Influence of Agronomic Practice on Total Phenols, Carotenoids, Chlorophylls Content, and Biological Activities in Dry Herbs Water Macerates
Abstract
1. Introduction
2. Results
2.1. Chemical Composition
2.1.1. Polyphenols
2.1.2. Chlorophyll and Carotenoids
2.2. Antimicrobial Activity
3. Discussion
4. Materials and Methods
4.1. Macerates Preparation
4.2. Chemical Analysis
4.2.1. The Content of Polyphenols
4.2.2. The Content of Chlorophylls and Carotenoids
4.3. Microbial Analysis
4.3.1. Microbial Strains and Culture Conditions
4.3.2. Antimicrobial Assay
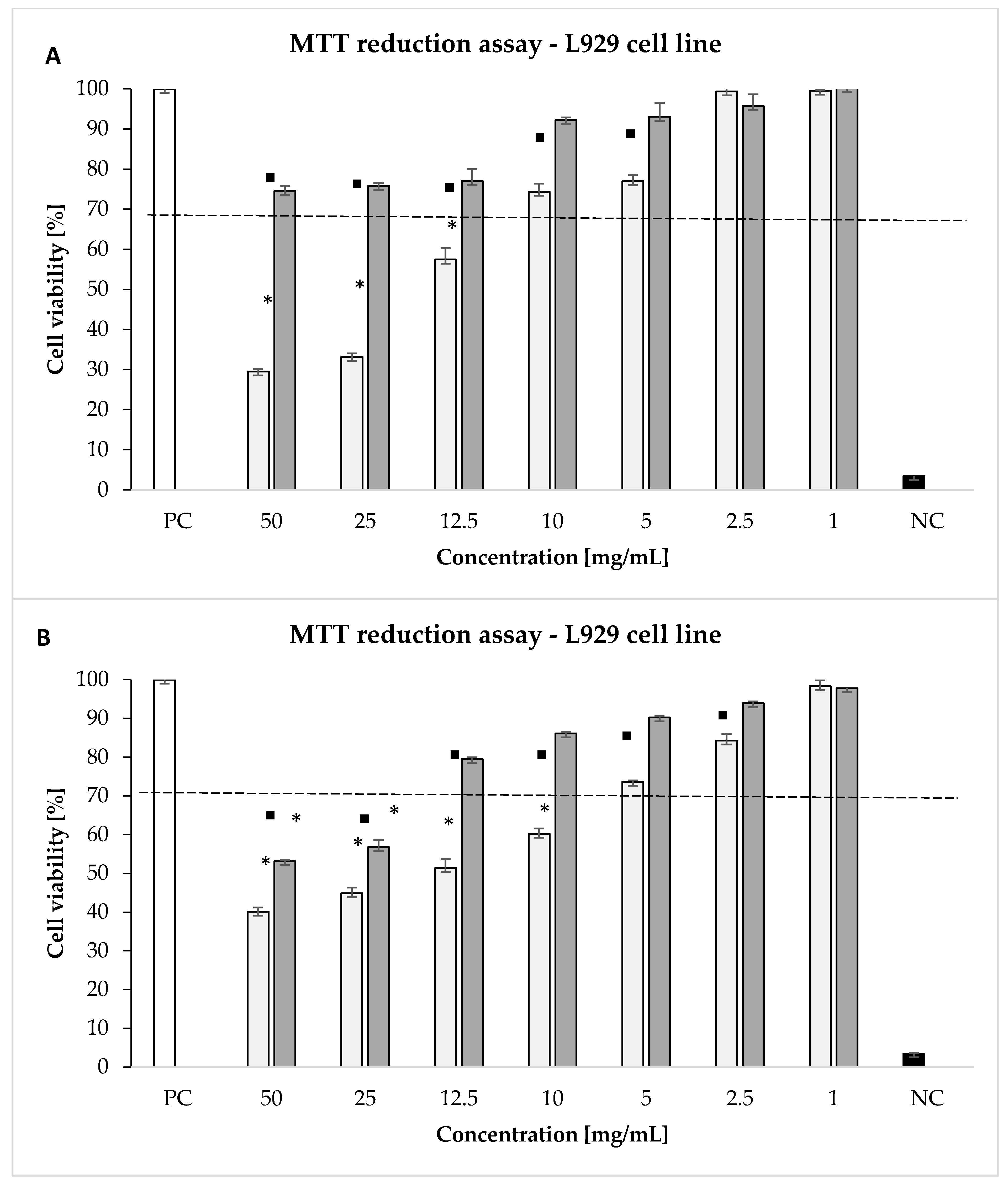
4.4. Cytotoxicity Studies
4.4.1. In Vitro Cell Culture
4.4.2. Measurements of Cellular Metabolic Activity and Global Growth Inhibition
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wogiatzi, E.; Gougoulias, N.; Papachatzis, A.; Vagelas, I.; Chouliaras, N. Greek Oregano Essential Oils Production, Phytotoxicity and Antifungal Activity. Biotech. Biotechnoll. Equip. 2009, 23, 1150–1152. [Google Scholar] [CrossRef]
- Stahl-Biskup, E.; Venskutonis, R.P. Thyme. In Handbook of Herbs and Spices; Peter, K.V., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 499–525. [Google Scholar]
- Sivicka, I.; Adamovičs, A.; Žukauska, I. Research of oregano (Origanum vulgare L.) inflorescence′s parameters. In Proceedings of the Annual 18th International Scientific Conference “Research for Rural Development”, Latvia University of Agriculture, Jelgava, Latvia, 16–18 May 2012; Volume 1, pp. 56–60. [Google Scholar]
- Mäkinen, S.M.; Pääkkönen, K.K. Processing, effects and use of oregano and marjoram in foodstuffs and in food preparation. In Oregano: The genera Origanum and Lippia; Kintzios, S.E., Ed.; Taylor and Francis: London, UK, 2002; Volume 25, pp. 217–233. [Google Scholar]
- Ložienė, K. Selection of fecund and chemically valuable clones of thyme (Thymus) species growing wild in Lithuania. Ind. Crop. Prod. 2009, 29, 502–508. [Google Scholar] [CrossRef]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microb. Biotech. 2017, 27, 429–438. [Google Scholar] [CrossRef]
- Faleiro, L.; Miguel, G.; Gomes, S.; Costa, L.; Venâncio, F.; Teixeira, A.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Antibacterial and Antioxidant Activities of Essential Oils Isolated from Thymbra capitata L. (Cav.) and Origanum vulgare L. J. Agric. Food Chem. 2005, 53, 8162–8168. [Google Scholar] [CrossRef]
- Nabissi, M.; Marinelli, O.; Morelli, M.B.; Nicotra, G.; Iannarelli, R.; Amantini, C.; Maggi, F. Thyme extract increases mucociliary-beating frequency in primary cell lines from chronic obstructive pulmonary disease patients. Biomed. Pharm. 2018, 105, 1248–1253. [Google Scholar] [CrossRef]
- Damtie, D.; Mekonnen, Y. Antibacterial activity of essential oils from Ethiopian thyme (Thymus serrulatus and Thymus schimperi) against tooth decay bacteria. PLoS ONE 2020, 15, e0239775. [Google Scholar] [CrossRef]
- Kędzia, A.; Dera-Tomaszewska, B.; Ziółkowska-Klinkosz, M.; Kędzia, A.W.; Kochańska, B.; Gębska, A. Activity of thyme oil (Oleum Thymi) against aerobic bacteria. Post Fitoter. 2012, 2, 67–71. [Google Scholar]
- Tellez-Monzón, L.A.; Nolazco-Cama, D.M. Estudio de la composición química del aceite esencial de orégano (Origanum vulgare spp.) de Tacna. Ing. Ind. 2017, 35, 195–205. [Google Scholar]
- Sim, J.X.F.; Khazandi, M.; Chan, W.Y.; Trott, D.J.; Deo, P. Antimicrobial activity of thyme oil, oregano oil, thymol and carvacrol against sensitive and resistant microbial isolates from dogs with otitis externa. Vet. Dermat. 2019, 30, 524. [Google Scholar] [CrossRef]
- Yamazaki, K.; Yamamoto, T.; Kawai, Y.; Inoue, N. Enhancement of antilisterial activity of essential oil constituents by nisin and diglycerol fatty acid ester. Food Microbiol. 2004, 21, 283–289. [Google Scholar] [CrossRef]
- Rota, C.; Carraminana, J.J.; Burillo, J.; Herrera, A. In vitro antimicrobial activity of essential oils from aromatic plants against selected foodborne pathogens. J. Food Protect. 2004, 67, 1252–1256. [Google Scholar] [CrossRef]
- Dorozko, J.; Kunkulberga, D.; Sivicka, I.; Kruma, Z. The influence of various drying methods on the quality of edible flower petals. FOODBALT 2019. In Proceedings of the 13th Baltic Conference on Food Science and Technology “Food, Nutrition, Well-Being”, Latvia University of Life Sciences and Technologies, Jelgava, Latvia, 2–3 May 2019; Volume 1, pp. 182–187. [Google Scholar]
- Moreira, M.D.R.; Ponce, A.; Del Valle, C.E.; Roura, S.I. Edible coatings on fresh squash slices: Effect of film drying temperature on the nutritional and microbiological quality. J. Food Proc. Preserv. 2009, 33, 226–236. [Google Scholar] [CrossRef]
- Kalwa, K.; Wyrostek, J. Ocena zawartości związków biologicznie aktywnych oraz zawartość i skład olejku eterycznego w melisie lekarskiej (Melissa officinalis L.). Post Nauk Technol. Przem Rol-Spoż 2018, 73, 54–65. [Google Scholar]
- Kapadiya, D.B.; Dabhi, B.K.; Aparnathi, K.D. Spices and Herbs as a Source of Natural Antioxidants. Food Int. J. Curr. Microbiol. App. Sci. 2016, 5, 280–288. [Google Scholar] [CrossRef]
- Jałoszyński, K.; Figiel, A.; Wojdyło, A. Drying kinetics and antioxidant activity of oregano. Acta Agroph. 2008, 11, 81–90. [Google Scholar]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants–a mini-review. J. Funct. Foods. 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Hallmann, E.; Sabała, P. Organic and Conventional Herbs Quality Reflected by Their Antioxidant Compounds Concentration. Appl. Sci. 2020, 10, 3468. [Google Scholar] [CrossRef]
- Burnett, S.E.; Mattson, N.S.; Williams, K.A. Substrates, and fertilisers for organic container production of herbs, vegetables, and herbaceous ornamental plants grown in greenhouses in the United States. Sci. Hortic. 2016, 208, 111–119. [Google Scholar] [CrossRef]
- Matłok, N.; Gorzelany, J.; Stepień, A.E.; Figiel, A.; Balawejder, M. Effect of Fertilization in Selected Phytometric Features and Contents of Bioactive Compounds in Dry Matter of Two Varieties of Basil (Ocimum basilicum L.). Sustainability 2019, 11, 6590. [Google Scholar] [CrossRef]
- Dragland, S.; Senoo, H.; Wake, K.; Holte, K.; Blomhoff, R. Several Culinary and Medicinal Herbs Are Important Sources of Dietary Antioxidants. J. Nutr. 2003, 133, 1286–1290. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; Alvarenga, J.F.R.; Leal, L.N.; Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin, and bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef]
- Taghipour, S.; Rahimi, A.; Zartoshti, M.R.; Arslan, Y. The effect of micronutrients on antioxidant properties of thyme (Thymus vulgaris L.) under humic acid using condition. YYU J. Agric. Sci. 2017, 27, 589–600. [Google Scholar]
- Matłok, N.; Stępień, A.E.; Gorzelany, J.; Wojnarowska-Nowak, R.; Balawejder, M. Effects of Organic and Mineral Fertilization on Yield and Selected Quality Parameters for Dried Herbs of Two Varieties of Oregano (Origanum vulgare L.). Appl. Sci. 2020, 10, 5503. [Google Scholar]
- Lv, J.; Huang, H.; Yu, L.; Whent, M.; Niu, Y.; Shi, H.; Yu, L.L. Phenolic composition and nutraceutical properties of organic and conventional cinnamon and peppermint. Food Chem. 2012, 132, 1442–1450. [Google Scholar] [CrossRef]
- Faller, A.L.K.; Fialho, E. The antioxidant capacity and polyphenol content of organic and conventional retail vegetables after domestic cooking. Food Rese. Int. 2009, 42, 210–215. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls A and B of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Garousi, F.; Veres, S.; Bódi, É.; Várallyay, S.; Kovács, B. Role of selenite and selenate uptake by maise plants in chlorophyll A and B content. Int. J. Biol. Biomol. Agric. Food Biot. Eng. 2015, 9, 625–668. [Google Scholar]
- Chen, B.H.; Chen, Y.Y. Stability of chlorophylls and carotenoids in sweet potato leaves during microwave cooking. J. Agric. Food Chem. 1993, 41, 1315–1320. [Google Scholar] [CrossRef]
- Onofrei, V.; Burducea, M.; Lobiuc, A.; Teliban, G.C.; Ranghiuc, G.; Robu, T. Influence of organic foliar fertilisation on antioxidant activity and content of polyphenols in Ocimum basilicum L. Acta Pol. Pharm. 2017, 74, 611–615. [Google Scholar]
- Manukyan, A. Secondary metabolites and their antioxidant capacity of Caucasian endemic thyme (Thymus transcaucasicus Ronn.) as affected by environmental stress. J. Appl. Res. Med. Arom. Plants 2019, 13, 100209. [Google Scholar] [CrossRef]
- Skubij, N.; Dzida, K. Effect of natural fertilisation and the type of substrate on the biological value of the thyme herb (Thymus vulgaris L.). Acta Sci. Pol. Hortorum. Cultus 2016, 15, 291–304. [Google Scholar]
- Tzima, K.; Brunton, N.P.; Rai, D.K. Evaluation of the impact of chlorophyll removal techniques on polyphenols in rosemary and thyme by-products. J. Food Biochem. 2020, 44, e13148. [Google Scholar] [CrossRef] [PubMed]
- Kulbat-Warycha, K.; Georgiadou, E.C.; Mańkowska, D.; Smolińska, B.; Fotopoulos, V.; Leszczyńska, J. Response to stress and allergen production caused by metal ions (Ni, Cu and Zn) in oregano (Origanum vulgare L.) plants. J. Biotechnol. 2020, 324, 171–182. [Google Scholar] [CrossRef] [PubMed]
- De Boer, E.; Spiegelenberg, W.M.; Janssen, F.W. Microbiology of spices and herbs. Antonie van Leeuwenhoek 1985, 51, 435–438. [Google Scholar] [CrossRef]
- Fogele, B.; Granta, R.; Valciņa, O.; Bērziņš, A. Occurrence and diversity of Bacillus cereus and moulds in spices and herbs. Food Control 2018, 83, 69–74. [Google Scholar] [CrossRef]
- Bankova, R.; Popova, T.P. Antimicrobial Activity in vitro of Aqueous Extracts of Oregano (Origanum vulgare L.) and Thyme (Thymus vulgaris L.). Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1–12. [Google Scholar] [CrossRef]
- de Sousa Lima, C.M.; Fujishima, M.A.T.; de Paula Lima, B.; Mastroianni, P.C.; Fábio, F.; de Sousa, O.; Oliveira da Silva, J. Microbial contamination in herbal medicines: A serious health hazard to elderly consumers. BMC Complement. Med. Ther. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Quadir, M.; Shahzadi, S.K.; Bashir, A.; Munir, A.; Shahzad, S. Evaluation of phenolic compounds and antioxidant and antimicrobial activities of some common herbs. Int. J. Anal. Chem. 2017, 2017, 1–6. [Google Scholar] [CrossRef]
- Kandasamy, M.; Nasimuddin, S.; Gnanadesikan, S.; Nithyalakshmi, J.; Vennimalai, S. Antibacterial activity of aqueous infusion and decoction of dried leaves of oregano (Origanum vulgare) on clinical bacterial isolates. Indian J. Microbiol. Res. 2017, 4, 442–447. [Google Scholar]
- Shan, B.; Cai, Y.-Z.; Brooks, J.D.; Corke, H. The in vitroantibacterial activity of dietary spice and medicinal herb extracts. Int. J. Food Microb. 2007, 117, 112–119. [Google Scholar] [CrossRef]
- Al-Alzoreky, N.S. Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. Int. J. Food Microbiol. 2009, 34, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.R. Tratado de Fitomedicina. Bases Clínicas y Farmacológicas; Isis Ediciones SRL: Buenos Aires, Brazil, 1998; pp. 1–1039. [Google Scholar]
- Soliman, K.M.; Badeaa, R.I. Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food Chem. Toxicol. 2002, 40, 1669–1675. [Google Scholar] [CrossRef]
- Bukovská, A.; Cikos, S.; Juhás, S.; Il’ková, G.; Rehák, P.; Koppel, J. Effects of a combination of thyme and oregano essential oils on TNBS-induced colitis in mice. Mediat. Inflamm. 2007, 2007, 23296. [Google Scholar] [CrossRef]
- de Oliveira, J.R.; de Jesus Viegas, D.; Martins, A.P.R.; Carvalho, C.A.T.; Soares, C.P.; Camargo, S.E.A.; de Oliveira, L.D. Thymus vulgaris L. extract has antimicrobial and anti-inflammatory effects in the absence of cytotoxicity and genotoxicity. Arch. Oral Biol. 2017, 82, 271–279. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Menichini, F.; Conforti, F.; Tundis, R.; Bonesi, M.; Saab, A.M.; Statti, G.A.; de Cindio, B.; Houghton, P.J.; Menichini, F.; et al. Chemical analysis, antioxidant, anti-inflammatory and anticholinesterase activities of Origanum ehrenbergii Boiss and Origanum syriacum L. essential oils. Food Chem. 2009, 117, 174–180. [Google Scholar] [CrossRef]
- Jamali, T.; Kavoosi, G.; Safavi, M.; Ardestani, S.K. In-vitro evaluation of apoptotic effect of OEO and thymol in 2D and 3D cell cultures and the study of their interaction mode with DNA. Sci. Rep. 2018, 25, 15787. [Google Scholar] [CrossRef]
- El-Babili, F.; Bouajila, J.; Souchard, J.P.; Bertrand, C.; Bellvert, F.; Fouraste, I.; Moulis, C.; Valentin, A. Oregano: Chemical analysis and evaluation of its antimalarial, antioxidant, and cytotoxic activities. J. Food Sci. 2011, 76, 512–518. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Zhiri, A.; Baudoux, D.; Idaomar, M. Antigenotoxic effects of three essential oils in diploid yeast Saccharomyces cerevisiae after treatments with UVC radiation, 8-MOP plus UVA and MMS. Mut. Res. 2006, 606, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Mezzoug, N.; Elhadri, A.; Dallouh, A.; Amkiss, S.; Skali, S.; Abrini, J.; Zhiri, A.; Baudoux, D.; Diallo, B.; El Jaziri, M. Investigation of the mutagenic and antimutagenic effects of Origanum compactum essential oil and some of its constituents. Mutat. Res. Genet. Toxicol. Environ. 2007, 629, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST) 2020. MIC Determination of Non-Fastidious and Fastidious Organisms. EUCAST Version 6. Available online: https://www.eucast.org/ast_of_bacteria/mic_determination/?no_cache=1 (accessed on 21 December 2020).
- Kamizela, A.; Gawdzik, B.; Urbaniak, M.; Lechowicz, Ł.; Białońska, A.; Kutniewska, S.E.; Chmiela, M. New γ-Halo-δ-lactones and δ-Hydroxy-γ-lactones with Strong Cytotoxic Activity. Molecules 2019, 24, 1875. [Google Scholar] [CrossRef] [PubMed]


| Herb | Cultivation Type | Polyphenols | Chlorophyll a | Chlorophyll b | Carotenoids | ||||
|---|---|---|---|---|---|---|---|---|---|
| mg GAE/mL Sample | μg/1 mL Sample | ||||||||
| oregano | ORG | 0.20 | a | 7.81 | cd | 12.32 | cd | 2.07 | bcd |
| CONV | 0.36 | bc | 4.84 | ab | 7.21 | ab | 1.03 | abcd | |
| thyme | ORG | 0.40 | bcd | 7.35 | cd | 11.40 | cd | 1.67 | abc |
| CONV | 0.42 | cd | 2.96 | ab | 4.56 | ab | 0.76 | abc | |
| Microorganisms | Water Macerates | Antimicrobial Reference Standard * | ||||
|---|---|---|---|---|---|---|
| Thyme CONV | Thyme ORG | Oregano CONV | Oregano ORG | Gentamicin | Fluconazole | |
| MIC/MBC (mg/mL) | MIC/MBC (µg/mL) | |||||
| Gram-Positive Bacteria | ||||||
| Staphylococcus aureus ATCC 25923 | 12.5/12.5 | >50/>50 | 6.25/6.25 | 50/50 | 0.5 | nt |
| Enterocococcus faecalis ATCC 29212 | 25/25 | >50/>50 | 25/25 | >50/>50 | 16 | nt |
| Bacillus cereus PCM 1948 | 6.25/6.25 | 12.5/12.5 | 3.125/3.125 | 6.25/6.25 | 0.25 | nt |
| Bacillus subtilis ATCC 6635 | 25/25 | 50/50 | 6.25/6.25 | 25/25 | 0.25 | nt |
| Listeria monocytogenes PCM 2191 | 50/50 | 50/50 | 25/25 | 25/25 | 0.25 | nt |
| Gram-Negative Bacteria | ||||||
| Escherichia coli ATCC 25922 | >50/>50 | >50/>50 | 50/50 | >50/>50 | 2 | nt |
| Pseudomonas aeruginosa ATCC 27853 | 0.625/0.625 | >50/>50 | 12.5/12.5 | >50/>50 | 2 | nt |
| Shigella flexneri ATCC 12022 | 50/50 | >50/>50 | >50/>50 | >50/>50 | nd ^ | nt |
| Salmonella Enteritidis ZMF 279 | 12.5/12.5 | >50 | 50/50 | >50/>50 | 0.25 | nt |
| Fungi | ||||||
| Candida albicans ATCC 10241 | >50/>50 | >50/>50 | >50/>50 | >50/>50 | nt | 5/>5 |
| Aspergillus brasiliensis ATCC 16404 | >50/>50 | >50/>50 | >50/>50 | >50/>50 | nt | 5/>5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikorska-Zimny, K.; Lisiecki, P.; Gonciarz, W.; Szemraj, M.; Ambroziak, M.; Suska, O.; Turkot, O.; Stanowska, M.; Rutkowski, K.P.; Chmiela, M.; et al. Influence of Agronomic Practice on Total Phenols, Carotenoids, Chlorophylls Content, and Biological Activities in Dry Herbs Water Macerates. Molecules 2021, 26, 1047. https://doi.org/10.3390/molecules26041047
Sikorska-Zimny K, Lisiecki P, Gonciarz W, Szemraj M, Ambroziak M, Suska O, Turkot O, Stanowska M, Rutkowski KP, Chmiela M, et al. Influence of Agronomic Practice on Total Phenols, Carotenoids, Chlorophylls Content, and Biological Activities in Dry Herbs Water Macerates. Molecules. 2021; 26(4):1047. https://doi.org/10.3390/molecules26041047
Chicago/Turabian StyleSikorska-Zimny, Kalina, Paweł Lisiecki, Weronika Gonciarz, Magdalena Szemraj, Maja Ambroziak, Olga Suska, Oliwia Turkot, Małgorzata Stanowska, Krzysztof P. Rutkowski, Magdalena Chmiela, and et al. 2021. "Influence of Agronomic Practice on Total Phenols, Carotenoids, Chlorophylls Content, and Biological Activities in Dry Herbs Water Macerates" Molecules 26, no. 4: 1047. https://doi.org/10.3390/molecules26041047
APA StyleSikorska-Zimny, K., Lisiecki, P., Gonciarz, W., Szemraj, M., Ambroziak, M., Suska, O., Turkot, O., Stanowska, M., Rutkowski, K. P., Chmiela, M., & Mielicki, W. (2021). Influence of Agronomic Practice on Total Phenols, Carotenoids, Chlorophylls Content, and Biological Activities in Dry Herbs Water Macerates. Molecules, 26(4), 1047. https://doi.org/10.3390/molecules26041047






