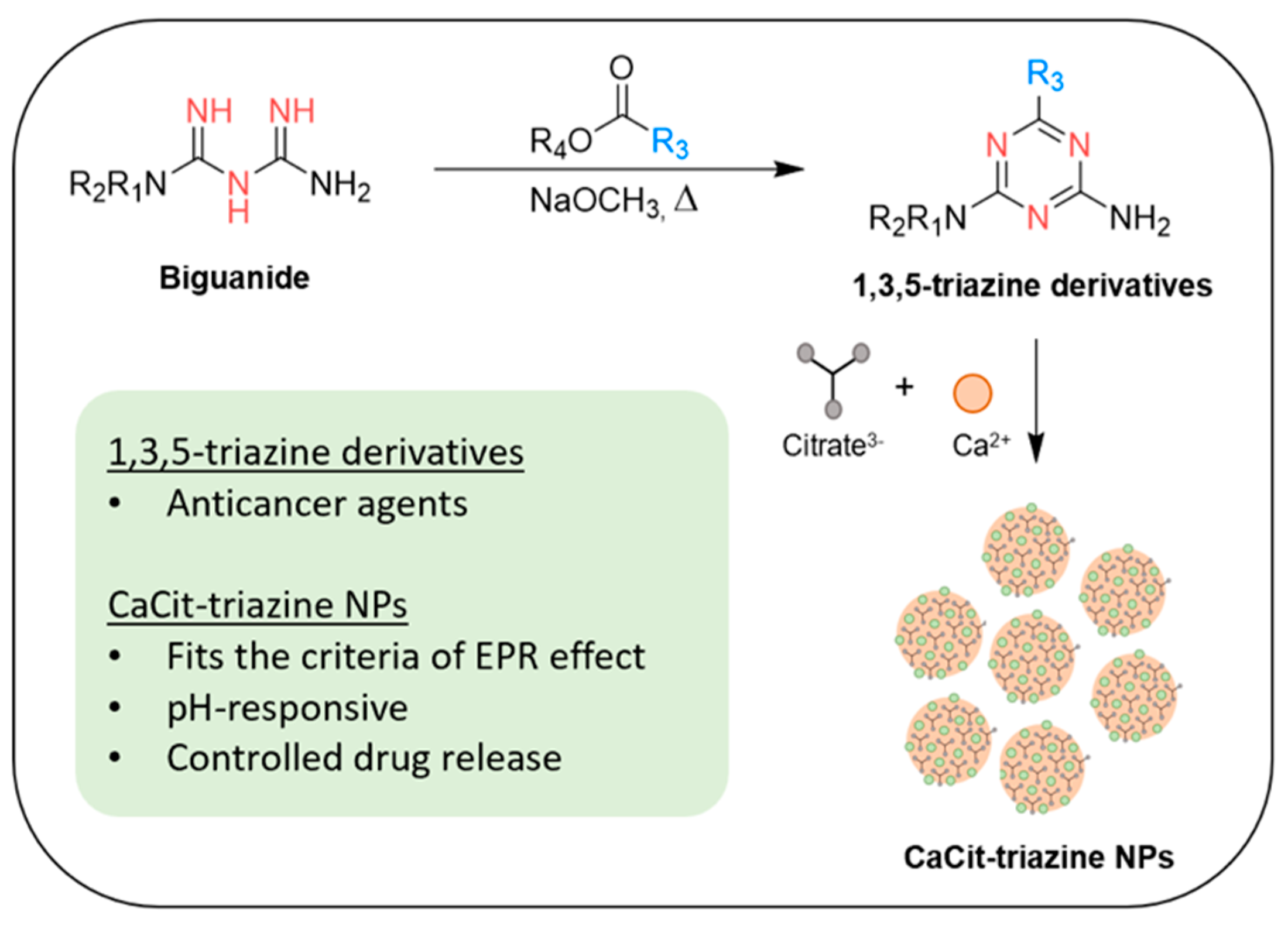
Biguanide-Based Synthesis of 1,3,5-Triazine Derivatives with Anticancer Activity and 1,3,5-Triazine Incorporated Calcium Citrate Nanoparticles
Abstract
1. Introduction
2. Results and Discussions
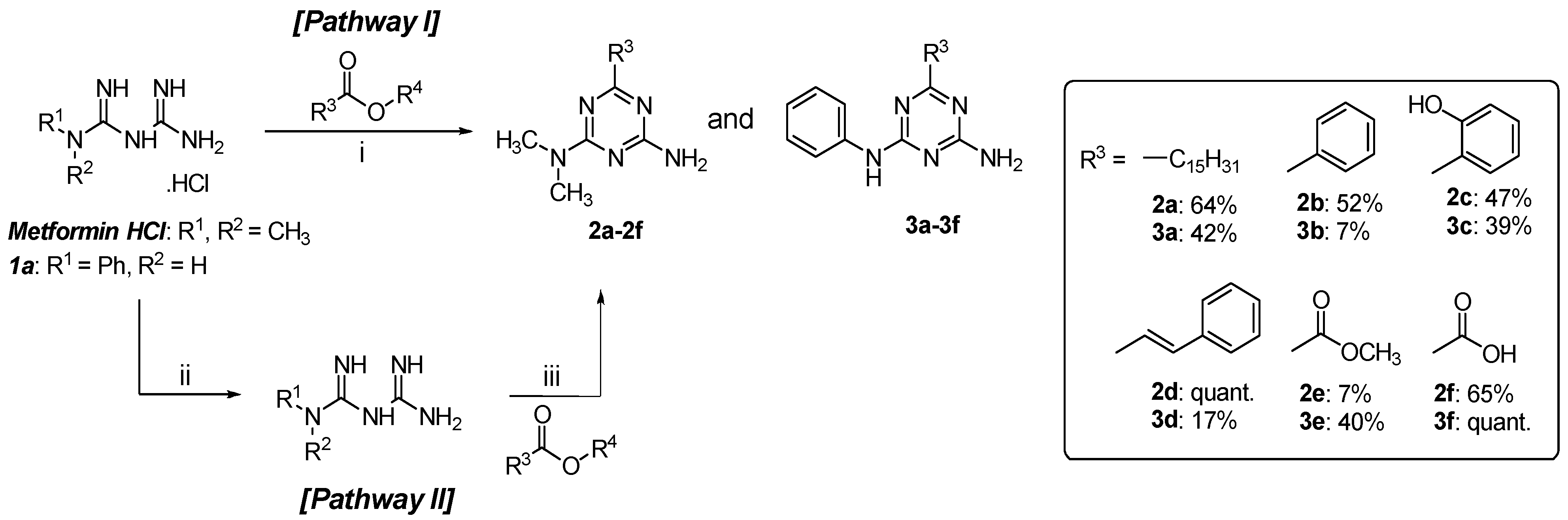
2.1. Chemistry
2.2. In Vitro Anticancer Activities
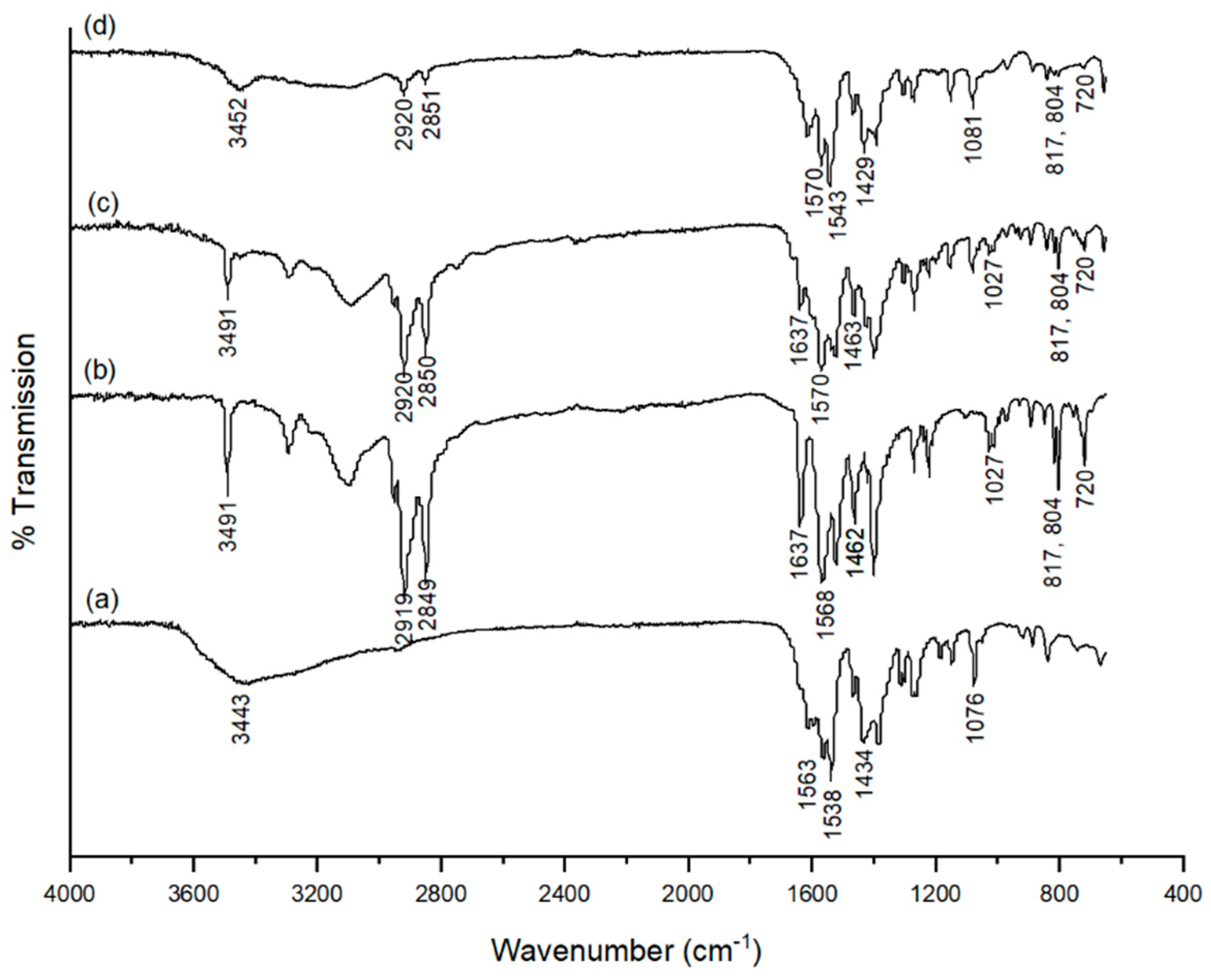
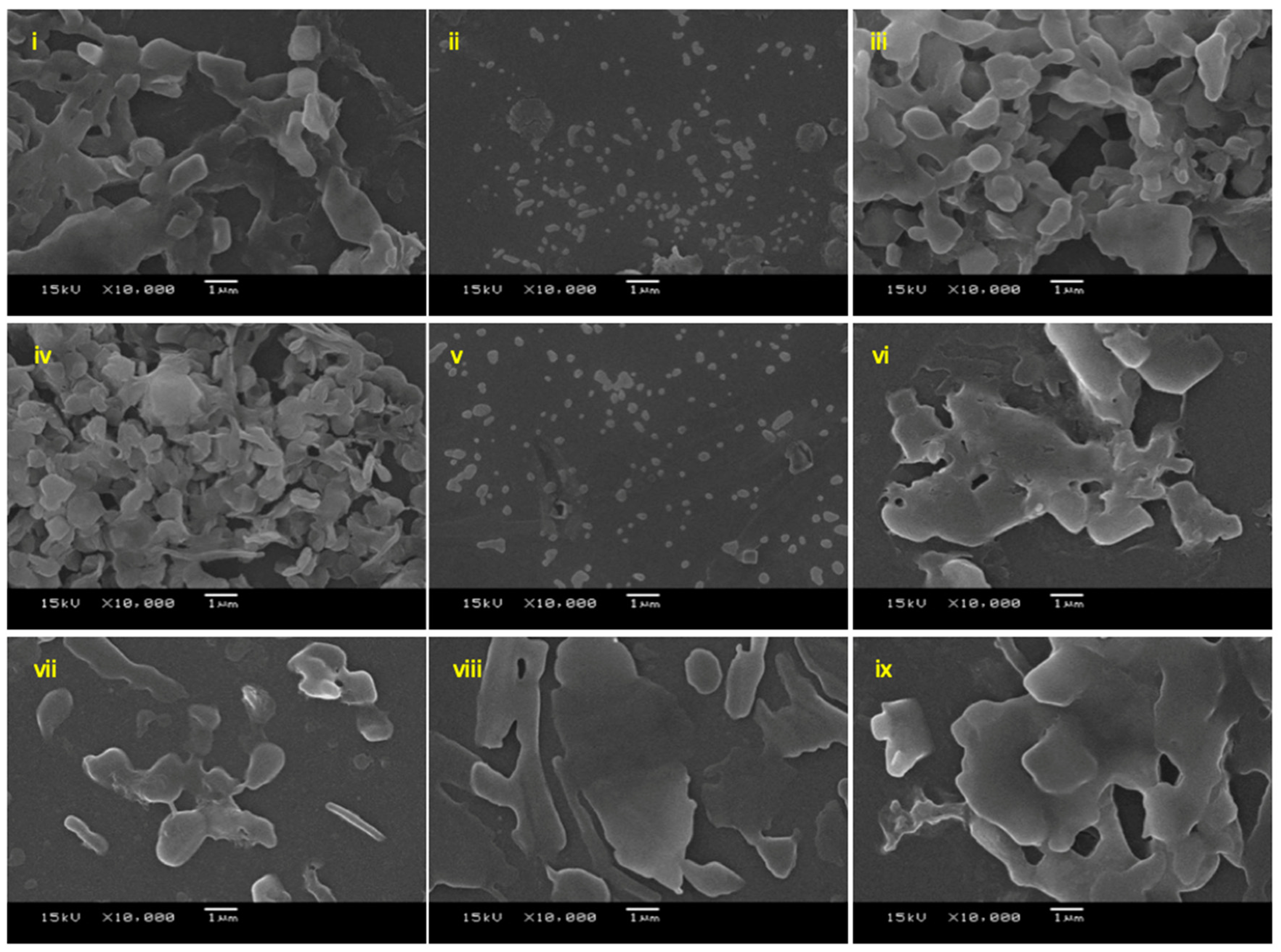
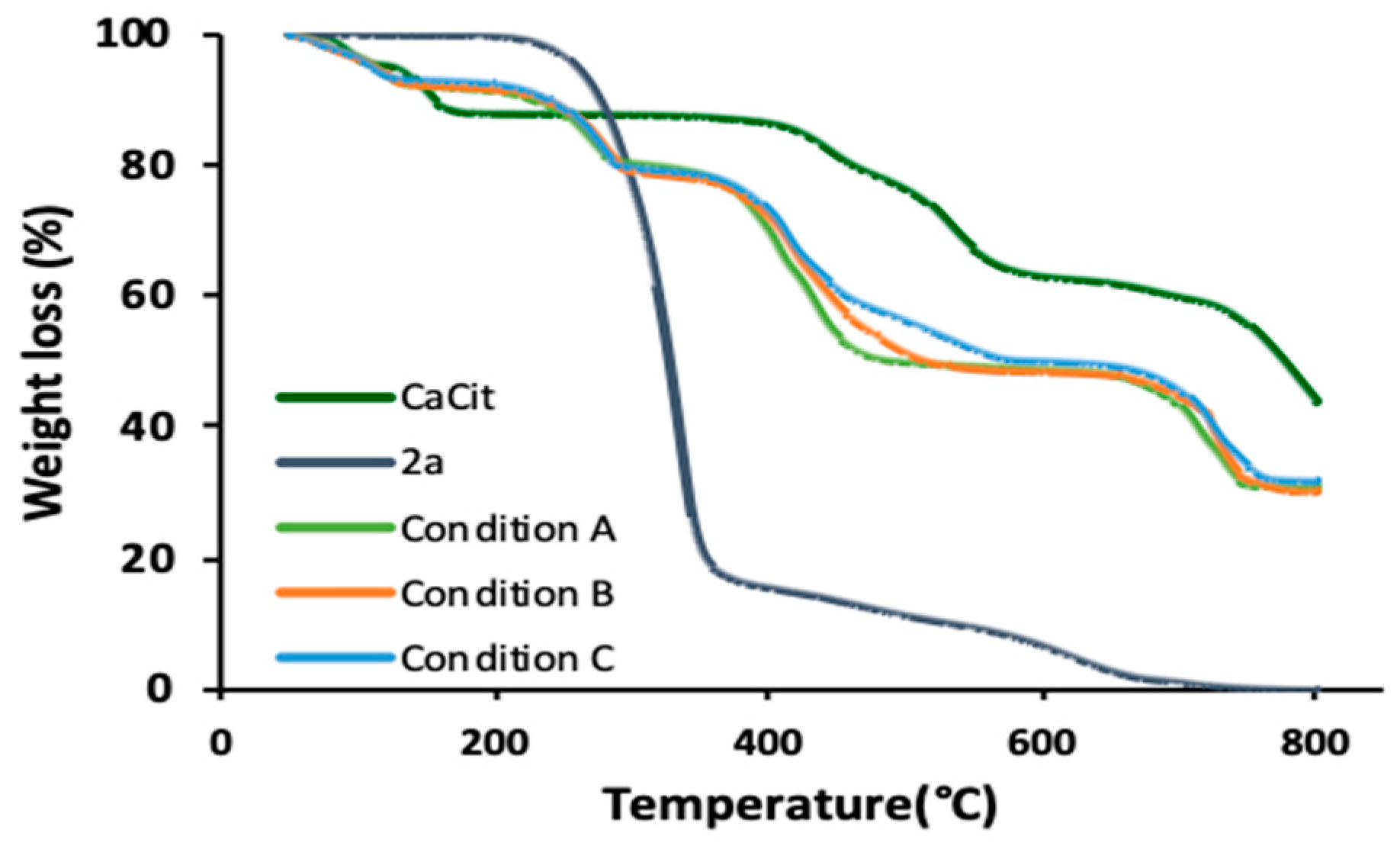
2.3. 1,3,5-Triazine Incorporated Calcium Citrate Nanoparticles
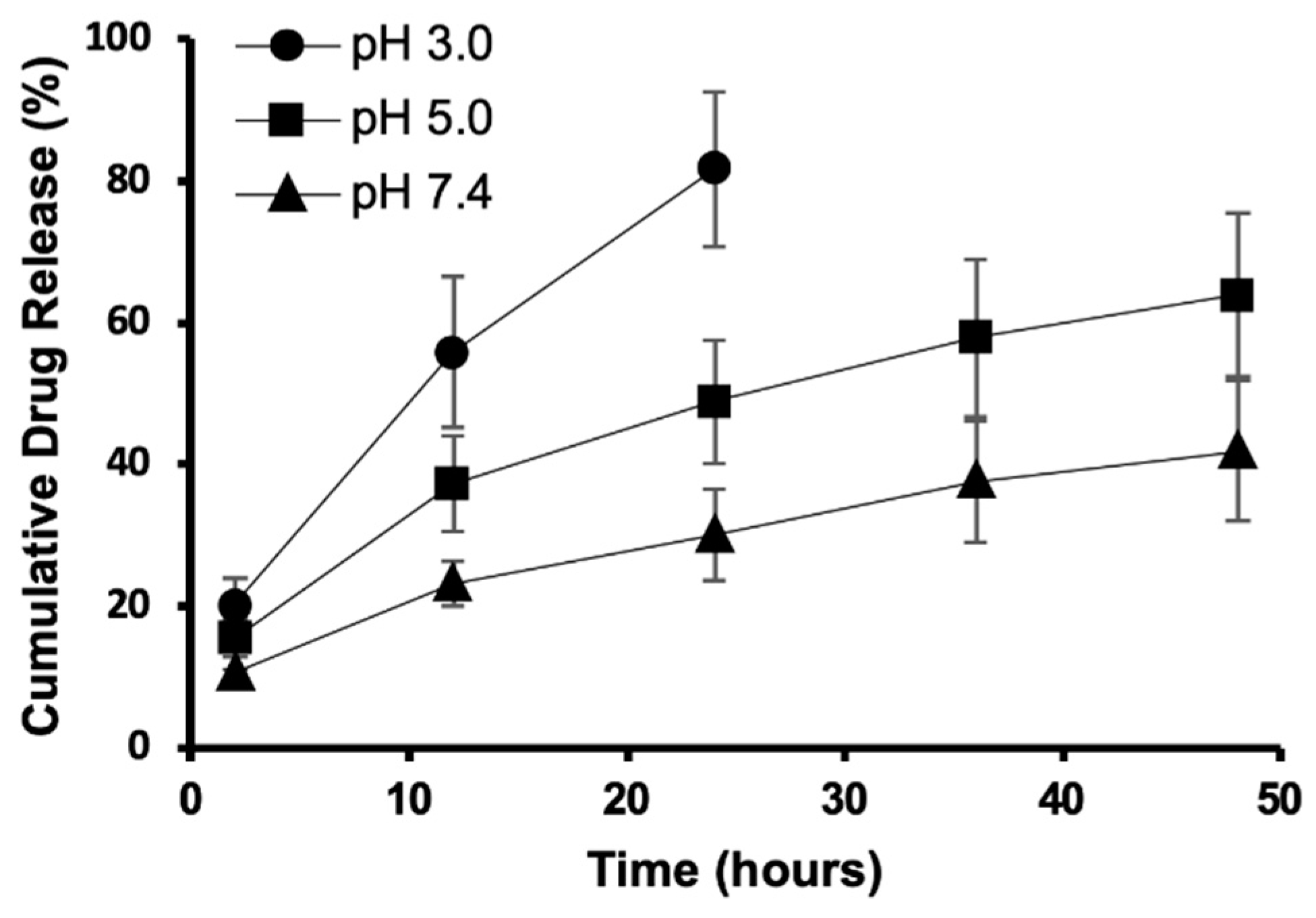
2.4. In Vitro Drug Release
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Chemical Synthesis
3.2.2. Characterisation of 1,3,5-Triazine Derivatives
3.2.3. In vitro Anticancer Activity of 1,3,5-Triazine Derivatives
3.2.4. Preparation of 1,3,5-Triazine Incorporated Calcium Citrate Nanoparticles (Condition A–C)
3.2.5. Characterisation of 1,3,5-Triazine Incorporated Calcium Citrate Nanoparticles
3.2.6. Drug Release Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. Ca: Cancerj. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Mohanty, C.; Sahoo, S.K. Ligand-based targeted therapy for cancer tissue. Expert Opin. Drug Deliv. 2009, 6, 285–304. [Google Scholar] [CrossRef]
- Banerjee, A.; Pathak, S.; Subramanium, V.D.; Dharanivasan, G.; Murugesan, R.; Verma, R.S. Strategies for targeted drug delivery in treatment of colon cancer: Current trends and future perspectives. Drug Discov. Today 2017, 22, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Singla, P.; Luxami, V.; Paul, K. Synthesis and in vitro evaluation of novel triazine analogues as anticancer agents and their interaction studies with bovine serum albumin. Eur. J. Med. Chem. 2016, 117, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Junaid, A.; Lim, F.P.L.; Tiekink, E.R.T.; Dolzhenko, A.V. Design, synthesis, and biological evaluation of new 6,N2-diaryl-1,3,5-triazine-2,4-diamines as anticancer agents selectively targeting triple negative breast cancer cells. Rsc Adv. 2020, 10, 25517–25528. [Google Scholar] [CrossRef]
- Junaid, A.; Lim, F.P.L.; Tiekink, E.R.T.; Dolzhenko, A.V. New One-Pot Synthesis of 1,3,5-Triazines: Three-Component Condensation, Dimroth Rearrangement, and Dehydrogenative Aromatization. Acs Comb. Sci. 2019, 21, 548–555. [Google Scholar] [CrossRef]
- Zeng, M.; Wang, T.; Cui, D.-M.; Zhang, C. Ruthenium-catalyzed synthesis of tri-substituted 1,3,5-triazines from alcohols and biguanides. Newj. Chem. 2016, 40, 8225–8228. [Google Scholar] [CrossRef]
- Zeng, M.; Xie, Z.P.; Cui, D.-M.; Zhang, C. Ruthenium-catalyzed synthesis of arylethyl 1,3,5-triazines from arylallyl alcohols and biguanides. Org. Biomol. Chem. 2018, 16, 6140–6145. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shen, B.; Liu, L.; Qiao, C. Metal free [4+1] and [5+1] annulation reactions to prepare heterocycles using DMF and its derivatives as one-carbon source. Tetrahedron Lett. 2020, 61, 151844. [Google Scholar] [CrossRef]
- Cao, H.; Liao, S.; Zhong, W.; Xiao, X.; Zhu, J.; Li, W.; Wu, X.; Feng, Y. Synthesis, Characterization, and Biological Evaluations of 1,3,5-Triazine Derivatives of Metformin Cyclization with Berberine and Magnolol in the Presence of Sodium Methylate. Molecules 2017, 22, 1752. [Google Scholar] [CrossRef] [PubMed]
- Makowska, A.; Sączewski, F.; Bednarski, P.J.; Sączewski, J.; Balewski, Ł. Hybrid Molecules Composed of 2,4-Diamino-1,3,5-triazines and 2-Imino-Coumarins and Coumarins. Synthesis and Cytotoxic Properties. Molecules 2018, 23, 1616. [Google Scholar] [CrossRef]
- Chaurasia, S.R.; Dange, R.; Bhanage, B.M. Graphene oxide as a carbo-catalyst for the synthesis of tri-substituted 1,3,5-triazines using biguanides and alcohols. Catal. Commun. 2020, 137, 105933. [Google Scholar] [CrossRef]
- Yao, W.; Duan, Z.-C.; Zhang, Y.; Sang, X.; Xia, X.-F.; Wang, D. Iridium Supported on Phosphorus-Doped Porous Organic Polymers: Active and Recyclable Catalyst for Acceptorless Dehydrogenation and Borrowing Hydrogen Reaction. Adv. Synth. Catal. 2019, 361, 5695–5703. [Google Scholar] [CrossRef]
- Koh, M.; Lee, J.-C.; Min, C.; Moon, A. A novel metformin derivative, HL010183, inhibits proliferation and invasion of triple-negative breast cancer cells. Bioorg. Med. Chem. 2013, 21, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Kothayer, H.; Spencer, S.M.; Tripathi, K.; Westwell, A.D.; Palle, K. Synthesis and in vitro anticancer evaluation of some 4,6-diamino-1,3,5-triazine-2-carbohydrazides as Rad6 ubiquitin conjugating enzyme inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 2030–2034. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Hickey, J.W.; Santos, J.L.; Williford, J.-M.; Mao, H.-Q. Control of polymeric nanoparticle size to improve therapeutic delivery. J. Control. Release 2015, 219, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.H.; Park, K. Targeted drug delivery to tumors: Myths, reality and possibility. J. Control. Release 2011, 153, 198–205. [Google Scholar] [CrossRef]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity Generated by the Tumor Microenvironment Drives Local Invasion. Cancer Res. 2013, 73, 1524. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, W.; Chen, Q.-Y.; Lin, Z.-Q.; Cheng, S.-W.; Kou, D.-Q.; Ying, X.-Z.; Shen, Y.; Cheng, X.-J.; Nie, P.-F.; et al. Effect of calcium citrate on bone integration in a rabbit femur defect model. Asian Pac. J. Trop. Med. 2012, 5, 310–314. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Gao, Y.; Zhong, L.; Zou, Q.; Lai, X. Preparation and properties of calcium citrate nanosheets for bone graft substitute. Bioengineered 2016, 7, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Oungeun, P.; Rojanathanes, R.; Pinsornsak, P.; Wanichwecharungruang, S. Sustaining Antibiotic Release from a Poly(methyl methacrylate) Bone-Spacer. Acs Omega 2019, 4, 14860–14867. [Google Scholar] [CrossRef]
- Rimsueb, N.; Cherdchom, S.; Aksornkitti, V.; Khotavivattana, T.; Sereemaspun, A.; Rojanathanes, R. Feeding Cells with a Novel “Trojan” Carrier: Citrate Nanoparticles. Acs Omega 2020, 5, 7418–7423. [Google Scholar] [CrossRef] [PubMed]
- Kothayer, H.; Morelli, M.; Brahemi, G.; Elshanawani, A.A.; Abu Kull, M.E.; El-Sabbagh, O.I.; Shekhar, M.P.V.; Westwell, A.D. Optimised synthesis of diamino-triazinylmethyl benzoates as inhibitors of Rad6B ubiquitin conjugating enzyme. Tetrahedron Lett. 2014, 55, 7015–7018. [Google Scholar] [CrossRef]
- Shapiro, S.L.; Parrino, V.A.; Freedman, L. Guanamines. VIII. 6-(Substituted Phenyl)guanamines. J. Org. Chem. 1961, 26, 3331–3334. [Google Scholar] [CrossRef]
- Mallon, R.; Feldberg, L.R.; Lucas, J.; Chaudhary, I.; Dehnhardt, C.; Santos, E.D.; Chen, Z.; dos Santos, O.; Ayral-Kaloustian, S.; Venkatesan, A.; et al. Antitumor Efficacy of PKI-587, a Highly Potent Dual PI3K/mTOR Kinase Inhibitor. Clin. Cancer Res. 2011, 17, 3193. [Google Scholar] [CrossRef]
- Moreno, L.M.; Quiroga, J.; Abonia, R.; Ramírez-Prada, J.; Insuasty, B. Synthesis of New 1,3,5-Triazine-Based 2-Pyrazolines as Potential Anticancer Agents. Molecules 2018, 23, 1956. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, P.; Xue, X.; He, S.; Kuang, Y.; Zhao, H.; Chen, S.; Zhi, Q.; Guo, X. Salinomycin induces apoptosis in cisplatin-resistant colorectal cancer cells by accumulation of reactive oxygen species. Toxicol. Lett. 2013, 222, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Kothayer, H.; Elshanawani, A.A.; Abu Kull, M.E.; El-Sabbagh, O.I.; Shekhar, M.P.V.; Brancale, A.; Jones, A.T.; Westwell, A.D. Design, synthesis and in vitro anticancer evaluation of 4,6-diamino-1,3,5-triazine-2-carbohydrazides and -carboxamides. Bioorg. Med. Chem. Lett. 2013, 23, 6886–6889. [Google Scholar] [CrossRef]
- Peng, H.; Li, K.; Wang, T.; Wang, J.; Wang, J.; Zhu, R.; Sun, D.; Wang, S. Preparation of hierarchical mesoporous CaCO3 by a facile binary solvent approach as anticancer drug carrier for etoposide. Nanoscale Res. Lett. 2013, 8, 321. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Gao, Y.; Zhong, L.Z.; Liu, Y.Q.; Liu, H.Q.; Zou, Q.; Lai, X.F. Facile Self-Assembly Synthesis of Hierarchical 3D Flowerlike Calcium Citrate Microspheres. J. Nano Res. 2017, 45, 185–192. [Google Scholar] [CrossRef]
- Garcia, A.C.; Vavrusova, M.; Skibsted, L.H. Supersaturation of calcium citrate as a mechanism behind enhanced availability of calcium phosphates by presence of citrate. Food Res. Int. 2018, 107, 195–205. [Google Scholar] [CrossRef]
- Um, N.; Hirato, T. Precipitation behavior of Ca(OH)2, Mg(OH)2, and Mn(OH)2 from CaCl2, MgCl2, and MnCl2 in NaOH-H2O solutions and study of lithium recovery from seawater via two-stage precipitation process. Hydrometallurgy 2014, 146, 142–148. [Google Scholar] [CrossRef]
- Al-Khaldi, M.H.; Nasr-El-Din, H.A.; Mehta, S.; Al-Aamri, A.D. Reaction of citric acid with calcite. Chem. Eng. Sci. 2007, 62, 5880–5896. [Google Scholar] [CrossRef]
- Ma, C.; Gerhard, E.; Lu, D.; Yang, J. Citrate chemistry and biology for biomaterials design. Biomaterials 2018, 178, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Iafisco, M.; Ramírez-Rodríguez, G.B.; Sakhno, Y.; Tampieri, A.; Martra, G.; Gómez-Morales, J.; Delgado-López, J.M. The growth mechanism of apatite nanocrystals assisted by citrate: Relevance to bone biomineralization. CrystEngComm 2015, 17, 507–511. [Google Scholar] [CrossRef]
- Xiao, H.; Hu, C.; Chen, C.; Tao, C.; Wu, Y.; Jiang, J. The advantage of alcohol–calcium method on the formation and the stability of vaterite against ethanol–water binary solvent method. J. Mater. Res. 2020, 35, 289–298. [Google Scholar] [CrossRef]
- Sand, K.K.; Rodriguez-Blanco, J.D.; Makovicky, E.; Benning, L.G.; Stipp, S.L.S. Crystallization of CaCO3 in Water–Alcohol Mixtures: Spherulitic Growth, Polymorph Stabilization, and Morphology Change. Cryst. Growth Des. 2012, 12, 842–853. [Google Scholar] [CrossRef]
- Hebeish, A.; El-Rafie, M.H.; El-Sheikh, M.A.; El-Naggar, M.E. Ultra-Fine Characteristics of Starch Nanoparticles Prepared Using Native Starch With and Without Surfactant. J. Inorg Organomet Polym Mater. 2014, 24, 515–524. [Google Scholar] [CrossRef]
- Hardiansyah, A.; Yang, M.-C.; Liu, T.-Y.; Kuo, C.-Y.; Huang, L.-Y.; Chan, T.-Y. Hydrophobic Drug-Loaded PEGylated Magnetic Liposomes for Drug-Controlled Release. Nanoscale Res. Lett. 2017, 12, 355. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Chen, T.; Wu, C.; Zhu, G.; Qiu, L.; Cui, C.; Hou, W.; Tan, W. Aptamer CaCO3 Nanostructures: A Facile, pH-Responsive, Specific Platform for Targeted Anticancer Theranostics. Chem. Asianj. 2015, 10, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, H.; Shi, W.; Zhang, J.; Feng, J.; Yang, X.; Wang, K.; Zhang, H.; Yang, L. Targeted delivery and pH-responsive release of doxorubicin to cancer cells using calcium carbonate/hyaluronate/glutamate mesoporous hollow spheres. J. Colloid Interface Sci. 2017, 502, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, C. Synthesis of the derivatives of 2-Amino-4-dimethylamino-1,3,5-triazine. J. Guangdong Coll. Pharm. 2005, 21, 2. [Google Scholar]
- Liu, C.; Lin, J.; Leftheris, K. A novel one-pot synthesis of N,6-disubstituted 1,3,5-triazine-4,6-diamines from isothiocyanates and amidines. Tetrahedron Lett. 2007, 48, 435–437. [Google Scholar] [CrossRef]
- Lebel, O.; Perron, M.-È.; Maris, T.; Zalzal, S.F.; Nanci, A.; Wuest, J.D. A New Class of Selective Low-Molecular-Weight Gelators Based on Salts of Diaminotriazinecarboxylic Acids. Chem. Mater. 2006, 18, 3616–3626. [Google Scholar] [CrossRef]
- Xu, M.; McCanna, D.J.; Sivak, J.G. Use of the viability reagent PrestoBlue in comparison with alamarBlue and MTT to assess the viability of human corneal epithelial cells. J. Pharmacol. Toxicol. Methods 2015, 71, 1–7. [Google Scholar] [CrossRef]
- Wu, J.-L.; Wang, C.-Q.; Zhuo, R.-X.; Cheng, S.-X. Multi-drug delivery system based on alginate/calcium carbonate hybrid nanoparticles for combination chemotherapy. Colloids Surf. B 2014, 123, 498–505. [Google Scholar] [CrossRef]
| 1,3,5-Triazine Derivatives | %Cytotoxicity at 100 µM 1 | IC50 (µM) 2 | ||
|---|---|---|---|---|
| SW620 | HCT116 | SW620 | HCT116 | |
| 1a | n.a. 3 | n.a. 3 | n.d. 4 | n.d. 4 |
| 2a | 96.02 ± 0.06 | 67.43 ± 1.82 | 59.13 ± 1.46 | 59.35 ± 1.66 |
| 2b | 15.86 ± 1.91 | 19.23 ± 5.94 | n.d. | n.d. |
| 2c | 53.85 ± 1.40 | 50.77 ± 1.83 | 25.25 ± 1.12 | 26.29 ± 1.07 |
| 2d | 27.39 ± 4.16 | 26.52 ± 3.77 | n.d. 4 | n.d. 4 |
| 2e | 11.41 ± 3.52 | 7.16 ± 2.11 | n.d. 4 | n.d. 4 |
| 2f | 5.64 ± 1.51 | 11.59 ± 4.03 | n.d. 4 | n.d. 4 |
| 3a | 54.96 ± 1.46 | 66.56 ± 2.58 | 54.55 ± 1.09 | 59.17 ± 1.48 |
| 3b | 52.60 ± 4.06 | 54.42 ± 8.48 | 38.37 ± 1.05 | 26.92 ± 1.31 |
| 3c | 56.85 ± 3.18 | 65.46 ± 1.07 | 22.80 ± 1.06 | 20.79 ± 1.07 |
| 3d | 87.47 ± 1.54 | 92.27 ± 0.04 | 54.10 ± 1.05 | 55.02 ± 1.04 |
| 3e | 12.14 ± 3.40 | 5.54 ± 3.65 | n.d. 4 | n.d. 4 |
| 3f | 15.36 ± 1.15 | 14.22 ± 2.81 | n.d. 4 | n.d. 4 |
| Cisplatin | 62.14 ± 5.05 | 78.20 ± 0.96 | 31.67 ± 1.13 | 19.18 ± 1.06 |
| Condition | Reaction Time (h) | SEM Size (nm) | Particle Size (nm) [PDI] | Zeta Potential (mV) | Drug Loading (%) | |
|---|---|---|---|---|---|---|
| A | 2 | 134 ± 18.87 | 369.30 ± 13.44 [0.464] | −8.36 | 9.6 1 | 13.5 2 |
| B | 5 | >1000 | n.d. 3 | n.d. 3 | 17.8 1 | n.d. 3 |
| C | 8 | 148 ± 23.69 | 300.30 ± 12.42 [0.322] | −7.71 | 16.3 1 | 15.6 2 |
 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalermnon, M.; Cherdchom, S.; Sereemaspun, A.; Rojanathanes, R.; Khotavivattana, T. Biguanide-Based Synthesis of 1,3,5-Triazine Derivatives with Anticancer Activity and 1,3,5-Triazine Incorporated Calcium Citrate Nanoparticles. Molecules 2021, 26, 1028. https://doi.org/10.3390/molecules26041028
Chalermnon M, Cherdchom S, Sereemaspun A, Rojanathanes R, Khotavivattana T. Biguanide-Based Synthesis of 1,3,5-Triazine Derivatives with Anticancer Activity and 1,3,5-Triazine Incorporated Calcium Citrate Nanoparticles. Molecules. 2021; 26(4):1028. https://doi.org/10.3390/molecules26041028
Chicago/Turabian StyleChalermnon, Monnaya, Sarocha Cherdchom, Amornpun Sereemaspun, Rojrit Rojanathanes, and Tanatorn Khotavivattana. 2021. "Biguanide-Based Synthesis of 1,3,5-Triazine Derivatives with Anticancer Activity and 1,3,5-Triazine Incorporated Calcium Citrate Nanoparticles" Molecules 26, no. 4: 1028. https://doi.org/10.3390/molecules26041028
APA StyleChalermnon, M., Cherdchom, S., Sereemaspun, A., Rojanathanes, R., & Khotavivattana, T. (2021). Biguanide-Based Synthesis of 1,3,5-Triazine Derivatives with Anticancer Activity and 1,3,5-Triazine Incorporated Calcium Citrate Nanoparticles. Molecules, 26(4), 1028. https://doi.org/10.3390/molecules26041028






