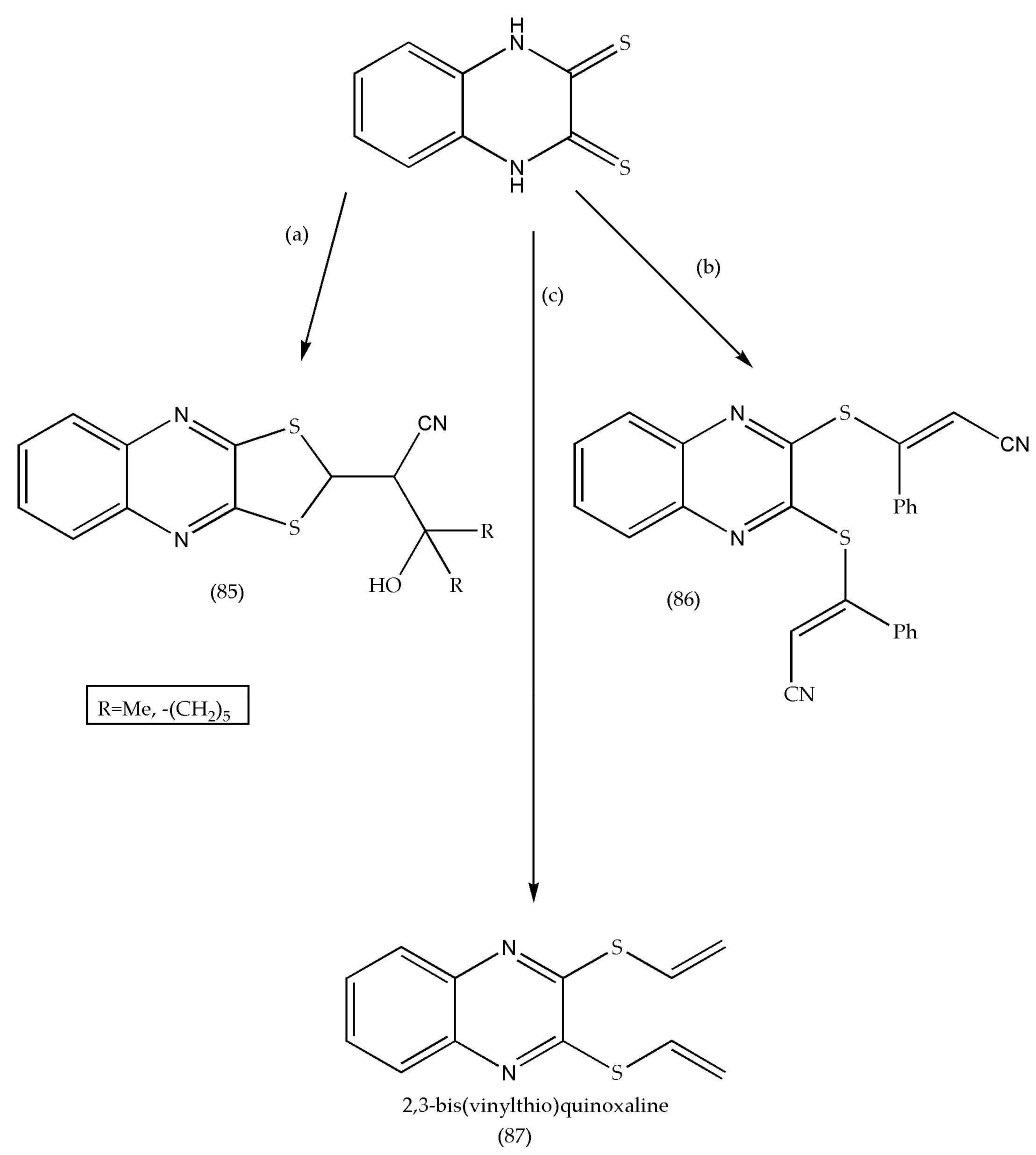
3.11. Synthesis of Quinoxaline-2,3(1H,4H)-Dithione Derivatives as Antimicrobial Agents
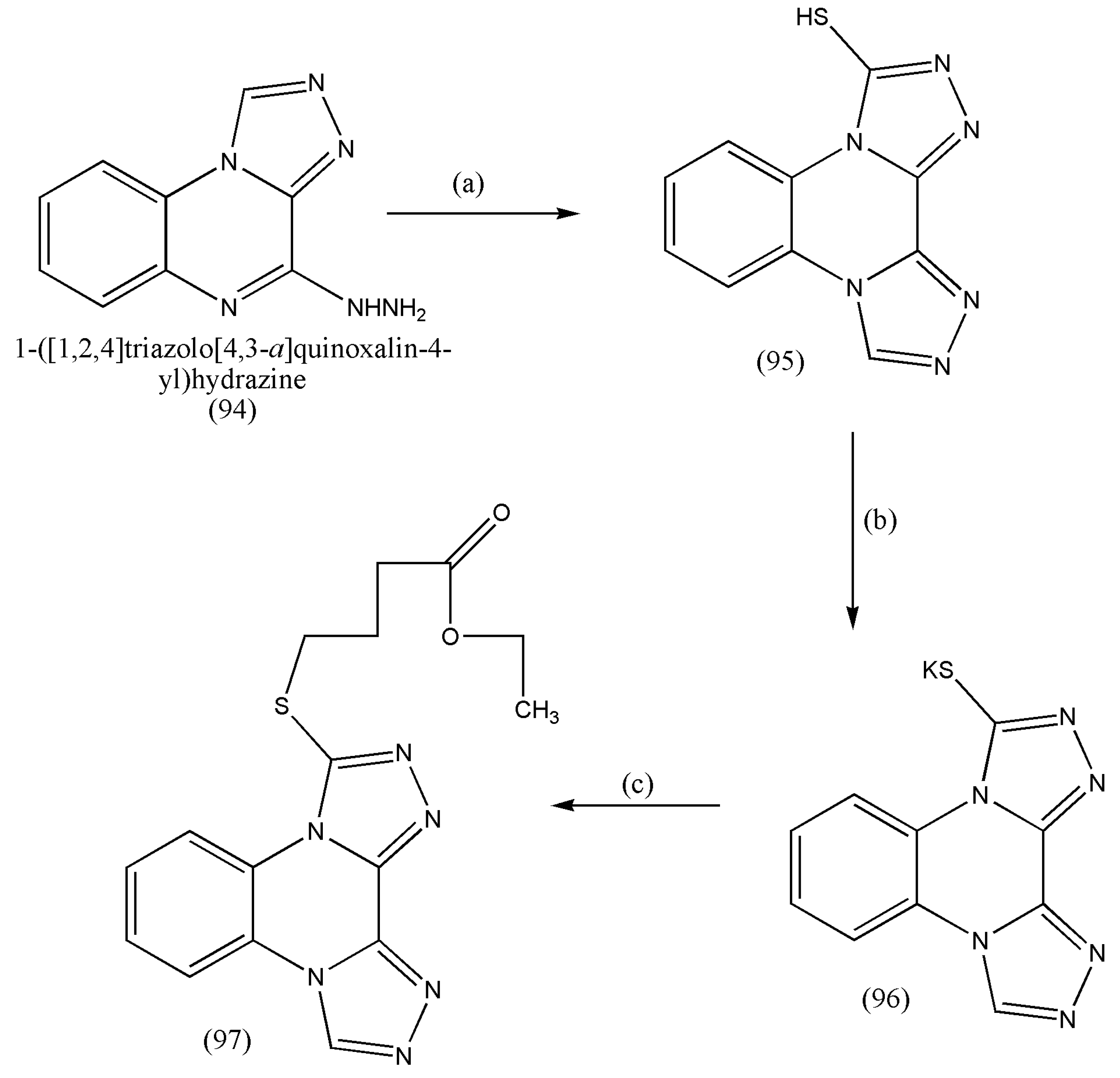
3.12. Synthesis of [1,2,4]triazolo[4,3-a]quinoxaline and bis([1,2,4]triazolo)[4,3-a:3′,4′-c]quinoxaline as Anti-Tumor Agents
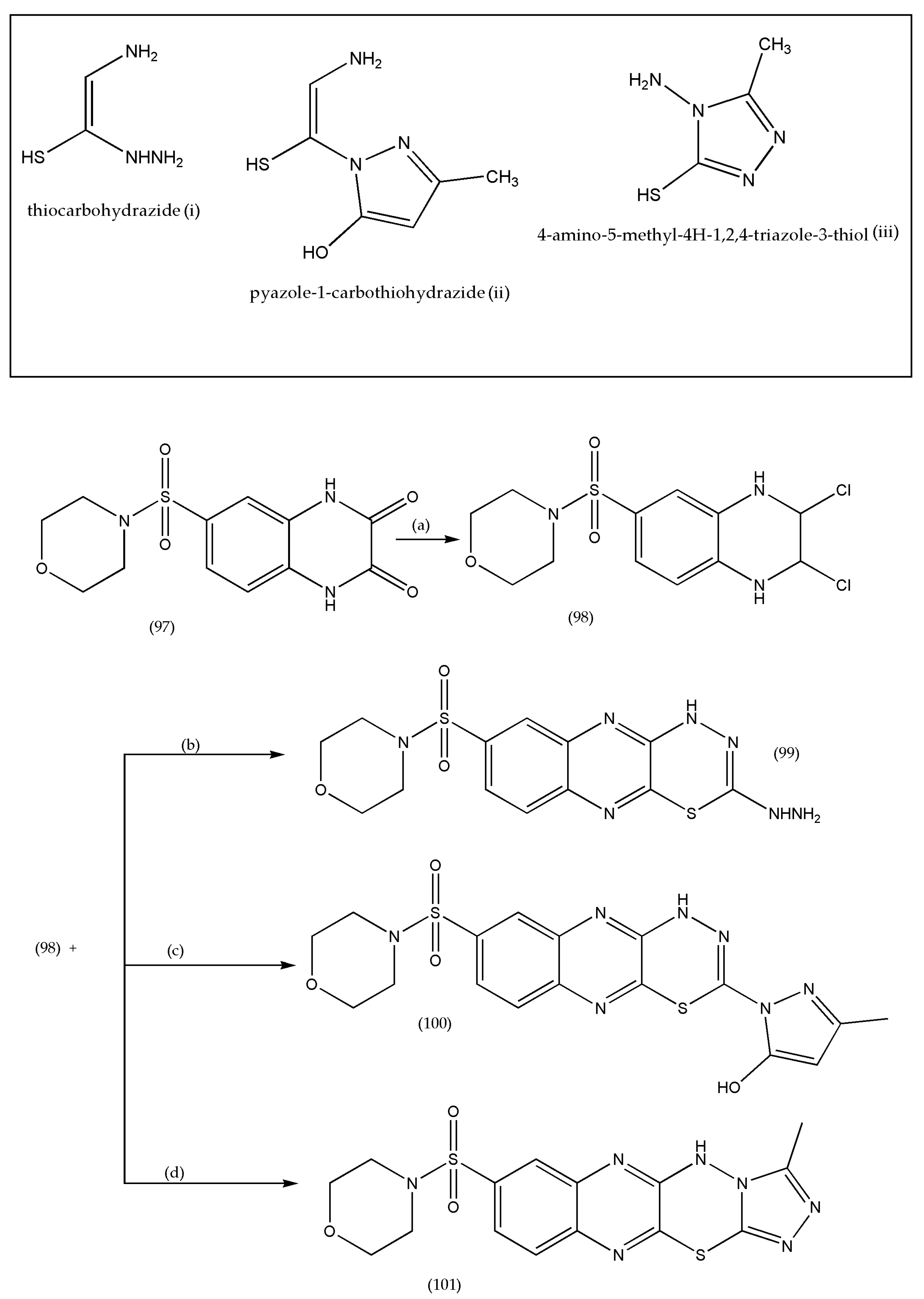
3.13. Synthesis of Thiadiazino and Thiazolo Quinoxaline Derivatives as Antibacterial/Antifungal Agents
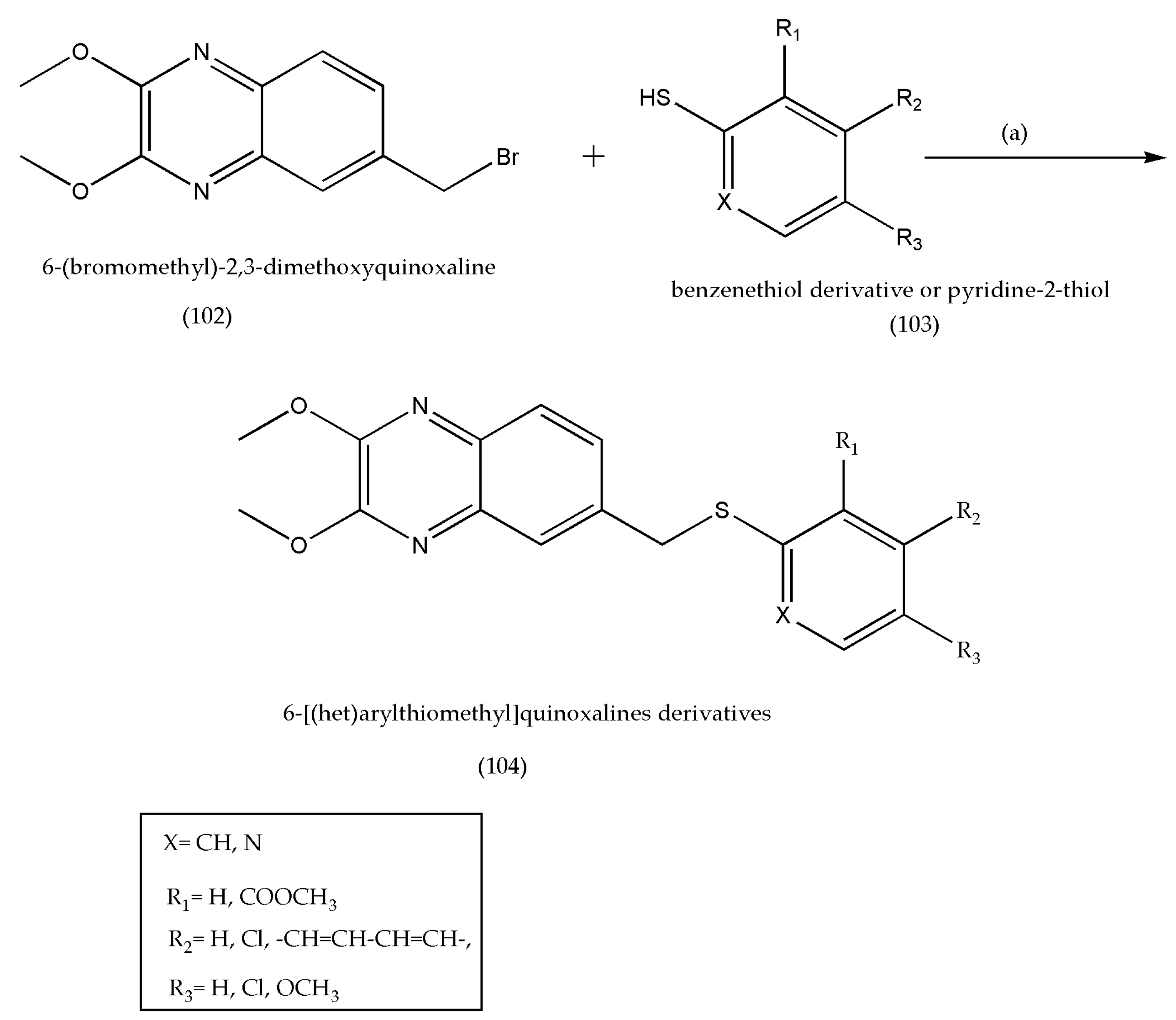
3.14. Synthesis of 6-[(het)arylthiomethyl]quinoxaline Derivatives as Antiviral Agents
3.15. Synthesis of Quinoxaline-2-carboxylate 1,4-dioxide Derivatives as Antimycobacterium Tuberculosis Agents
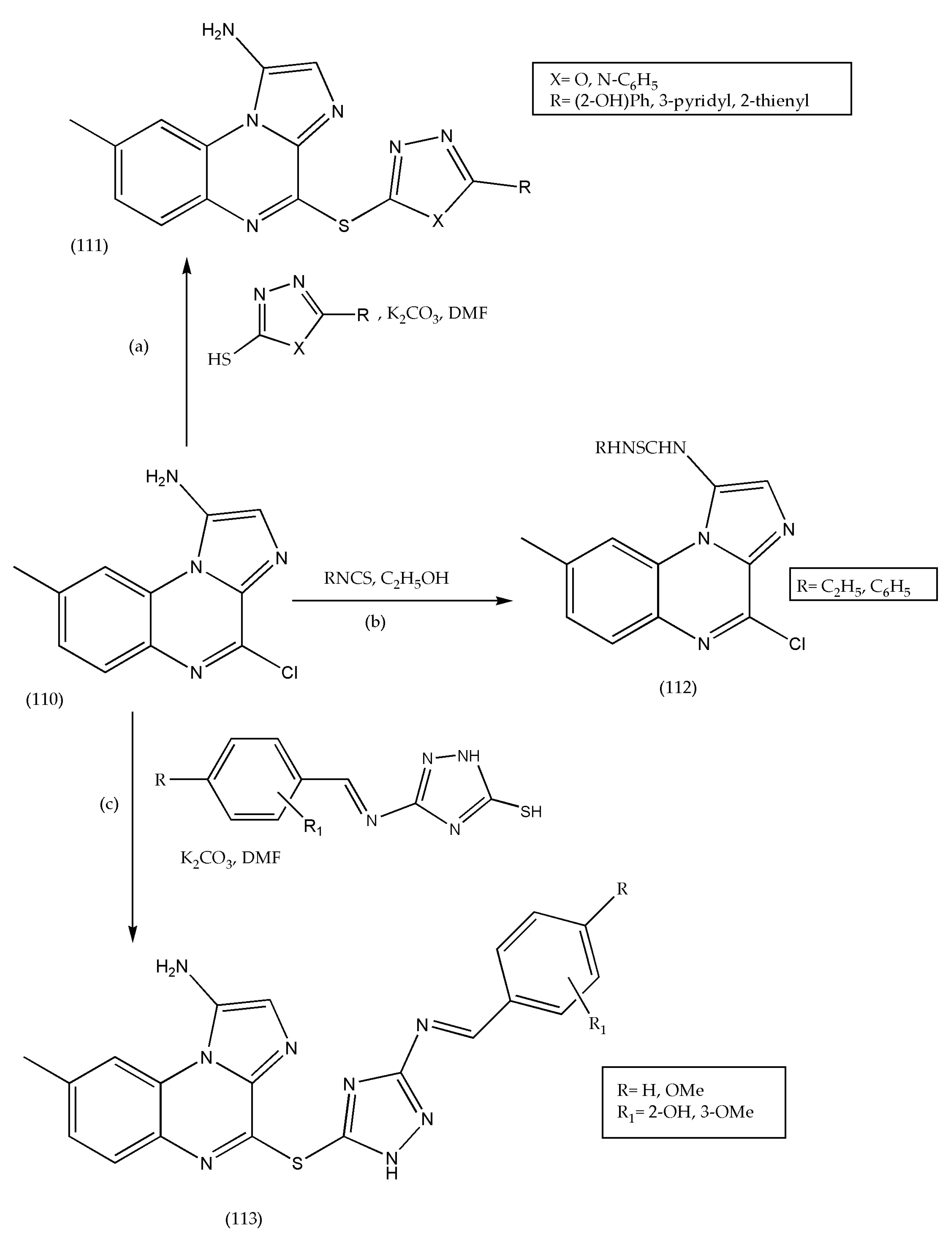
3.16. Synthesis of [1,2,4]triazolo[4,3-a]quinoxaline Derivatives as Antiviral/Antimicrobial Agents
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Irfan, A.; Sabeeh, I.; Umer, M.; Naqvi, A.Z.; Fatima, H.; Yousaf, S.; Fatima, Z. A review on the therapeutic potential of quinoxaline derivatives. World J. Pharm. Res. 2017, 6, 47–68. [Google Scholar] [CrossRef]
- Cheeseman, G.W.H.; Cookson, R.F. Chemistry of Heterocyclic Compounds; Wiley-Interscience: Hoboken, NJ, USA, 1979; Volume 35. [Google Scholar]
- Pereira, J.A.; Pessoa, A.S.M.; Cordeiro, M.N.D.S.; Fernandes, R.; Prudêncio, C.; Noronha, J.P.; Vieira, M. Quinoxaline, its derivatives and applications: A state of the art review. Eur. J. Med. Chem. 2015, 97, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Niknam, K.; Saberi, D.; Mohagheghnejad, M. Silica bonded S-sulfonic acid: A recyclable catalyst for the synthesis of quinoxalines at room temperature. Molecules 2009, 14, 1915–1926. [Google Scholar] [CrossRef]
- Thakuria, H.; Das, G. One-pot efficient green synthesis of 1,4-dihydro-quinoxaline-2,3-dione derivatives. J. Chem. Sci. 2006, 118, 425–428. [Google Scholar] [CrossRef]
- da Costa, C.F.; Nora de Souza, M.V.; Brandao Gomes, C.R.; Facchinetti, V. Microwave-Assisted Synthesis of Quinoxalines-A Review. Curr. Microwave Chem. 2017, 4, 277–286. [Google Scholar] [CrossRef]
- Rostamizadeh, S.; Jafari, S. The synthesis of quinoxalines under microwave irradiation. Indian J. Heterocycl. Chem. 2001, 10, 303–304. [Google Scholar]
- Arde, S.M.; Patil, A.D.; Mane, A.H.; Salokhe, P.R.; Salunkhe, R.S. Synthesis of quinoxaline, benzimidazole and pyrazole derivatives under the catalytic influence of biosurfactant-stabilized iron nanoparticles in water. Res. Chem. Intermed. 2020, 46, 5069–5086. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; Gouda, M.A.; Badria, F.A. synthesis of some new pyrimido [2′, 1′: 2, 3] thiazolo [4, 5-b] quinoxaline derivatives as anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2010, 45, 1976–1981. [Google Scholar] [CrossRef]
- Barea, C.; Pabón, A.; Galiano, S.; Pérez-Silanes, S.; González, G.; Deyssard, C.; Monge, A.; Deharo, E.; Aldana, I. Antiplasmodial and leishmanicidal activities of 2-cyano-3-(4-phenylpiperazine-1-carboxamido) quinoxaline 1,4-dioxide derivatives. Molecules 2012, 17, 9451–9461. [Google Scholar] [CrossRef]
- Sarges, R.; Howard, H.R.; Browne, R.G.; Lebel, L.A.; Seymour, P.A.; Koe, B.K. 4-Amino[1,2,4]triazolo[4,3-a]quinoxalines. A novel class of potent adenosine receptor antagonists and potential rapid-onset antidepressants. J. Med. Chem. 1990, 33, 2240–2254. [Google Scholar] [CrossRef]
- Montana, M.; Montero, V.; Khoumeri, O.; Vanelle, P. Quinoxaline derivatives as antiviral agents: A systematic review. Molecules 2020, 25, 2784. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.; Pinheiro, C.; Fernandes, R.; Noronha, J.; Prudêncio, C. Antimicrobial activity of quinoxaline 1,4-dioxide with 2- and 3-substituted derivatives. Microbiol. Res. 2014, 169, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Husain, A.; Madhesia, D. Recent advances in pharmacological activities of quinoxaline derivatives. J. Pharm. Res. 2011, 4, 924–929. [Google Scholar]
- Carta, A.; Paglietti, G.; Nikookar, M.E.R.; Sanna, P.; Sechi, L.; Zanetti, S. Novel substituted quinoxaline 1,4-dioxides with in vitro antimycobacterial and anticandida activity. Eur. J. Med. Chem. 2002, 37, 355–366. [Google Scholar] [CrossRef]
- Mohd, H.A.; Al-Tawfiq, J.A.; Memish, Z.A. Middle East respiratory syndrome coronavirus (MERS-CoV) origin and animal reservoir. Virol. J. 2016, 13, 1–7. [Google Scholar] [CrossRef]
- Waring, M.J.; Ben-Hadda, T.; Kotchevar, A.T.; Ramdani, A.; Touzani, R.; Elkadiri, S.; Hakkou, A.; Bouakka, M.; Ellis, T. 2,3-bifunctionalized quinoxalines: Synthesis, DNA interactions and evaluation of anticancer, anti-tuberculosis and antifungal activity. Molecules 2002, 7, 641–656. [Google Scholar] [CrossRef]
- Coltart, C.E.M.; Lindsey, B.; Ghinai, I.; Johnson, A.M.; Heymann, D.L. The Ebola outbreak, 2013–2016: Old lessons for new epidemics. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160297. [Google Scholar] [CrossRef]
- Perlman, S. Another decade, another coronavirus. N. Engl. J. Med. 2020, 382, 760–762. [Google Scholar] [CrossRef]
- Hasaninejad, A.; Zare, A.; Shekouhy, M.; Moosavi-Zare, A.R. Bentonite clay K-10 as an efficient reagent for the synthesis of quinoxaline derivatives at room temperature. E-J. Chem. 2009, 6, S247–S253. [Google Scholar] [CrossRef]
- Anastas, P.; Warner, J. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998; Volume 30. [Google Scholar]
- Malek, B.; Bahammou, I.; Zimou, O.; El Hallaoui, A.; Ghailane, R.; Boukhris, S.; Souizi, A. Eco-friendly synthesis of quinoxaline derivatives using mineral fertilizers as heterogeneous catalysts. J. Turk. Chem. Soc. Sect. A Chem. 2020, 7, 427–440. [Google Scholar] [CrossRef]
- Nageswar, Y.V.D.; Reddy, K.H.V.; Ramesh, K.; Murthy, S.N. Recent developments in the synthesis of quinoxaline derivatives by green synthetic approaches. Org. Prep. Proced. Int. 2013, 45, 1–27. [Google Scholar] [CrossRef]
- More, S.V.; Sastry, M.N.V.; Yao, C.-F. Cerium (iv) ammonium nitrate (CAN) as a catalyst in tap water: A simple, proficient and green approach for the synthesis of quinoxalines. Green Chem. 2006, 8, 91–95. [Google Scholar] [CrossRef]
- An, Z.; Wu, M.; Ni, J.; Qi, Z.; Yu, G.; Yan, R.; Zhao, L.-B. FeCl 3 -Catalyzed synthesis of pyrrolo[1,2-a]quinoxaline derivatives from 1-(2-aminophenyl)pyrroles through annulation and cleavage of cyclic ethers. Chem. Commun. 2017, 53, 11572–11575. [Google Scholar] [CrossRef] [PubMed]
- Begue, J.P.; Bonnet-Delpon, D.; Crousse, B. Fluorinated alcohols: A new medium for the selective and clean reaction. Synlett 2004, 35, 18–29. [Google Scholar] [CrossRef]
- Povey, J.F.; Smales, C.M.; Hassard, S.J.; Howard, M.J. Comparison of the effects of 2, 2, 2-trifluoroethanol on peptide and protein structure and function. J. Struct. Boil. 2007, 157, 329338. [Google Scholar] [CrossRef] [PubMed]
- Khaksar, S.; Rostamnezhad, F. A novel one-pot synthesis of quinoxaline derivatives in fluorinated alcohols. Bull. Korean Chem. Soc. 2012, 33, 2581–2584. [Google Scholar] [CrossRef]
- Brown, D.J.; Taylor, E.C.; Ellman, J.A. Quinoxalines, Supplement 2; John Wiley & Sons: Denmark, UK, 2004; Volume 107. [Google Scholar]
- Wadavrao, S.B.; Ghogare, R.; Narsaiah, A.V. A simple and efficient protocol for the synthesis of quinoxalines catalyzed by pyridine. Org. Commun. 2013, 6, 23. [Google Scholar]
- Atghia, S.V.; Beigbaghlou, S.S. Nanocrystalline titania-based sulfonic acid (TiO2-Pr-SO3H) as a new, highly efficient, and recyclable solid acid catalyst for the preparation of quinoxaline derivatives. J. Nanostructure Chem. 2013, 3, 38. [Google Scholar] [CrossRef]
- Jafarpour, M.; Rezaeifard, A.; Danehchin, M. Easy access to quinoxaline derivatives using alumina as an effective and reusable catalyst under solvent-free conditions. Appl. Catal. A Gen. 2011, 394, 48–51. [Google Scholar] [CrossRef]
- Huang, T.-K.; Wang, R.; Shi, L.; Lu, X.-X. Montmorillonite K-10: An efficient and reusable catalyst for the synthesis of quinoxaline derivatives in water. Catal. Commun. 2008, 9, 1143–1147. [Google Scholar] [CrossRef]
- Krishnakumar, B.; Swaminathan, M. Solvent free synthesis of quinoxalines, dipyridophenazines and chalcones under microwave irradiation with sulfated Degussa titania as a novel solid acid catalyst. J. Mol. Catal. A Chem. 2011, 350, 16–25. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Kanagaraj, K.; Tharmaraj, V. Zn2+-K10-clay (clayzic) as an efficient water-tolerant, solid acid catalyst for the synthesis of benzimidazoles and quinoxalines at room temperature. Tetrahedron Lett. 2011, 52, 69–73. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, C. Zirconium(IV)-modified silica gel: Preparation, characterization and catalytic activity in the synthesis of some biologically important molecules. Catal. Commun. 2011, 12, 327–331. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Wang, J.X.; Sun, Y.J.; Zhan, H.W. Synthesis of quinoxaline derivatives catalyzed by PEG-400. Chin. Chem. Lett. 2010, 21, 395–398. [Google Scholar] [CrossRef]
- Heravi, M.M.; Bakhtiari, K.; Bamoharram, F.F.; Tehrani, M.H. Wells-dawson type heteropolyacid catalyzed synthesis of quinoxaline derivatives at room temperature. Mon. Für Chem. Chem. Mon. 2007, 138, 465–467. [Google Scholar] [CrossRef]
- Ajaikumar, S.; Pandurangan, A. Efficient synthesis of quinoxaline derivatives over ZrO2/MxOy (M=Al, Ga, In and La) mixed metal oxides supported on MCM-41 mesoporous molecular sieves. Appl. Catal. A Gen. 2009, 357, 184–192. [Google Scholar] [CrossRef]
- Shaabani, A.; Rezayan, A.H.; Behnam, M.; Heidary, M. Green chemistry approaches for the synthesis of quinoxaline derivatives: Comparison of ethanol and water in the presence of the reusable catalyst cellulose sulfuric acid. Comptes Rendus Chim. 2009, 12, 1249–1252. [Google Scholar] [CrossRef]
- Cai, J.-J.; Zou, J.; Pan, X.-Q.; Zhang, W. Gallium(III) triflate-catalyzed synthesis of quinoxaline derivatives. Tetrahedron Lett. 2008, 49, 7386–7390. [Google Scholar] [CrossRef]
- Pereira, M.D.F.; Thiéry, V. One-pot synthesis of pyrrolo[1,2-a]quinoxaline derivatives via iron-promoted aryl nitro reduction and aerobic oxidation of alcohols. Org. Lett. 2012, 14, 4754–4757. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, L.; Yang, Y.; Zha, Z.; Wang, Z. Unexpected activated carbon-catalyzed pyrrolo[1,2-a]quinoxalines synthesis in water. Chin. Chem. Lett. 2019, 30, 1379–1382. [Google Scholar] [CrossRef]
- Tang, X.-Y.; Gong, Y.; Huo, H.-R. Metal-free synthesis of pyrrolo[1,2-a]quinoxalines mediated by TEMPO oxoammonium salts. Synthesis 2018, 50, 2727–2740. [Google Scholar] [CrossRef]
- Allan, P.N.; Ostrowska, M.I.; Patel, B. Acetic acid catalyzed one-pot synthesis of pyrrolo[1, 2-a]quinoxaline derivatives. Synlett 2019, 3019, 2148–2152. [Google Scholar]
- De Clercq, E. (Ed.) Advances in Antiviral Drug Design; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Bini, E.J.; Weinshel, E.H. Severe exacerbation of asthma: A new side effect of interferon-α in patients with asthma and chronic hepatitis C. Mayo Clin. Proc. 1999, 74, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Wills, R.J.; Dennis, S.; Spiegel, H.E.; Gibson, D.M.; Nadler, P.I. Interferon kinetics and adverse reactions after IV infusion, IM and SQ routes of administration. Clin. Pharmacol. Ther. 1984, 35, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Shibinskaya, M.O.; Lyakhov, S.A.; Mazepa, A.V.; Andronati, S.A.; Turov, A.V.; Zholobak, N.M.; Spivak, N.Y. Synthesis, cytotoxicity, antiviral activity and interferon inducing ability of 6-(2-aminoethyl)-6H-indolo [2, 3-b] quinoxalines. Eur. J. Med. Chem. 2010, 45, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Sepkowitz, K.A. AIDS—The first 20 years. N. Engl. J. Med. 2001, 344, 1764–1772. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, C.; Karmochkine, M.; Laureillard, D.; Tisserand, P.; Bélec, L.; Weiss, L.; Piketty, C.; Si-Mohamed, A. Drug resistance profiles for the HIV integrase gene in patients failing raltegravir salvage therapy*. HIV Med. 2008, 9, 765–770. [Google Scholar] [CrossRef]
- Patel, S.B.; Patel, B.D.; Pannecouque, C.; Bhatt, H.G. Design, synthesis and anti-HIV activity of novel quinoxaline derivatives. Eur. J. Med. Chem. 2016, 117, 230–240. [Google Scholar] [CrossRef]
- Loughran, H.M.; Han, Z.; Wrobel, J.E.; Decker, S.E.; Ruthel, G.; Freedman, B.D.; Harty, R.N.; Reitz, A.B. Quinoxaline-based inhibitors of Ebola and Marburg VP40 egress. Bioorg. Med. Chem. Lett. 2016, 26, 3429–3435. [Google Scholar] [CrossRef]
- Ali, I.A.I.; Al-Masoudi, I.A.; Aziz, N.M.; Al-Masoudi, N.A. New acyclic quinoxaline nucleosides. Synthesis and anti-HIV activity. Nucleosides Nucleotides Nucleic Acids 2008, 27, 146–156. [Google Scholar] [CrossRef]
- Barrio, J.R.; Bryant, J.D.; Keyser, G.E. A direct method for the preparation of 2-hydroxyethoxymethyl derivatives of guanine, adenine, and cytosine. J. Med. Chem. 1980, 23, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Gan, X.; Zhou, D.; Meneghetti, F.; Zeng, S.; Hu, D. Synthesis and antiviral activity of novel 1,4-pentadien-3-one derivatives containing a 1,3,4-thiadiazole moiety. Molecules 2017, 22, 658. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Guo, T.; Chen, M.; Su, S.; He, J.; Tang, X.; Jiang, S.; Xue, W. Synthesis, antiviral and antibacterial activities and action mechanism of penta-1,4-dien-3-one oxime ether derivatives containing a quinoxaline moiety. N. J. Chem. 2019, 43, 16461–16467. [Google Scholar] [CrossRef]
- El Rayes, S.M.; Aboelmagd, A.; Gomaa, M.S.; Ali, I.A.I.; Fathalla, W.; Pottoo, F.H.; Alam Khan, F. Convenient synthesis and anticancer activity of methyl 2-[3-(3-Phenyl-quinoxalin-2-ylsulfanyl)propanamido]alkanoates and N-Alkyl 3-((3-Phenyl-quinoxalin-2-yl)sulfanyl)propanamides. ACS Omega 2019, 4, 18555–18566. [Google Scholar] [CrossRef] [PubMed]
- Noolvi, M.N.; Patel, H.; Bhardwaj, V.; Chauhan, A. Synthesis and in vitro antitumor activity of substituted quinazoline and quinoxaline derivatives: Search for anticancer agent. Eur. J. Med. Chem. 2011, 46, 2327–2346. [Google Scholar] [CrossRef]
- El Newahie, A.M.S.; Nissan, Y.M.; Ismail, N.S.M.; El Ella, D.A.A.; Khojah, S.M.; Abouzid, K.A.; El Ella, D.A. Design and synthesis of new quinoxaline derivatives as anticancer agents and apoptotic inducers. Molecules 2019, 24, 1175. [Google Scholar] [CrossRef]
- Gris, J.; Glisoni, R.; Fabian, L.; Fernández, C.G.-C.; Moglioni, A.G. Synthesis of potential chemotherapic quinoxalinone derivatives by biocatalysis or microwave-assisted Hinsberg reaction. Tetrahedron Lett. 2008, 49, 1053–1056. [Google Scholar] [CrossRef]
- Wang, S.; Yan, J.; Wang, X.; Yang, Z.; Lin, F.; Zhang, T. Synthesis and evaluation of the α-glucosidase inhibitory activity of 3-[4-(phenylsulfonamido)benzoyl]-2H-1-benzopyran-2-one derivatives. Eur. J. Med. Chem. 2010, 45, 1250–1255. [Google Scholar] [CrossRef]
- Kakuta, H.; Zheng, X.; Oda, H.; Harada, S.; Sugimoto, Y.; Sasaki, K.; Tai, A. Cyclooxygenase-1-selective inhibitors are attractive candidates for analgesics that do not cause gastric damage. Design and in vitro/in vivo evaluation of a benzamide-type cyclooxygenase-1 selective inhibitor. J. Med. Chem. 2008, 51, 2400–2411. [Google Scholar] [CrossRef]
- Dai, Y.; Hartandi, K.; Ji, Z.; Ahmed, A.A.; Albert, D.H.; Bauch, J.L.; Bouska, J.J.; Bousquet, P.F.; Cunha, G.A.; Glaser, K.B.; et al. Discovery of N-(4-(3-Amino-1H-indazol-4-yl)phenyl)-N′-(2-fluoro-5-methylphenyl)urea (ABT-869), a 3-aminoindazole-based orally active multitargeted receptor tyrosine kinase inhibitor. J. Med. Chem. 2007, 50, 1584–1597. [Google Scholar] [CrossRef]
- Singh, D.P.; Deivedi, S.K.; Hashim, S.R.; Singhal, R.G. Synthesis and antimicrobial activity of some new quinoxaline derivatives. Pharmaceuticals 2010, 3, 2416–2425. [Google Scholar] [CrossRef] [PubMed]
- Bronckaers, A.; Gago, F.; Balzarini, J.; Liekens, S. The dual role of thymidine phosphorylase in cancer development and chemotherapy. Med. Res. Rev. 2009, 29, 903–953. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Gamboa, A.; Balzarini, J.; Esnouf, R.; De Clercq, E.; Camarasa, M.J.; Pérez-Pérez, M.J. Design, synthesis, and enzymatic evaluation of multisubstrate analogue inhibitors of Escherichia coli thymidine phosphorylase. J. Med. Chem. 2000, 43, 971–983. [Google Scholar] [CrossRef]
- Edmonds, M.; Delluva, A.M.; Wilson, D.W. The metabolism of purines and pyrimidines by growing yeast. J. Biol. Chem. 1952, 197, 1177–1257. [Google Scholar]
- Krenitsky, T.A.; Barclay, M.; Jacquez, J.A. Specificity of mouse uridine phosphorylase. Chromatography, purification, and properties. J. Biol. Chem. 1964, 239, 805–812. [Google Scholar]
- Almandil, N.B.; Taha, M.; Farooq, R.K.; Alhibshi, A.; Ibrahim, M.; Anouar, E.H.; Gollapalli, M.; Rahim, F.; Nawaz, M.; Shah, S.A.A.; et al. Synthesis of thymidine phosphorylase inhibitor based on quinoxaline derivatives and their molecular docking study. Molecules 2019, 24, 1002. [Google Scholar] [CrossRef]
- Essassi, E.M.; Ahoya, C.A.; Bouhfid, R.; Daouda, B.; Hançali, A.; Zouihri, H.; Zerzouf, A.; El Aouad, R. Synthesis and antibacterial activity of new spiro[thiadiazoline-quinoxaline] derivatives. Arkivoc 2011, 2011, 217–226. [Google Scholar] [CrossRef]
- Park, K.L.; Ko, N.Y.; Lee, J.H.; Kim, D.K.; Kim, H.S.; Kim, A.-R.; Her, E.; Kim, B.; Kim, H.S.; Moon, E.-Y.; et al. 4-Chlorotetrazolo[1,5-a]quinoxaline inhibits activation of Syk kinase to suppress mast cells in vitro and mast cell-mediated passive cutaneous anaphylaxis in mice. Toxicol. Appl. Pharmacol. 2011, 257, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, B.; Prasanna, B.; Ravinder, M. One pot synthesis of substituted bistetrazolo-[1, 5-a: 5′, 1′-c]-quinoxalines. Chem. Sci. Trans. 2013, 2, 1074–1077. [Google Scholar] [CrossRef][Green Version]
- Phillips, M.A. CCCXVII.—The formation of 2-substituted benziminazoles. J. Chem. Soc. 1928, 2393–2399. [Google Scholar] [CrossRef]
- Saha, S.; Chant, D.; Welham, J.; McGrath, J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005, 2, e141. [Google Scholar] [CrossRef] [PubMed]
- Lewine, R.R.; Fogg, L.; Meltzer, H.Y. Assessment of negative and positive symptoms in schizophrenia. Schizophr. Bull. 1983, 9, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, K.V.G.C.; Rao, V.S.; Deuther-Conrad, W.; Sridhar, D.; Nagesh, H.N.; Kumar, V.S.; Kumar, M.M.K. Design, synthesis, and preliminary in vitro and in vivo pharmacological evaluation of 4-{4-[2-(4-(2-substitutedquinoxalin-3-yl) piperazin-1-yl) ethyl] phenyl} thiazoles as atypical antipsychotic agents. Med. Chem. Res. 2013, 22, 1660–1673. [Google Scholar] [CrossRef]
- Meltzer, H.Y. What’s atypical about atypical antipsychotic drugs? Curr. Opin. Pharmacol. 2004, 4, 53–57. [Google Scholar] [CrossRef]
- Baashen, M. Quinoxaline-2,3(1H,4H)-dithione: Synthesis and reactions. Phosphorus Sulfur Silicon Relat. Elem. 2018, 193, 350–357. [Google Scholar] [CrossRef]
- Podsiadły, R.; Sokołowska, J. Synthesis of novel oxidizable polymerization sensitizers based on the dithiinoquinoxaline skeleton. Dye. Pigment. 2012, 92, 1300–1307. [Google Scholar] [CrossRef]
- Taeger, E.; El-Hewehi, Z. Synthese von in 5-Stellung mono-und disubstituierten 2-Mercapto-4-phenyl-Δ2-1, 3, 4-thiadiazolinen und 2, 3-Dimercaptochinoxalin. J. Für Prakt. Chem. 1962, 18, 255–261. [Google Scholar] [CrossRef]
- Ibrahim, M.; Taghour, M.; Metwaly, A.; Belal, A.; Mehany, A.; Elhendawy, M.; Radwan, M.; Yassin, A.; El-Deeb, N.; Hafez, E.; et al. Design, synthesis, molecular modeling and anti-proliferative evaluation of novel quinoxaline derivatives as potential DNA intercalators and topoisomerase II inhibitors. Eur. J. Med. Chem. 2018, 155, 117–134. [Google Scholar] [CrossRef]
- Kim, Y.B.; Kim, Y.H.; Park, J.Y.; Kim, S.K. Synthesis and biological activity of new quinoxaline antibiotics of echinomycin analogues. Bioorg. Med. Chem. Lett. 2004, 14, 541–544. [Google Scholar] [CrossRef]
- Ammar, Y.A.; Farag, A.A.; Ali, A.M.; Hessein, S.A.; Askar, A.A.; Fayed, E.A.; Elsisi, D.M.; Ragab, A. Antimicrobial evaluation of thiadiazino and thiazolo quinoxaline hybrids as potential DNA gyrase inhibitors; design, synthesis, characterization and morphological studies. Bioorg. Chem. 2020, 99, 103841. [Google Scholar] [CrossRef]
- Woodruff, J.F. Viral myocarditis. A review. Am. J. Pathol. 1980, 101, 425. [Google Scholar] [PubMed]
- Kandolf, R.; Sauter, M.; Aepinus, C.; Schnorr, J.-J.; Selinka, H.-C.; Klingel, K. Mechanisms and consequences of enterovirus persistence in cardiac myocytes and cells of the immune system. Virus Res. 1999, 62, 149–158. [Google Scholar] [CrossRef]
- Rotbart, H.A. Enteroviral infections of the central nervous system. Clin. Infect. Dis. 1995, 20, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Carta, A.; Sanna, G.; Briguglio, I.; Madeddu, S.; Vitale, G.; Piras, S.; Corona, P.; Peana, A.; Laurini, E.; Fermeglia, M.; et al. Quinoxaline derivatives as new inhibitors of coxsackievirus B5. Eur. J. Med. Chem. 2018, 145, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Awad, I.M. Synthesis and application of novel sulpha drugs based on quinoxaline-2-one and/or quinoxaline-2-thione. J. Chem. Technol. Biotechnol. 1992, 53, 227–236. [Google Scholar] [CrossRef]
- Dirlam, J.P.; Presslitz, J.E.; Williams, B.J. Synthesis and antibacterial activity of some 3-[(alkylthio)methyl]quinoxaline 1-oxide derivatives. J. Med. Chem. 1983, 26, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Jaso, A.; Zarranz, B.; Aldana, I.; Monge, A. Synthesis of new quinoxaline-2-carboxylate 1, 4-dioxide derivatives as anti-mycobacterium tuberculosis agents. J. Med. Chem. 2005, 48, 2019–2025. [Google Scholar] [CrossRef]
- Henen, M.A.; El Bialy, S.A.A.; Goda, F.E.; Nasr, M.N.A.; Eisa, H.M. [1,2,4]Triazolo[4,3-a]quinoxaline: Synthesis, antiviral, and antimicrobial activities. Med. Chem. Res. 2012, 21, 2368–2378. [Google Scholar] [CrossRef]

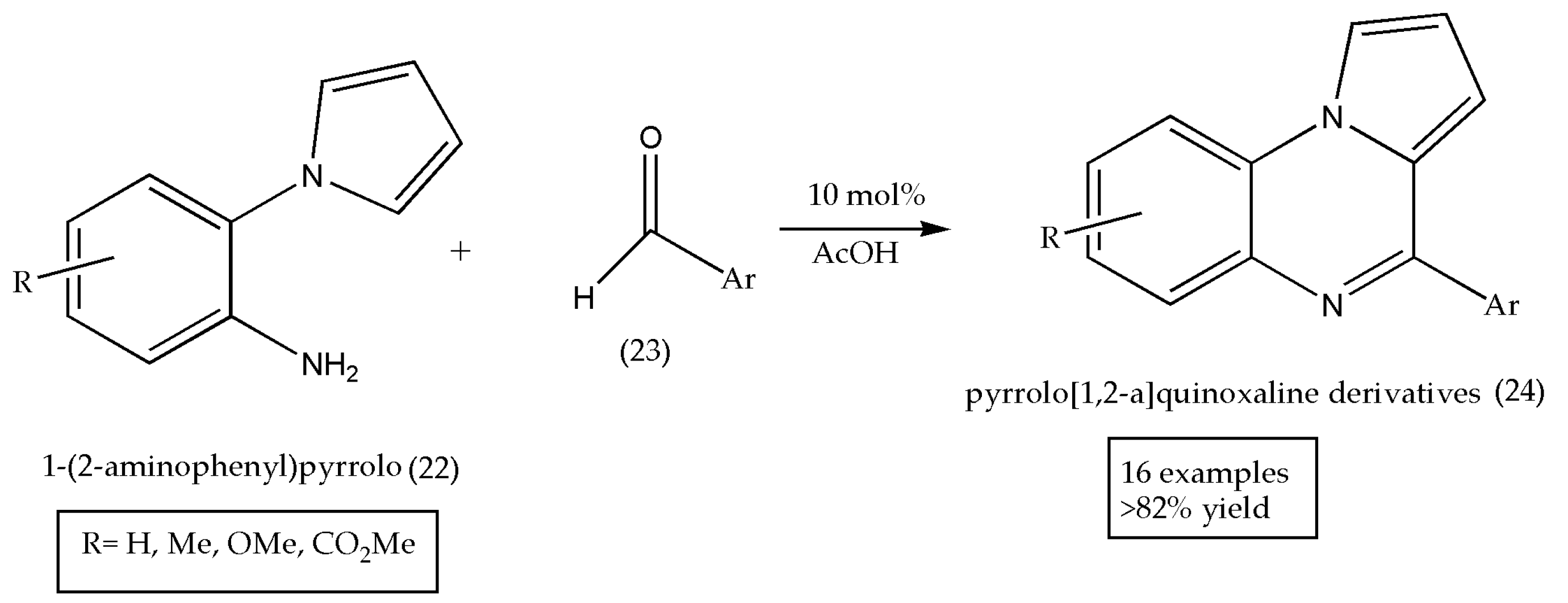
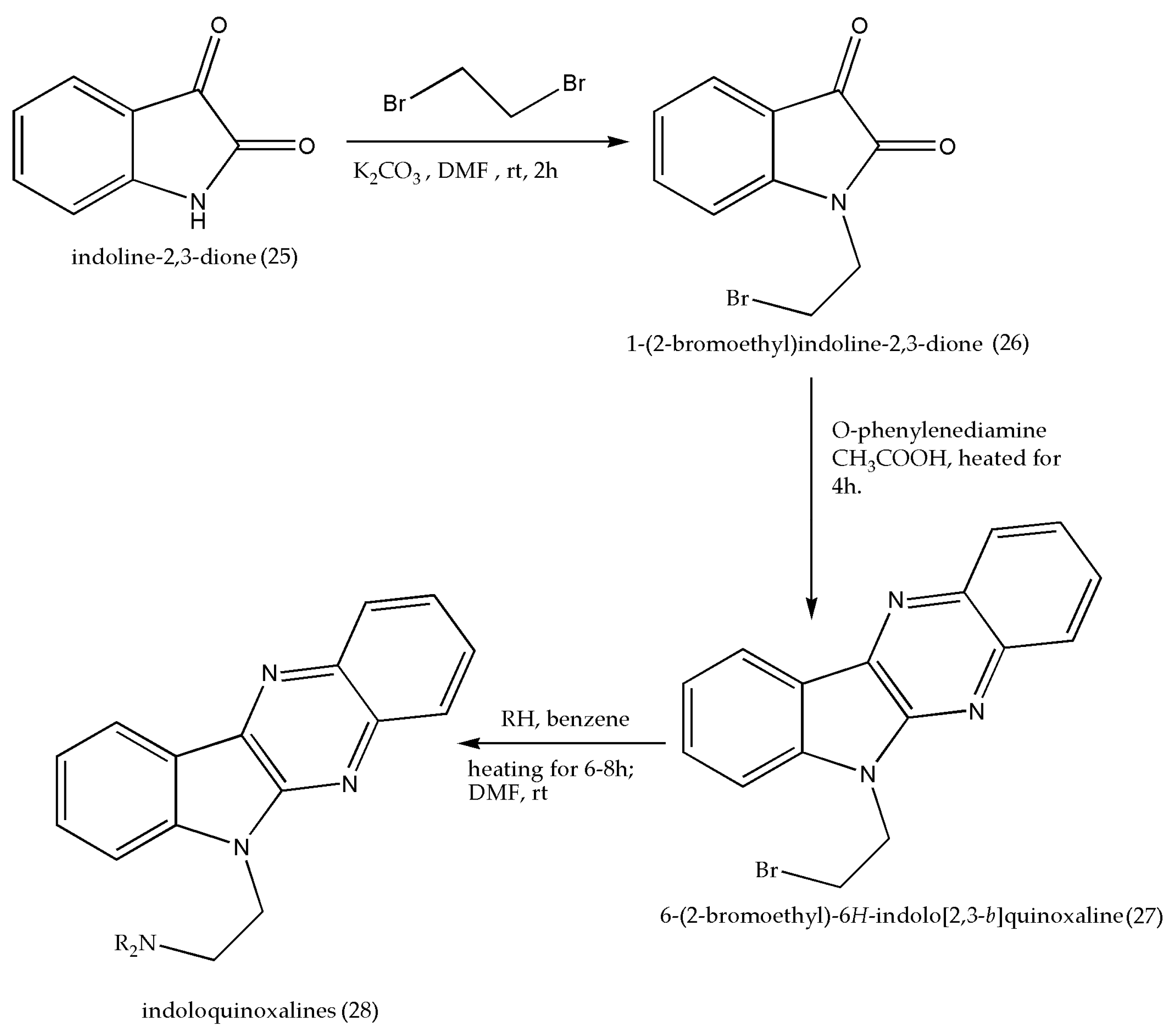
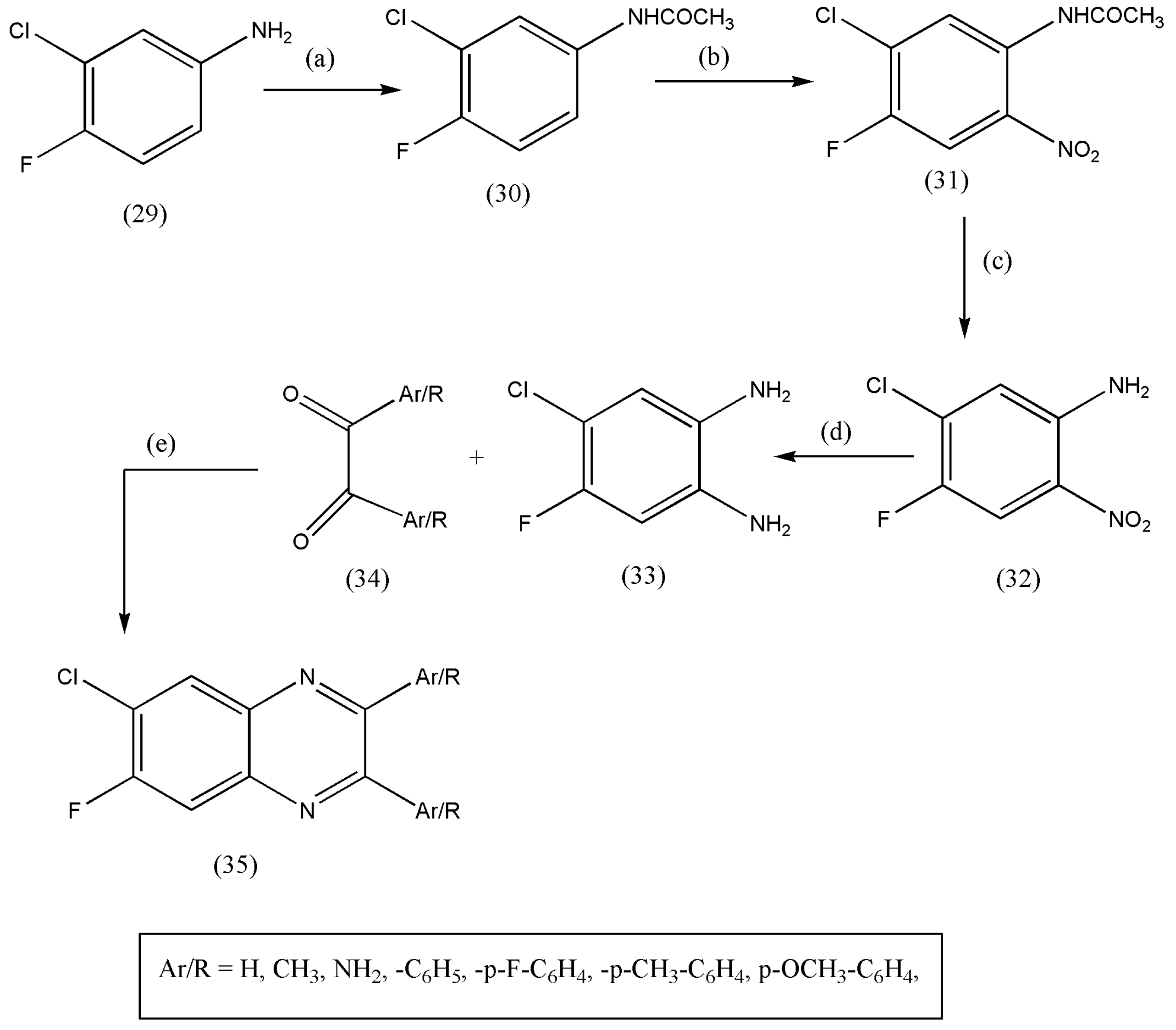

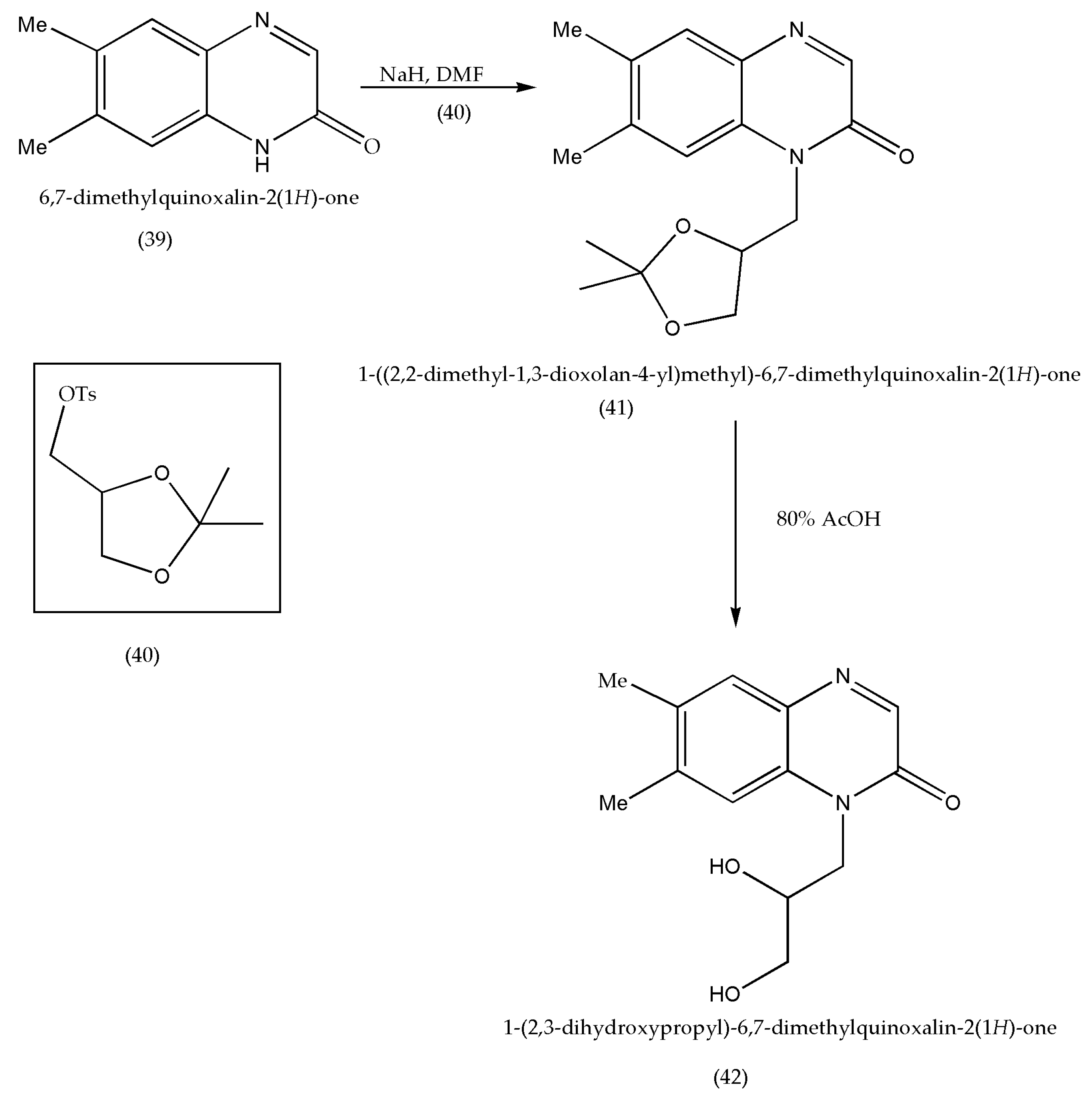





| Entry | Grams of Clay | Time in Minutes | Isolated Yields% |
|---|---|---|---|
| 1. | 0.5 | 720 | 38 |
| 2. | 1 | 120 | 43 |
| 3. | 1.5 | 90 | 65 |
| 4. | 2 | 60 | 93 |
| 5. | 2.5 | 20 | 95 |
| 6. | 3 | 20 | 95 |
| Entry | Solvent | Time, Min | Isolated Yields% |
|---|---|---|---|
| 1 | EtOH | 20 | 95 |
| 2 | MeOH | 30 | 91 |
| 3 | H2O | 120 | trace |
| 4 | CHCl3 | 30 | 87 |
| 5 | CH2Cl2 | 30 | 82 |
| 6 | MeCN | 35 | 92 |
| 7 | THF | 30 | 89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khatoon, H.; Abdulmalek, E. Novel Synthetic Routes to Prepare Biologically Active Quinoxalines and Their Derivatives: A Synthetic Review for the Last Two Decades. Molecules 2021, 26, 1055. https://doi.org/10.3390/molecules26041055
Khatoon H, Abdulmalek E. Novel Synthetic Routes to Prepare Biologically Active Quinoxalines and Their Derivatives: A Synthetic Review for the Last Two Decades. Molecules. 2021; 26(4):1055. https://doi.org/10.3390/molecules26041055
Chicago/Turabian StyleKhatoon, Hena, and Emilia Abdulmalek. 2021. "Novel Synthetic Routes to Prepare Biologically Active Quinoxalines and Their Derivatives: A Synthetic Review for the Last Two Decades" Molecules 26, no. 4: 1055. https://doi.org/10.3390/molecules26041055
APA StyleKhatoon, H., & Abdulmalek, E. (2021). Novel Synthetic Routes to Prepare Biologically Active Quinoxalines and Their Derivatives: A Synthetic Review for the Last Two Decades. Molecules, 26(4), 1055. https://doi.org/10.3390/molecules26041055







