8-O-(E-p-methoxycinnamoyl)harpagide Inhibits Influenza A Virus Infection by Suppressing Intracellular Calcium
Abstract
1. Introduction
2. Results
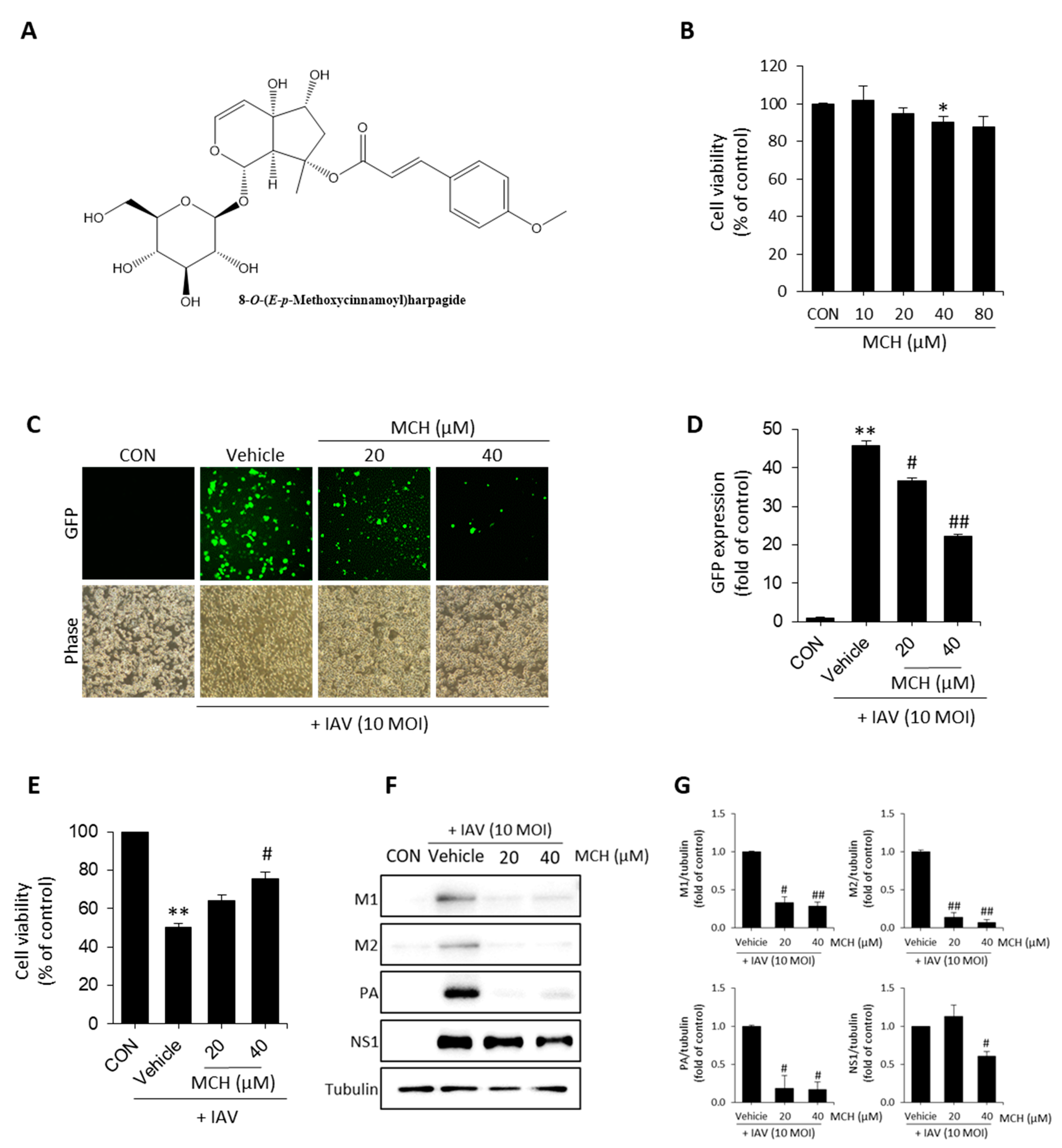
2.1. MCH Inhibits IAV Infection in Human Lung Cells
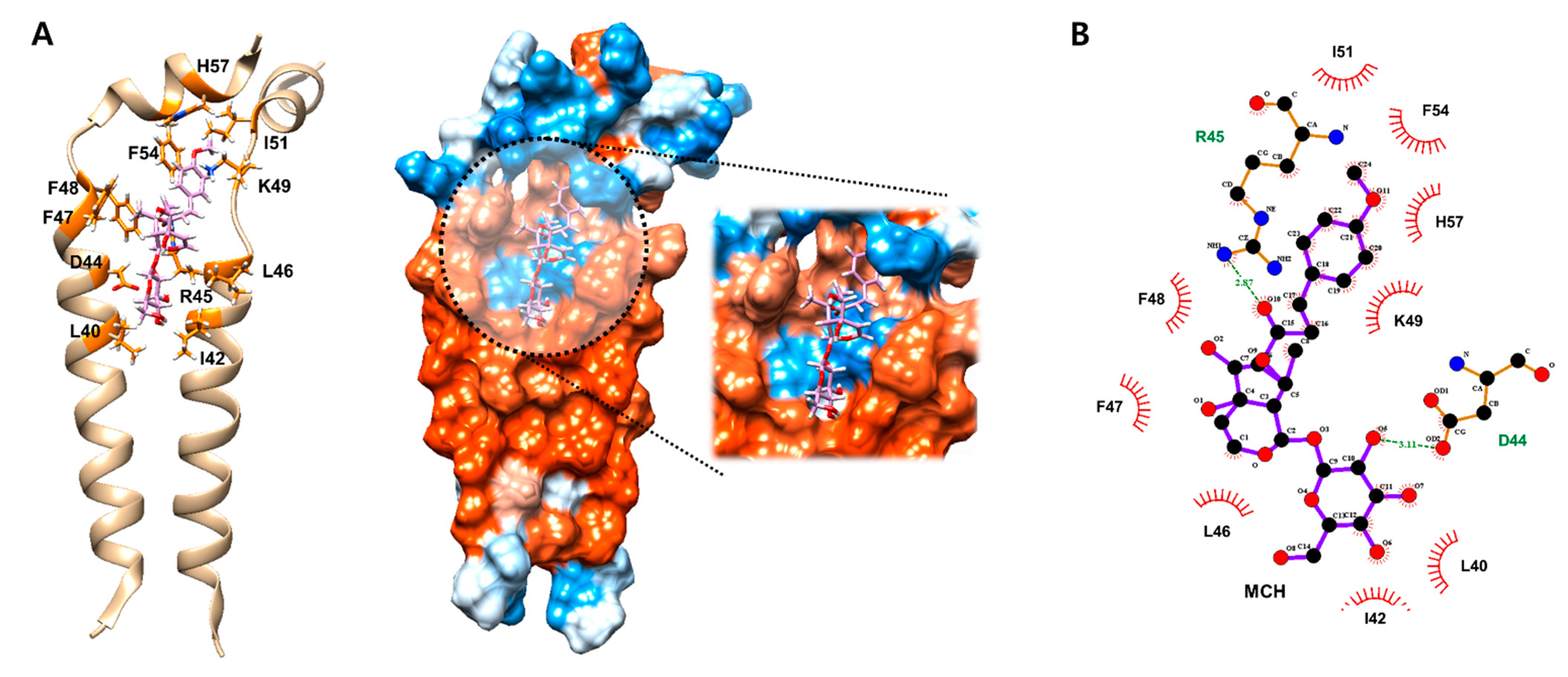
2.2. MCH May Inhibit the Function of Ion Channel by Binding onto M2 Protein
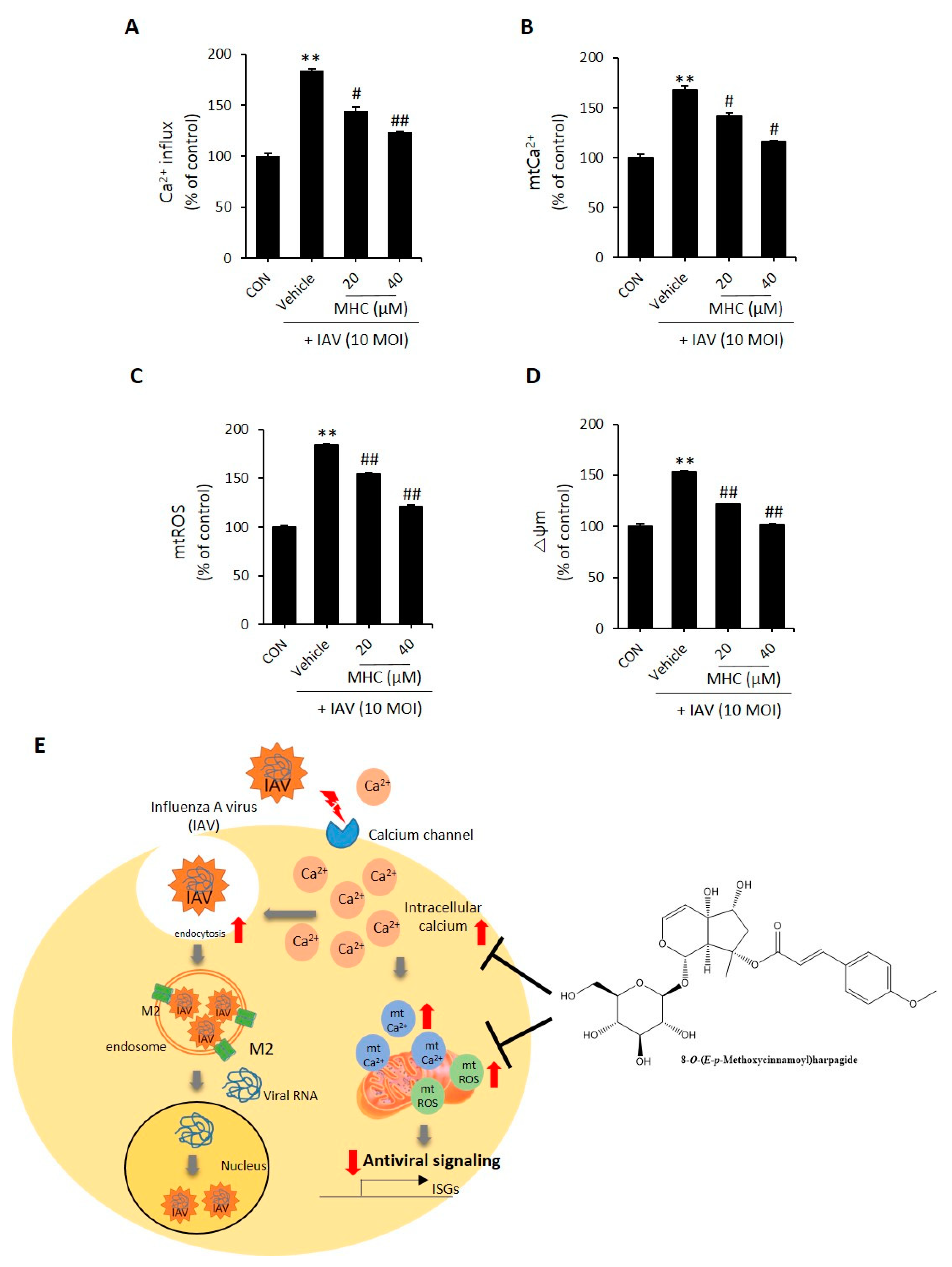
2.3. MCH Decreased Intracellular Calcium and Mitochondrial Stress in IAV-infected A549 Cells
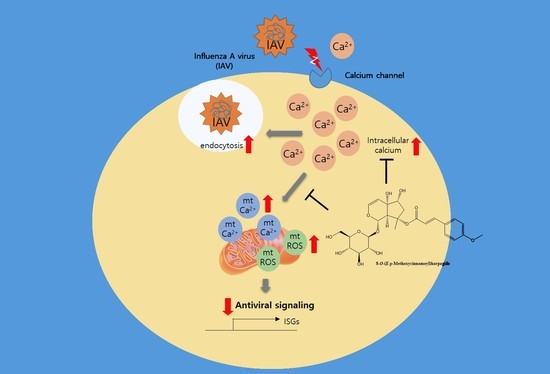
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell and Virus
4.3. Cell Viability and Antiviral Effect
4.4. Flow Cytometry Analysis
4.5. Immunoblotting
4.6. In Silico
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaitonde, D.Y.; Moore, F.C.; Morgan, M.K. Inflenza: Diagnosis and treatment. Am. Fam. Physician. 2019, 100, 751–758. [Google Scholar]
- Chen, X.; Cao, R.; Zhong, W. Host Calcium Channels and Pumps in Viral Infections. Cells 2019, 9, 94. [Google Scholar] [CrossRef]
- Fujioka, Y.; Nishide, S.; Ose, T.; Suzuki, T.; Kato, I.; Fukuhara, H.; Fujioka, M.; Horiuchi, K.; Satoh, A.O.; Nepal, P.; et al. A sialylated voltage-dependent Ca2+ channel binds hemagglutinin and mediates influenza a virus entry into mammalian cells. Cell Host Microbe 2018, 23, 809–818. [Google Scholar] [CrossRef]
- Hoffmann, M.; Pöhlmann, S. Cell entry of influenza A viruses: Sweet talk between HA and CaV1. 2. Cell Host Microbe 2018, 23, 697–699. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, Y.; Tsuda, M.; Nanbo, A.; Hattori, T.; Sasaki, J.; Sasaki, T.; Miyazaki, T.; Ohba, Y. A Ca2+-dependent signalling circuit regulates influenza A virus internalization and infection. Nat. Commun. 2013, 4, 2763. [Google Scholar] [CrossRef]
- Shin, N.R.; Lee, A.Y.; Song, J.H.; Yang, S.Y.; Park, I.K.; Lim, J.O.; Jung, T.Y.; Ko, J.K.; Kim, J.C.; Lim, K.S.; et al. Scrophularia buergeriana attenuates allergic inflammation by reducing NF-κB activation. Phytomedicine 2020, 67, 153159. [Google Scholar] [CrossRef]
- Lee, H.J.; Spandidos, D.A.; Tsatsakis, A.; Margina, D.; Izotov, B.N.; Yang, S.H. Neuroprotective effects of Scrophularia buergeriana extract against glutamate-induced toxicity in SH-SY5Y cells. Int. J. Mol. Med. 2019, 43, 2144–2152. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Kang, S.Y.; Lee, K.Y.; Kim, S.H.; Mrkelonis, G.J.; Oh, T.H.; Kim, Y.C. Anti-amnestic activity of E-p-methoxycinnamic acid from Scrophularia buergeriana. Cogn. Brain Res. 2003, 17, 454–461. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, K.Y.; Koo, K.A.; Sung, S.H.; Lee, N.G.; Kim, J.; Kim, Y.C. Four new neuroprotective iridoid glycosides from Scrophularia buergeriana roots. J. Nat. Prod. 2002, 65, 1696–1699. [Google Scholar] [CrossRef]
- Jeong, E.J.; Lee, K.Y.; Kim, S.H.; Sung, S.H.; Kim, Y.C. Cognitive-enhancing and antioxidant activities of iridoid glycosides from Scrophularia buergeriana in scopolamine-treated mice. Eur. J. Pharmacol. 2008, 588, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Hyser, J.M.; Estes, M.K. Pathophysiological Consequences of calcium-conducting viroporins. Annu. Rev. Virol. 2015, 2, 473–496. [Google Scholar] [CrossRef] [PubMed]
- Schnell, J.R.; Chou, J.J. Structure and mechanism of the M2 proton channel of influenza A virus. Nat. Cell Biol. 2008, 451, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Feno, S.; Butera, G.; Reane, D.V.; Rizzuto, R.; Raffaello, A. Crosstalk between calcium and ROS in pathophysiological conditions. Oxidative Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef]
- Wang, C.; Gong, X.; Bo, A.; Zhang, L.; Zhang, M.; Zang, E.; Zhang, C.; Li, M.-H. Iridoids: Research advances in their phytochemistry, biological activities, and pharmacokinetics. Molecule 2020, 25, 287. [Google Scholar] [CrossRef]
- Behbahani, M. Evaluation of anti-HIV-1 activity of a new iridoid glycoside isolated from Avicenna marina, in vitro. Int. Immunopharmacol. 2014, 23, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Bao, L.; Li, C.; Sun, J.; Zhao, R.; Cui, X. Antiviral activity of iridoid glycosides extracted from fructus gardeniae against influenza A virus by PACT-dependent suppression of viral RNA replication. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Tzakou, O.; Mylonas, P.; Vagias, C.; Petrakis, P.V. Iridoid glucosides with insecticidal activity from Galium melannatherum. Z. Naturforsch. 2007, 62, 597–602. [Google Scholar] [CrossRef]
- Choi, J.-G.; Kim, Y.S.; Kim, J.H.; Chung, H.-S. Antiviral activity of ethanol extract of Geranii Herba and its components against influenza viruses via neuraminidase inhibition. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Kim, Y.S.; Li, W.; Kim, J.H.; Chung, H.-S.; Choi, J.-G. Anti-Influenza Activity of an Ethyl Acetate Fraction of a Rhus verniciflua Ethanol Extract by Neuraminidase Inhibition. Oxidative Med. Cell. Longev. 2020, 2020, 1–15. [Google Scholar] [CrossRef]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39, W270–W277. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, E.-B.; Yang, H.-J.; Kim, Y.-S.; Li, W.; Choi, J.-G. 8-O-(E-p-methoxycinnamoyl)harpagide Inhibits Influenza A Virus Infection by Suppressing Intracellular Calcium. Molecules 2021, 26, 1029. https://doi.org/10.3390/molecules26041029
Kwon E-B, Yang H-J, Kim Y-S, Li W, Choi J-G. 8-O-(E-p-methoxycinnamoyl)harpagide Inhibits Influenza A Virus Infection by Suppressing Intracellular Calcium. Molecules. 2021; 26(4):1029. https://doi.org/10.3390/molecules26041029
Chicago/Turabian StyleKwon, Eun-Bin, Hye-Jin Yang, Young-Soo Kim, Wei Li, and Jang-Gi Choi. 2021. "8-O-(E-p-methoxycinnamoyl)harpagide Inhibits Influenza A Virus Infection by Suppressing Intracellular Calcium" Molecules 26, no. 4: 1029. https://doi.org/10.3390/molecules26041029
APA StyleKwon, E.-B., Yang, H.-J., Kim, Y.-S., Li, W., & Choi, J.-G. (2021). 8-O-(E-p-methoxycinnamoyl)harpagide Inhibits Influenza A Virus Infection by Suppressing Intracellular Calcium. Molecules, 26(4), 1029. https://doi.org/10.3390/molecules26041029