Post Synthetic Modification of Planar Antiaromatic Hexaphyrin (1.0.1.0.1.0) by Regio-Selective, Sequential SNAr
Abstract
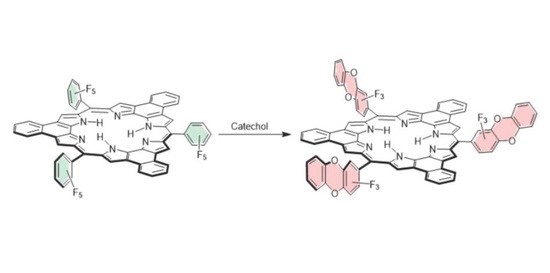
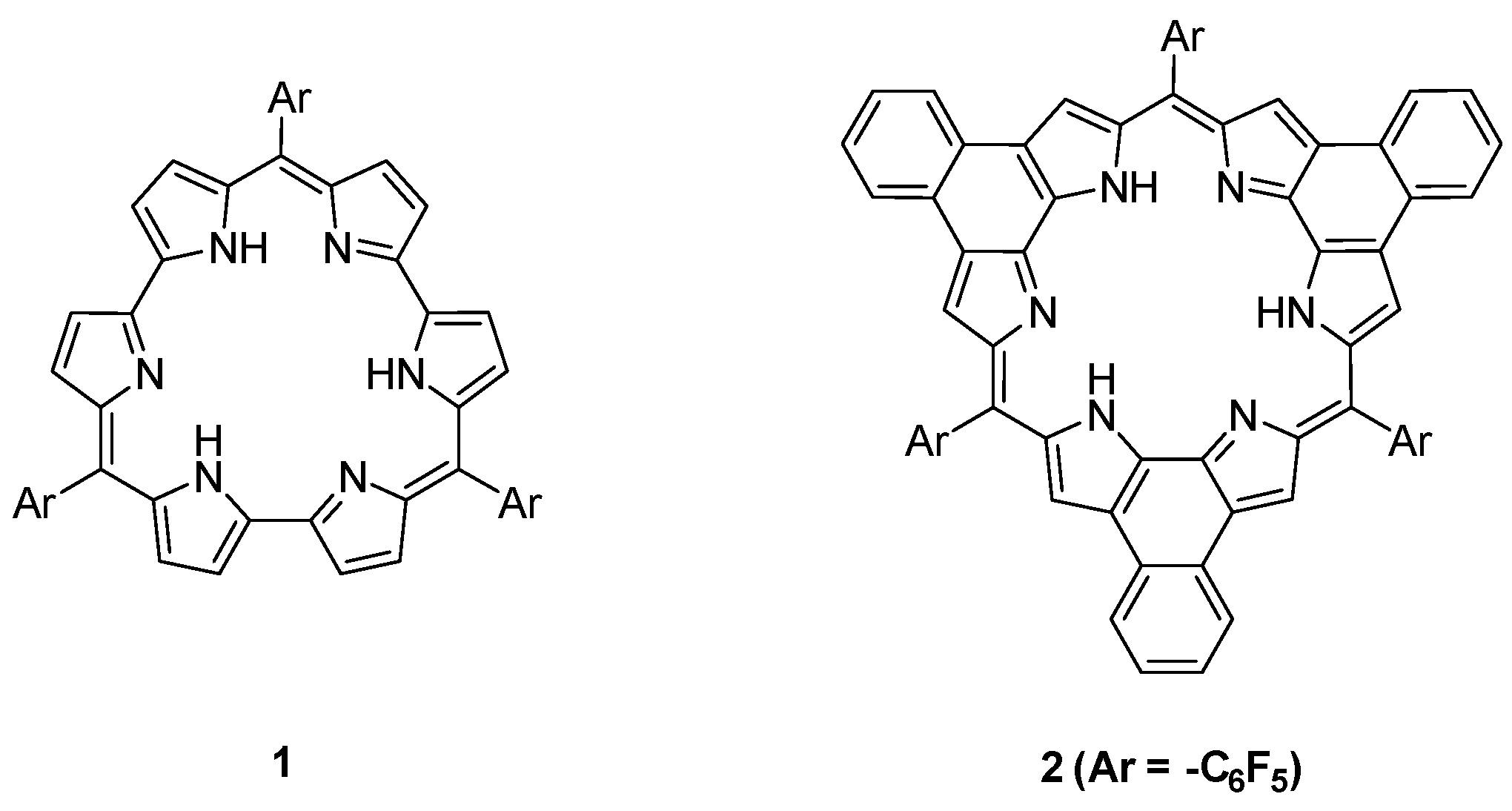
:1. Introduction
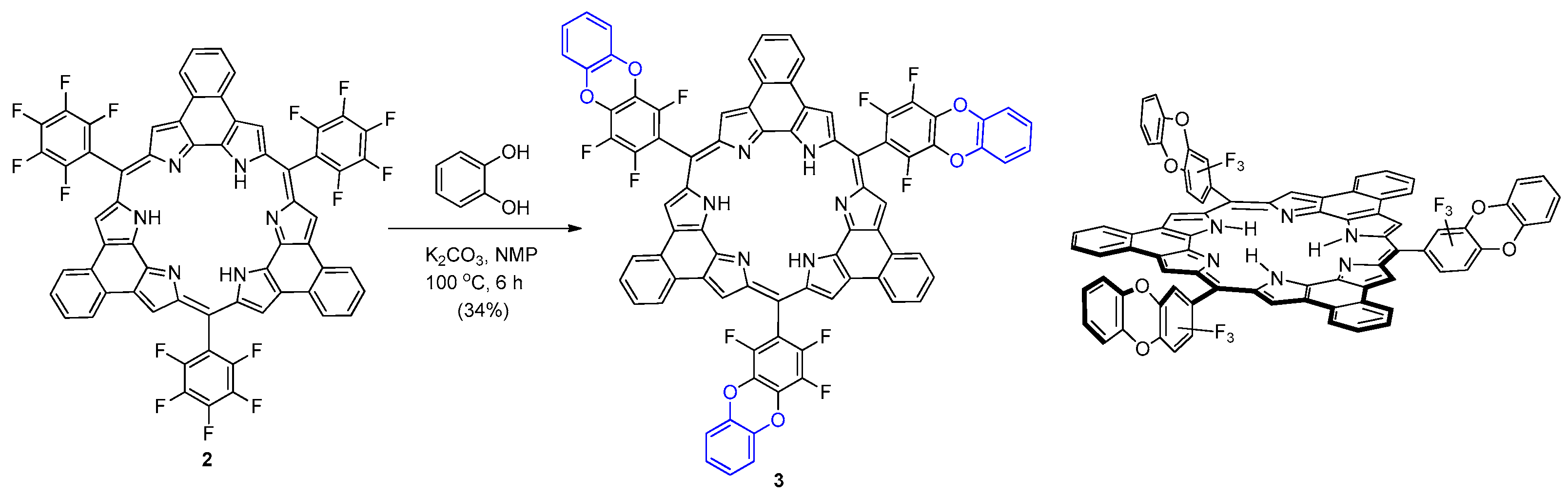
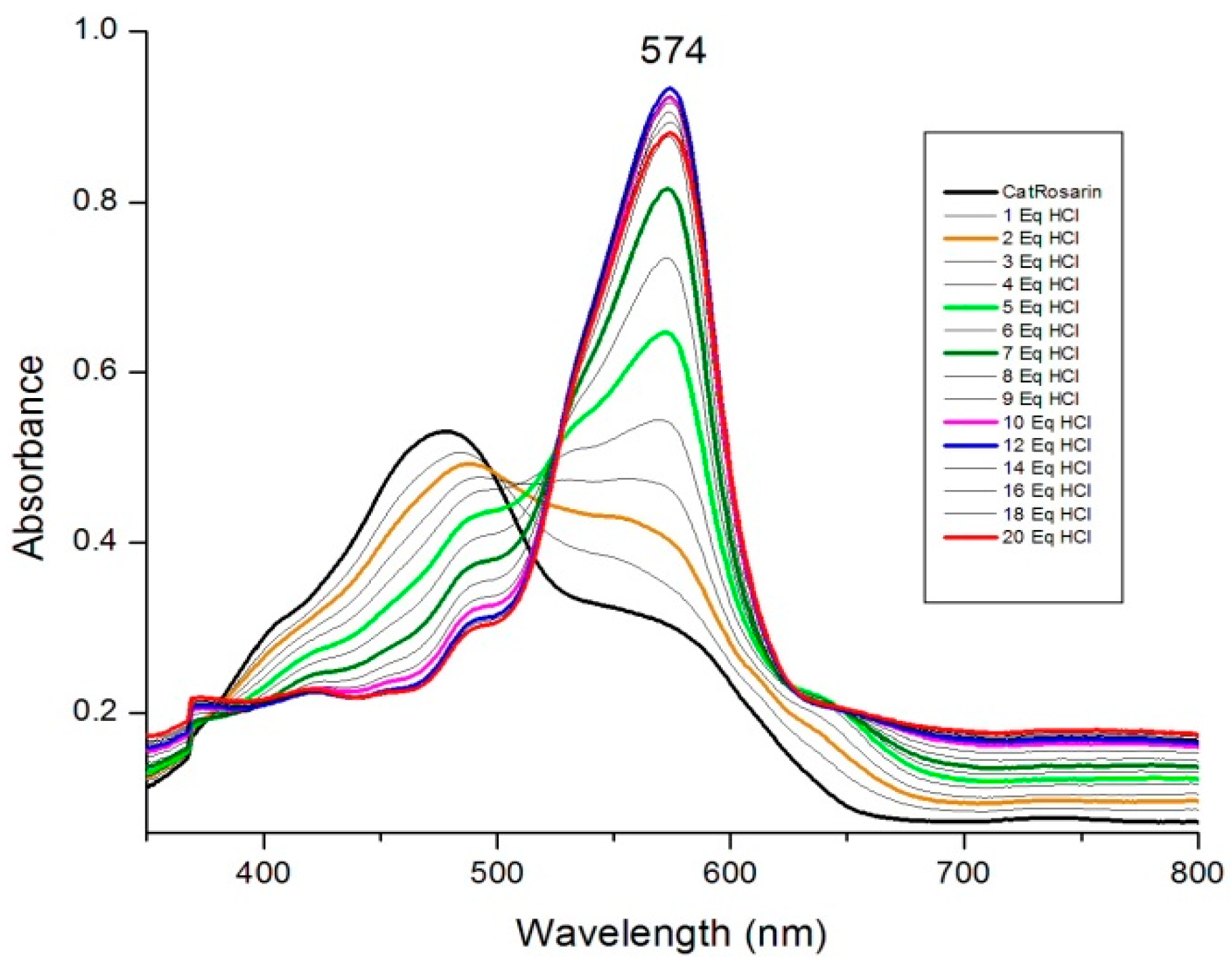
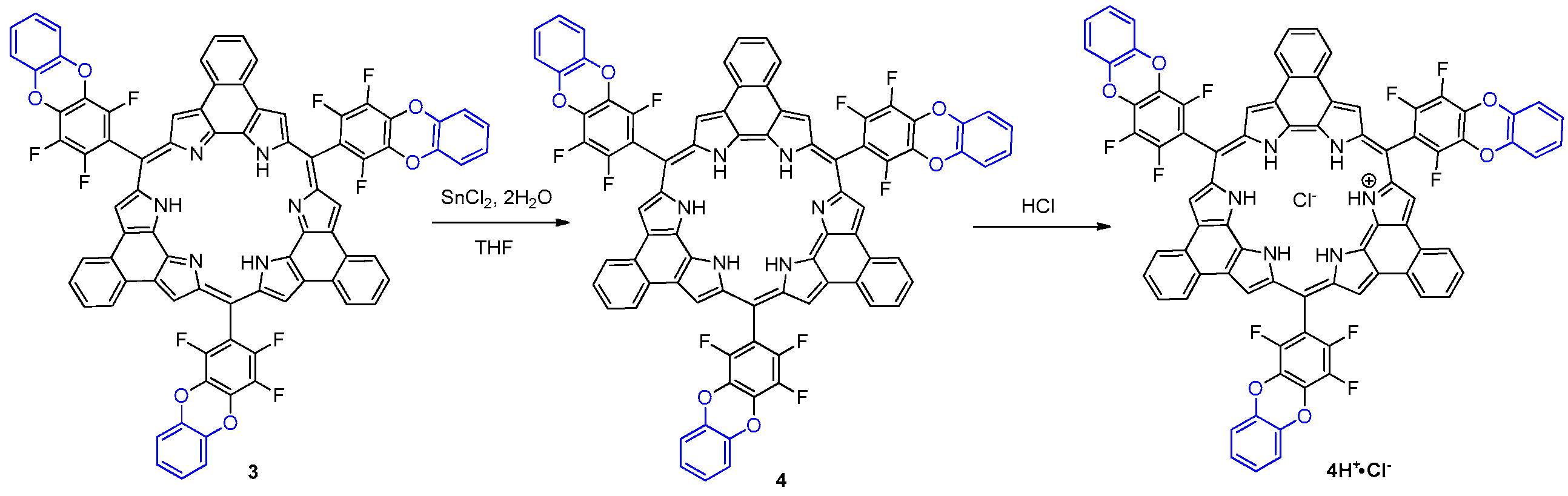
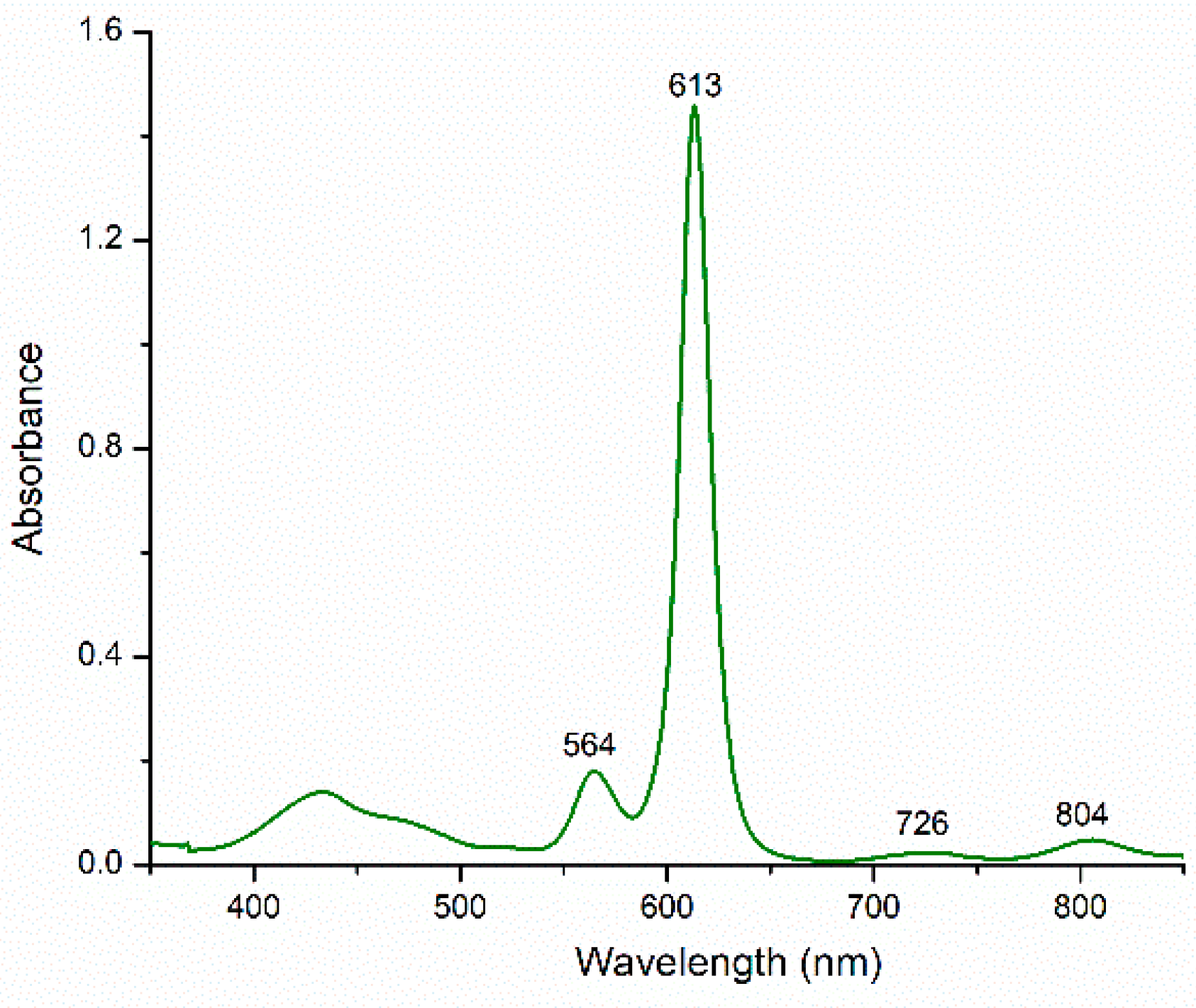
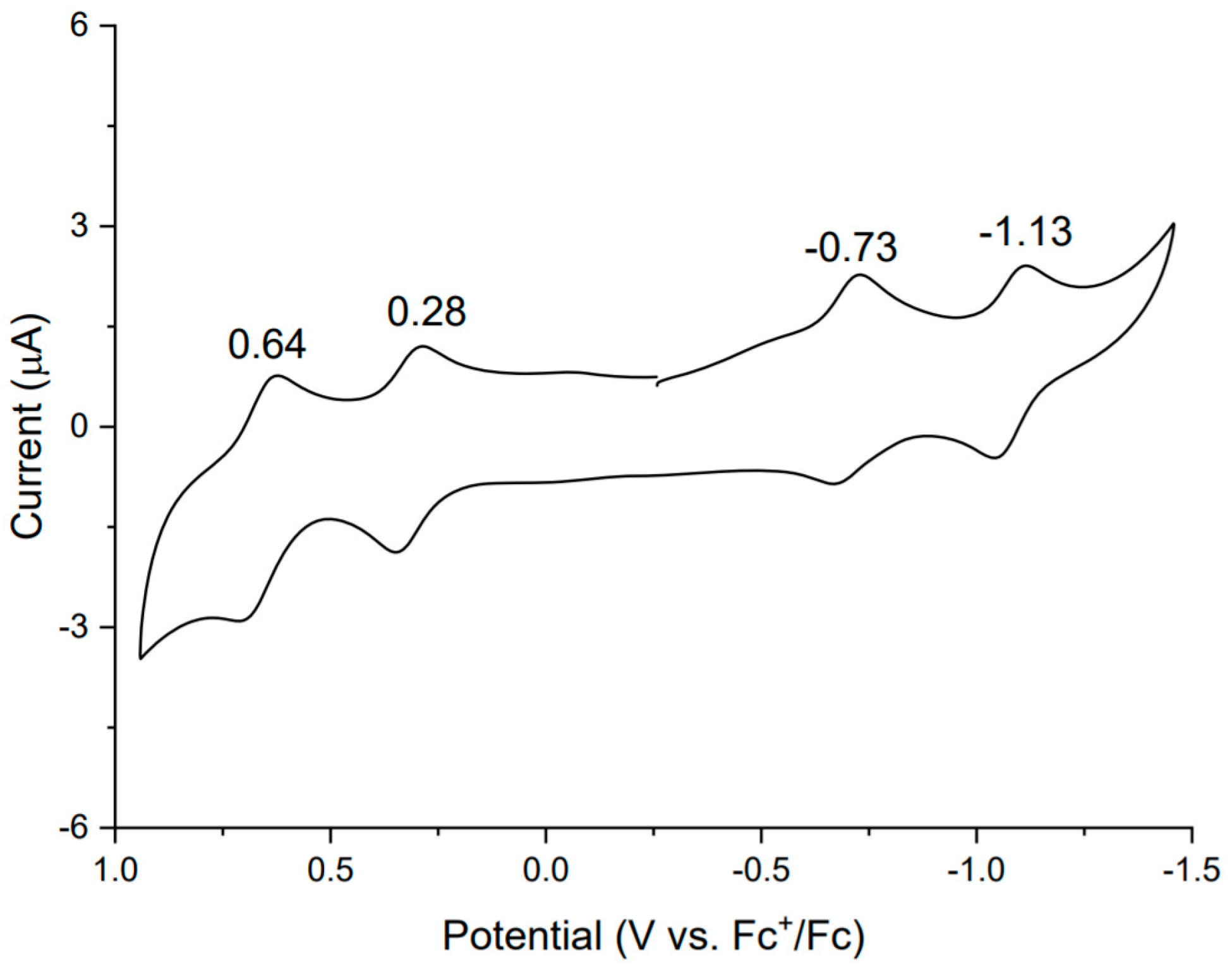
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of Rosarrin 3
3.2. Reduction of Rosarrin 3 to 4H+•Cl−
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tanaka, T.; Osuka, A. Chemistry of meso-Aryl substituted expanded porphyrins: Aromaticity and molecular twist. Chem. Rev. 2017, 117, 2584–2640. [Google Scholar] [CrossRef] [PubMed]
- Setsune, J.-I. 2,2′-bipyrrole-based porphyrinoids. Chem. Rev. 2017, 117, 3044–3101. [Google Scholar] [CrossRef] [PubMed]
- Szyszko, B.; Bialek, M.J.; Pacholska-Dudziak, E.; Latos-Grazynski, L. Flexible porphyrinods. Chem. Rev. 2017, 117, 2839–2909. [Google Scholar] [CrossRef] [PubMed]
- Sessler, J.L.; Seidel, D. Synthetic expanded porphyrin chemistry. Angew. Chem., Int. Ed. 2003, 42, 5134–5175. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Z.S.; Osuka, A.; Kim, D. Möbius aromaticity and antiaromaticity in expanded porphyrins. Nat. Chem. 2009, 1, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, T.; Kim, T.; Soya, T.; Neya, S.; Oh, J.; Kim, D.; Osuka, A. Conformational fixation of a rectangular antiaromatic [28]Hexaphyrin using rationally installed peripheral straps. Chem. Eur. J. 2016, 22, 4413–4417. [Google Scholar] [CrossRef] [PubMed]
- Sessler, J.L.; Weghorn, S.J.; Morishima, T.; Rosingana, M.; Lynch, V.; Lee, V. Rosarin: A new, easily prepared hexapyrrolic expanded porphyrin. J. Am. Chem. Soc. 1992, 114, 8306–8307. [Google Scholar] [CrossRef]
- Ishida, M.; Kim, S.-J.; Preihs, C.; Ohkubo, K.; Lim, J.M.; Lee, B.S.; Park, J.S.; Lynch, V.M.; Roznyatovskiy, V.; Sarma, T.; et al. Protonation-coupled redox reactions in planar antiaromatic meso-pentafluorophenyl-substituted o phenylene-bridged annulated rosarins. Nat. Chem. 2013, 5, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Samala, S.; Dutta, R.; He, Q.; Park, Y.; Chandra, B.; Lynch, M.V.; Jung, Y.M.; Sesslar, J.L.; Lee, C.-H. A robust bis-rhodium(I) complex of π-extended planar, anti-aromatic hexaphyrin[1.0.1.0.1.0]. Chem. Commun. 2020, 56, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Firmansyah, D.; Hong, S.-J.; Dutta, R.; He, Q.; Bae, J.; Jo, H.; Kim, H.; Ok, K.M.; Lynch, V.M.; Byon, H.R.; et al. Trapping of Stable [4n+1] π-Electron Species from Peripherally Substituted, Conformationally Rigid, Antiaromatic Hexaphyrins. Chem.-Eur. J. 2019, 25, 3525–3531. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Dutta, R.; Cha, Y.-V.; Hong, S.-J.; Oh, J.; Firmansyah, D.; Jo, H.; Ok, K.M.; Lee, C.-H.; Kim, D. Noncovalent Intermolecular Interaction in Cofacially Stacked 24π Antiaromatic Hexaphyrin Dimer. Chem.-Eur. J. 2020, 26, 16434–16440. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.I.T.; Tome, A.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. 5,10,15,20-tetrakis(pentafluorophenyl)porphyrin: A versatile platform to novel porphyrinic materials. J. Porphyr. Phthalocyanins 2011, 15, 1117–1133. [Google Scholar] [CrossRef]
- McKeown, N.B.; Hanif, S.; Msayib, K.; Tattershall, C.E.; Budd, P.M. Porphyrin-based nanoporous network polymers. Chem. Commun. 2002, 2782–2783. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.d.C.; Cunha, A.C.; de Souza, M.C.B.V.; Tome, A.C.; Neves, M.G.P.M.S.; Ferreira, V.F.; Cavaleiro, J.A.S. Synthesis of porphyrin–quinolone conjugates. Tetrahedron Lett. 2008, 49, 7268–7270. [Google Scholar] [CrossRef]
- Silva, A.M.G.; Tome, A.C.; Neves, M.G.P.M.S.; Silva, A.M.S.; Cavaleiro, J.A.S. meso-Tetraarylporphyrins as dipolarophiles in 1,3-dipolar cycloaddition reactions. Chem. Commun. 1999, 1767–1768. [Google Scholar] [CrossRef]
- Tome, J.P.C.; Neves, M.G.P.M.S.; Tome, A.C.; Cavaleiro, J.A.S.; Mendonc, A.F.; Pegado, I.N.; Duarte, R.; Valdeira, M.L. Synthesis of glycoporphyrin derivatives and their antiviral activity against herpes simplex virus types 1 and 2. Bioorg. Med. Chem. 2005, 13, 3878–3888. [Google Scholar] [CrossRef] [PubMed]
- Kvicala, J.; Benes, M.; Paleta, O.; Kral, V. Regiospecific nucleophilic substitution in 2,3,4,5,6-pentafluorobiphenyl as model compound for supramolecular systems. Theoretical study of transition states and energy profiles, evidence for tetrahedral SN2 mechanism. J. Fluor. Chem. 2010, 131, 1327–1337. [Google Scholar] [CrossRef]
- Gutsche, C.S.; Ortwerth, M.; Grafe, S.; Flanagan, K.J.; Senge, M.O.; Reissig, H.-U.; Kulak, N.; Wiehe, A. Nucleophilic Aromatic Substitution on Pentafluorophenyl-Substituted Dipyrranes and Tetrapyrroles as a Route to Multifunctionalized Chromophores for Potential Application in Photodynamic Therapy. Chem. Eur. J. 2016, 22, 13953–13964. [Google Scholar] [CrossRef] [PubMed]
- Fukuzumi, S.; Ohkubo, K.; Ishida, M.; Preihs, C.; Chen, B.; Borden, W.T.; Kim, D.; Sessler, J.L. Formation of Ground State Triplet Diradicals from Annulated Rosarin Derivatives by Triprotonation. J. Am. Chem. Soc. 2015, 137, 9780–9783. [Google Scholar] [CrossRef] [PubMed]
- Roznyatovskiy, V.; Roznyatovskaya, N.V.; Weyrauch, H.; Pinkwart, K.; Tubke, J.; Sessler, J.L. Naphthobipyrrole: Versatile synthesis and electropolymerization. J. Org. Chem. 2010, 75, 8355–8362. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutta, R.; Chandra, B.; Hong, S.-J.; Park, Y.; Jung, Y.M.; Lee, C.-H. Post Synthetic Modification of Planar Antiaromatic Hexaphyrin (1.0.1.0.1.0) by Regio-Selective, Sequential SNAr. Molecules 2021, 26, 1025. https://doi.org/10.3390/molecules26041025
Dutta R, Chandra B, Hong S-J, Park Y, Jung YM, Lee C-H. Post Synthetic Modification of Planar Antiaromatic Hexaphyrin (1.0.1.0.1.0) by Regio-Selective, Sequential SNAr. Molecules. 2021; 26(4):1025. https://doi.org/10.3390/molecules26041025
Chicago/Turabian StyleDutta, Ranjan, Brijesh Chandra, Seong-Jin Hong, Yeonju Park, Young Mee Jung, and Chang-Hee Lee. 2021. "Post Synthetic Modification of Planar Antiaromatic Hexaphyrin (1.0.1.0.1.0) by Regio-Selective, Sequential SNAr" Molecules 26, no. 4: 1025. https://doi.org/10.3390/molecules26041025
APA StyleDutta, R., Chandra, B., Hong, S.-J., Park, Y., Jung, Y. M., & Lee, C.-H. (2021). Post Synthetic Modification of Planar Antiaromatic Hexaphyrin (1.0.1.0.1.0) by Regio-Selective, Sequential SNAr. Molecules, 26(4), 1025. https://doi.org/10.3390/molecules26041025