Synthesis of Nitroaromatic Compounds via Three-Component Ring Transformations
Abstract
:1. Introduction
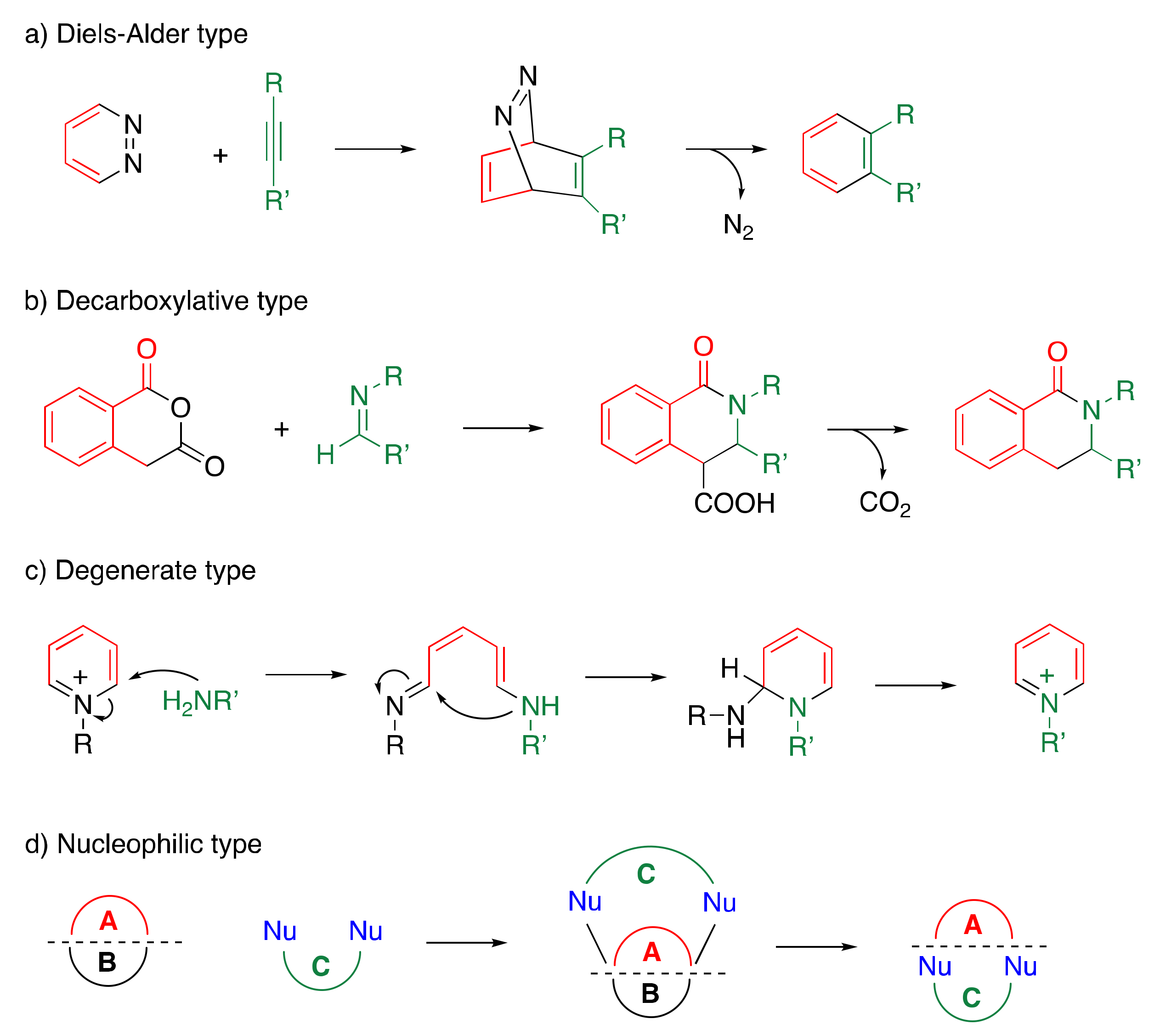
1.1. Ring Transformation
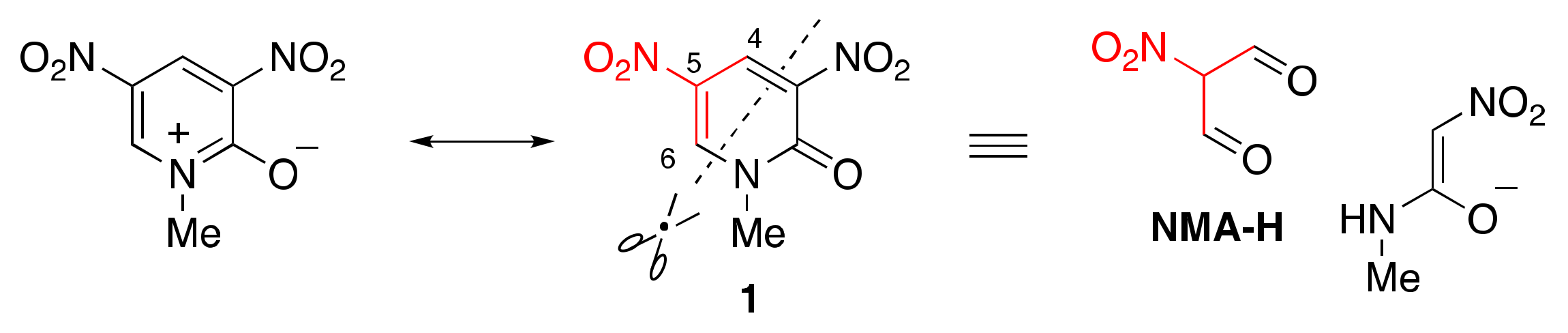
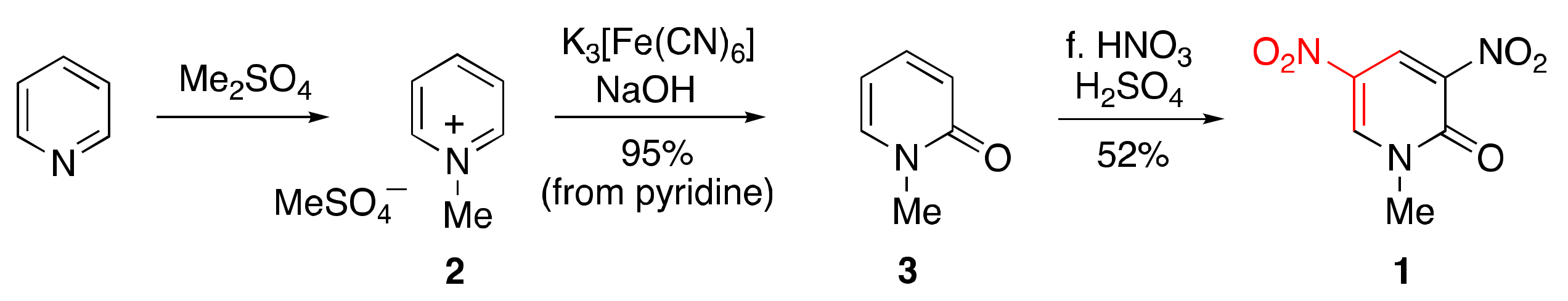
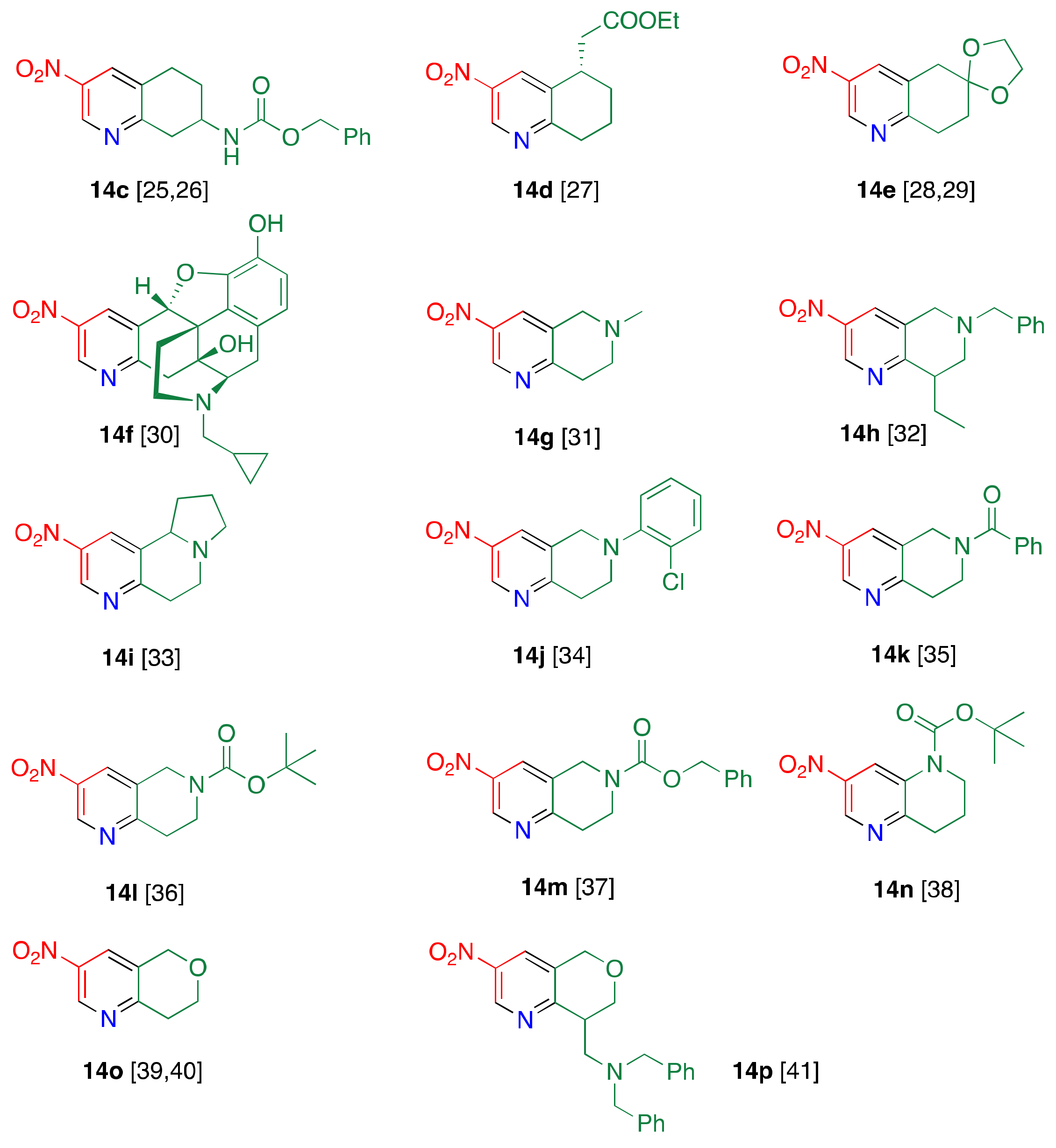
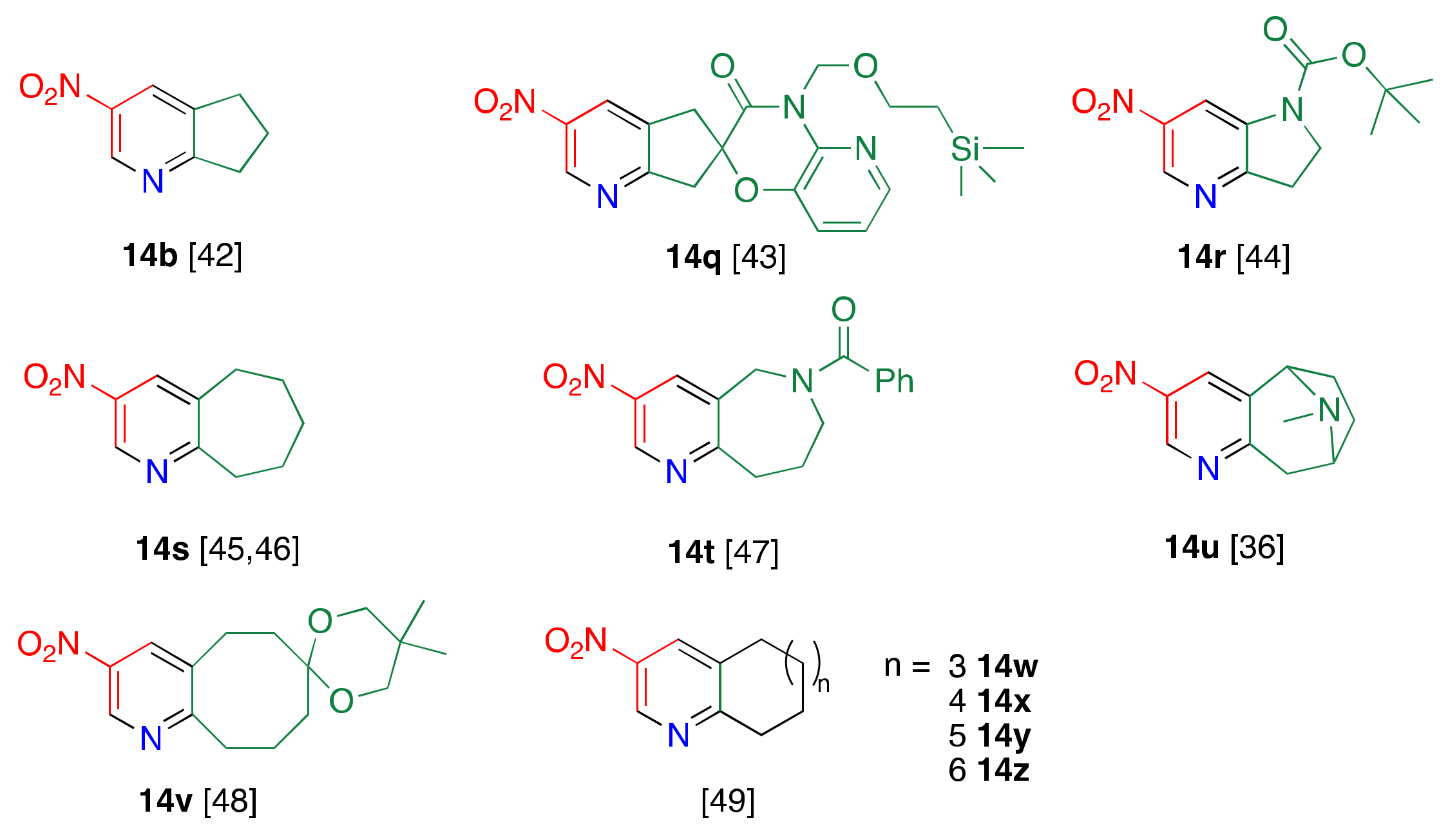
1.2. Suitable Substrate for Nucleophilic-Type Ring Transformation
2. Ring Transformation of 1 with Carbon Dinucleophiles
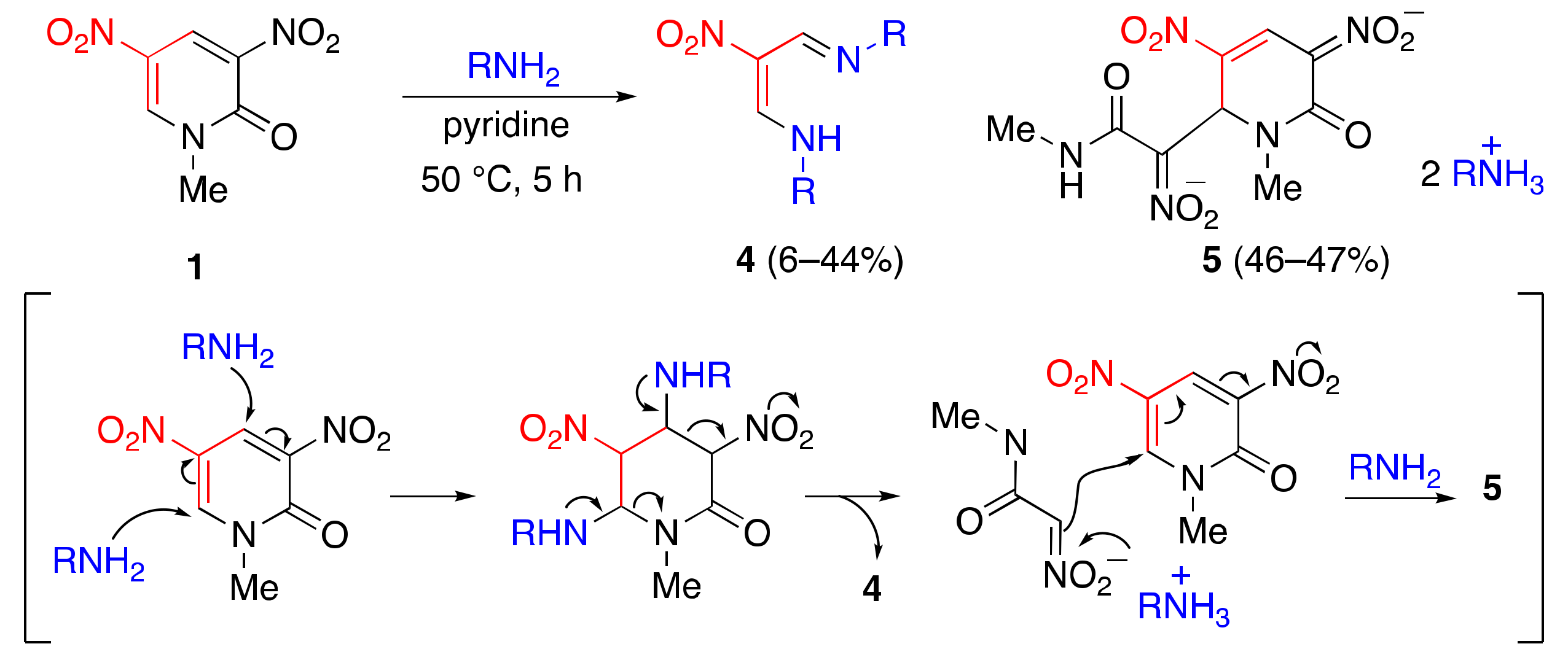
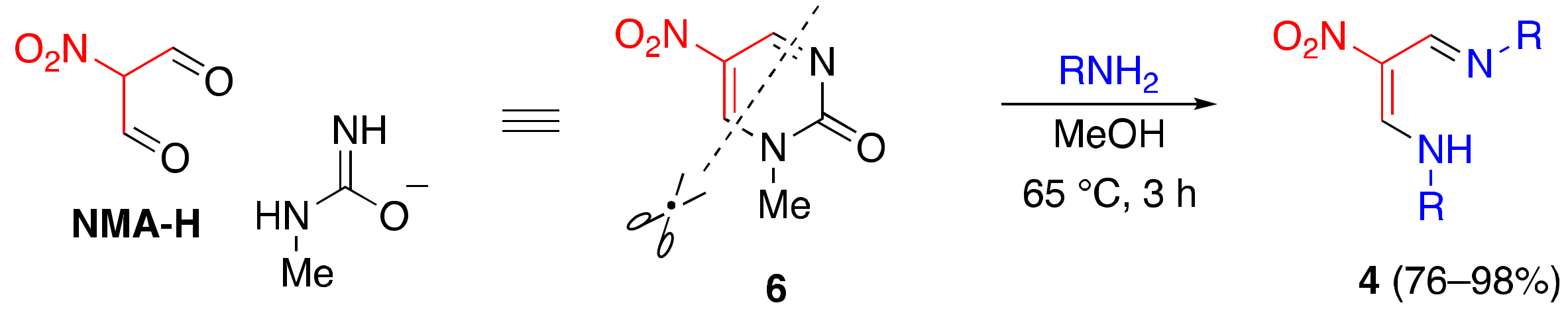
2.1. Aminolysis of Dinitropyridone 1
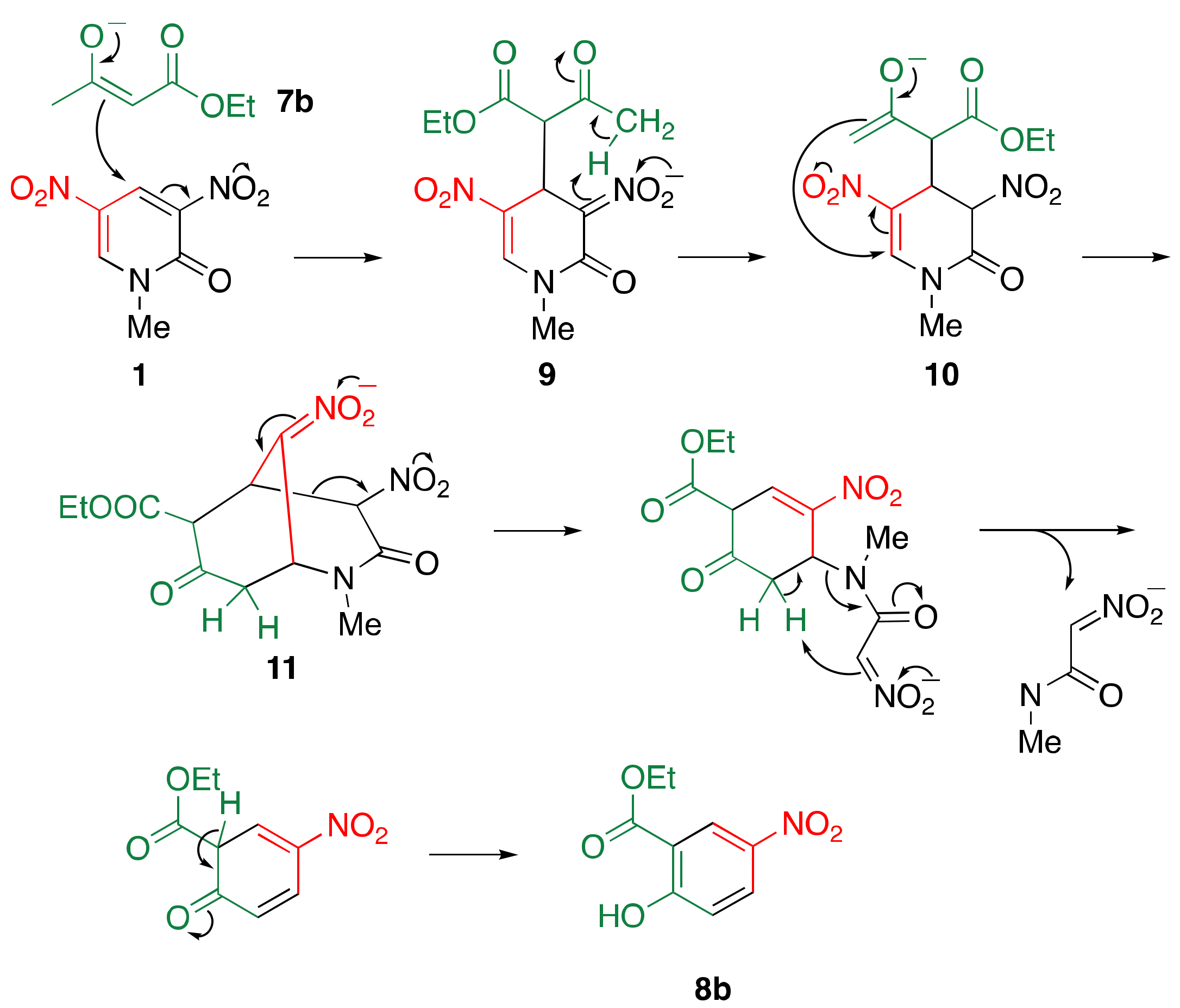
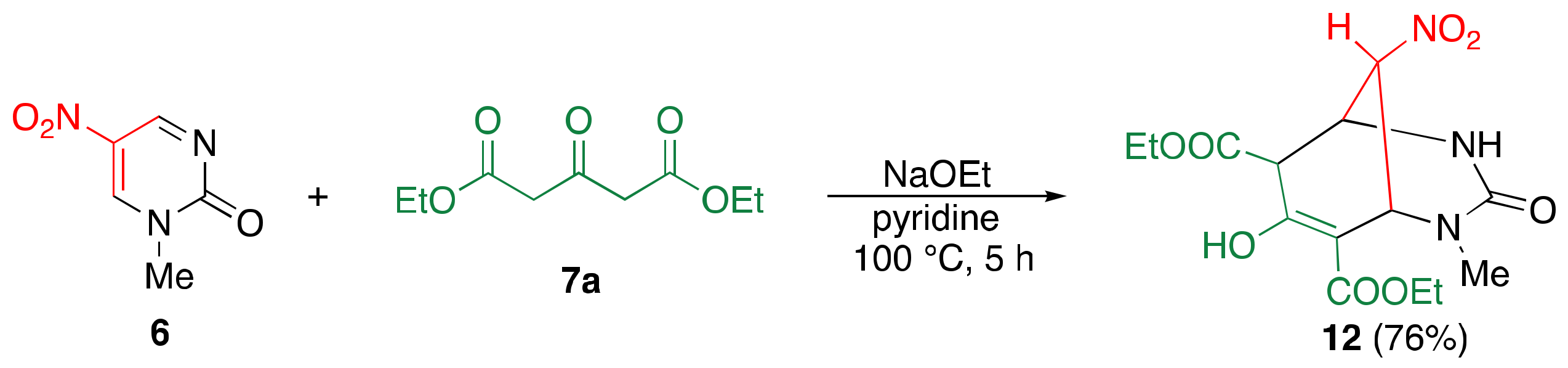
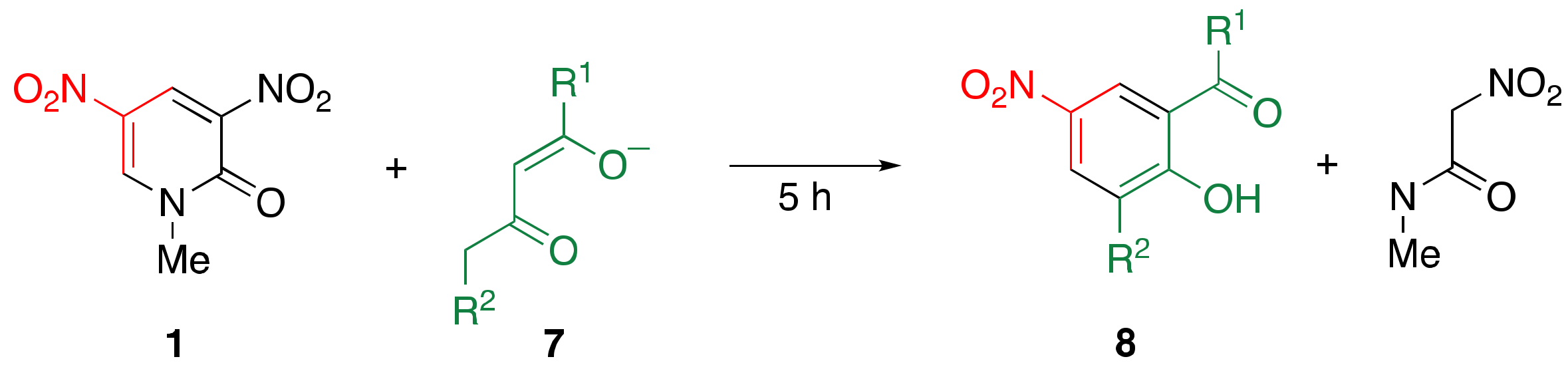
2.2. Reaction of Dinitropyridone 1 with 1,3-dicarbonyl Compounds
3. Three-Component Ring Transformation (TCRT)
3.1. General Concept of TCRT
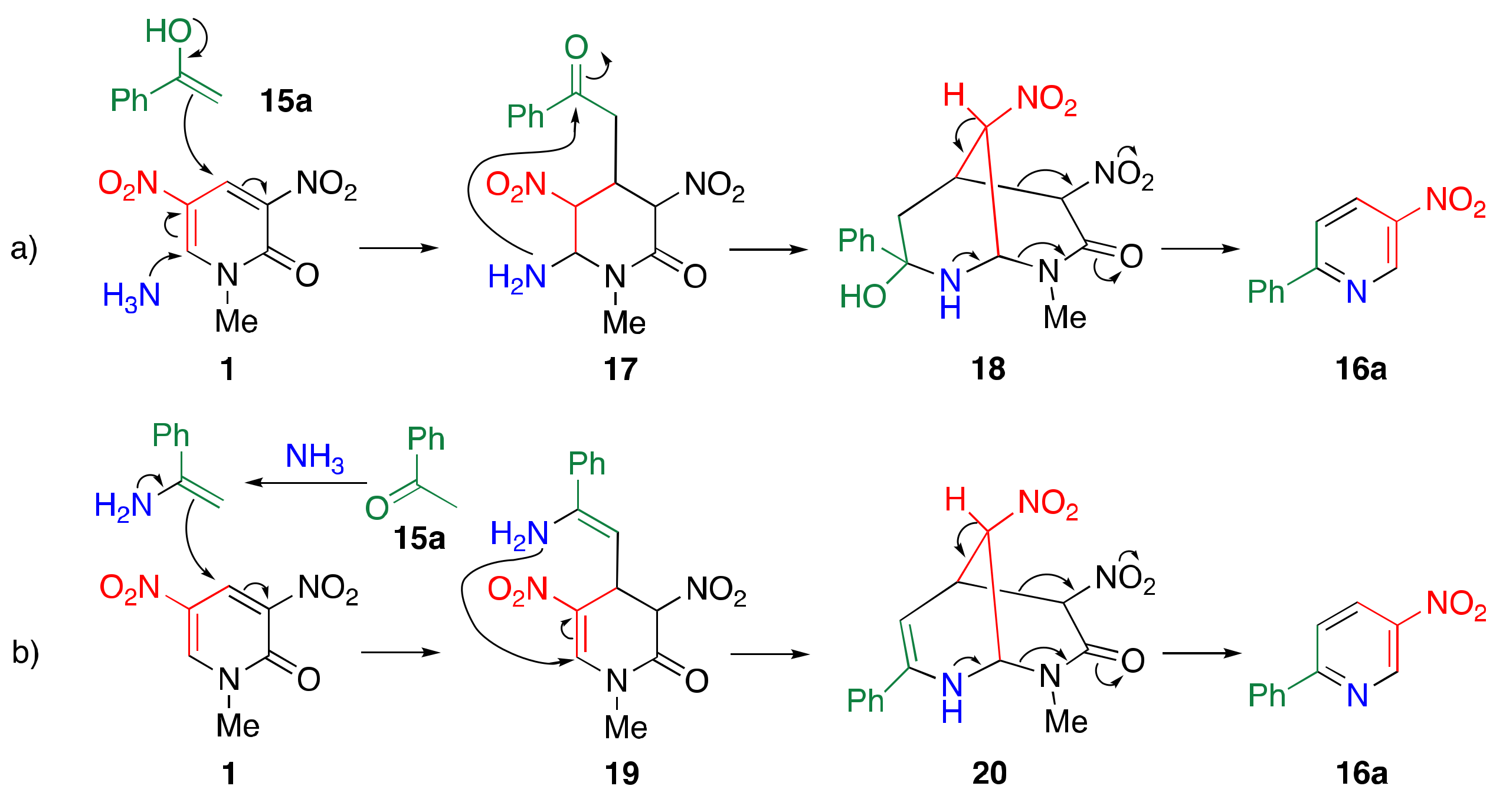
3.2. TCRT Using Ammonia as the Nitrogen Source
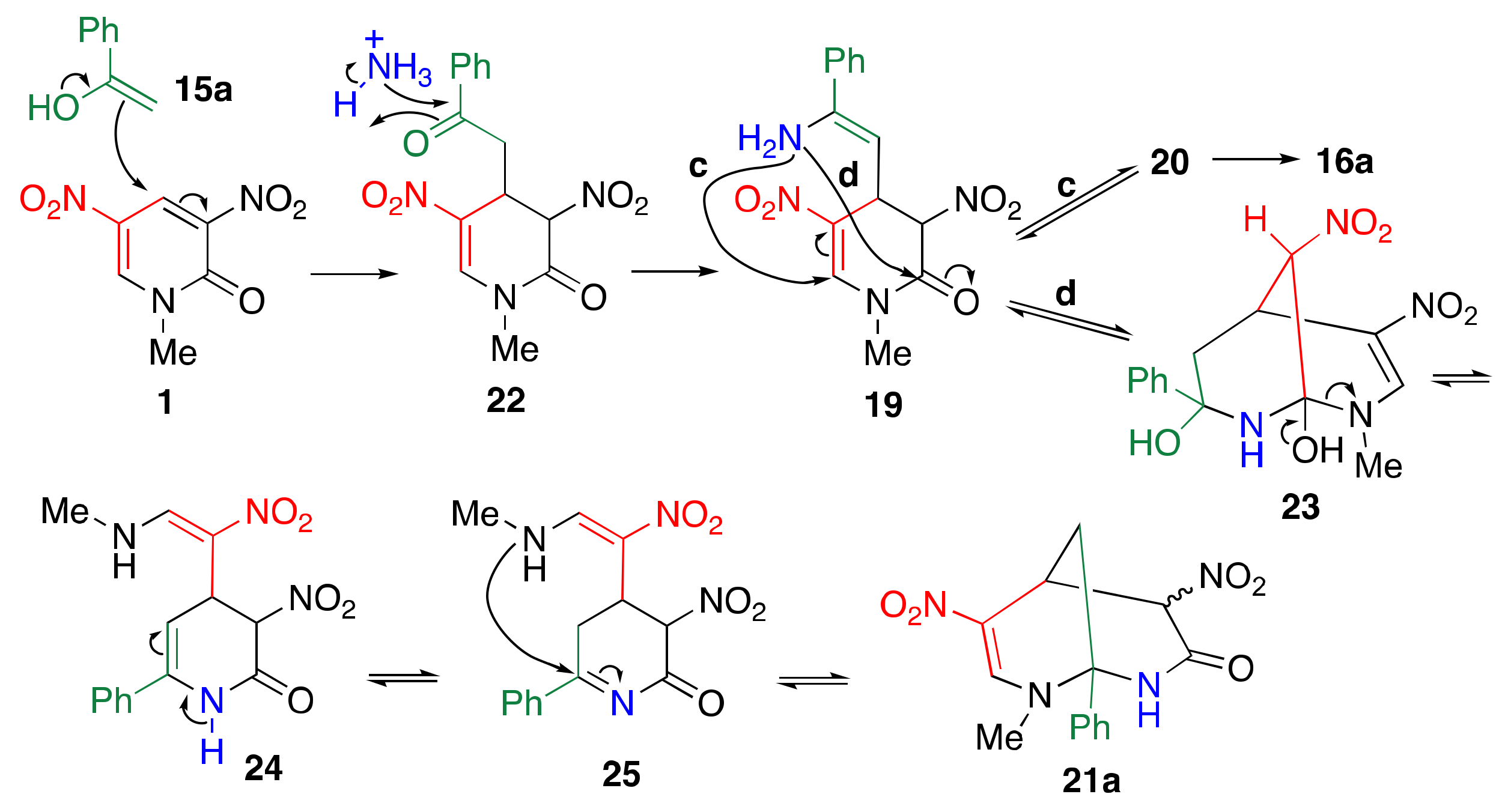
3.3. Reaction Mechanism of TCRT
3.4. TCRT Using Ammonium Acetate as the Nitrogen Source
3.5. Preparation of 3-substituted 5-nitropyridines 31 by TCRT
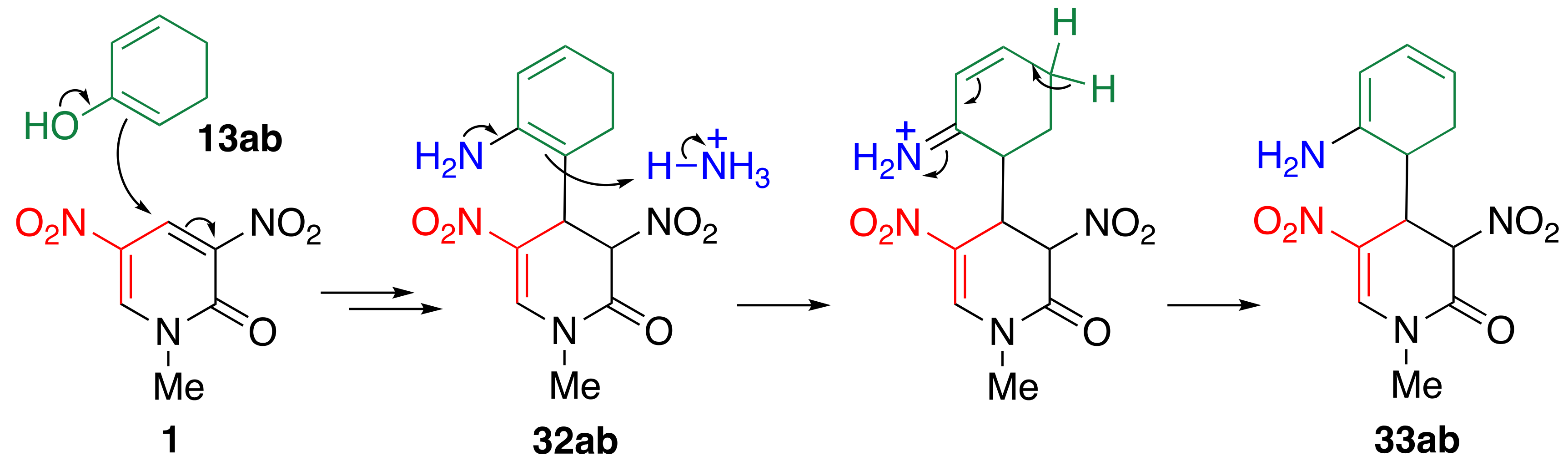
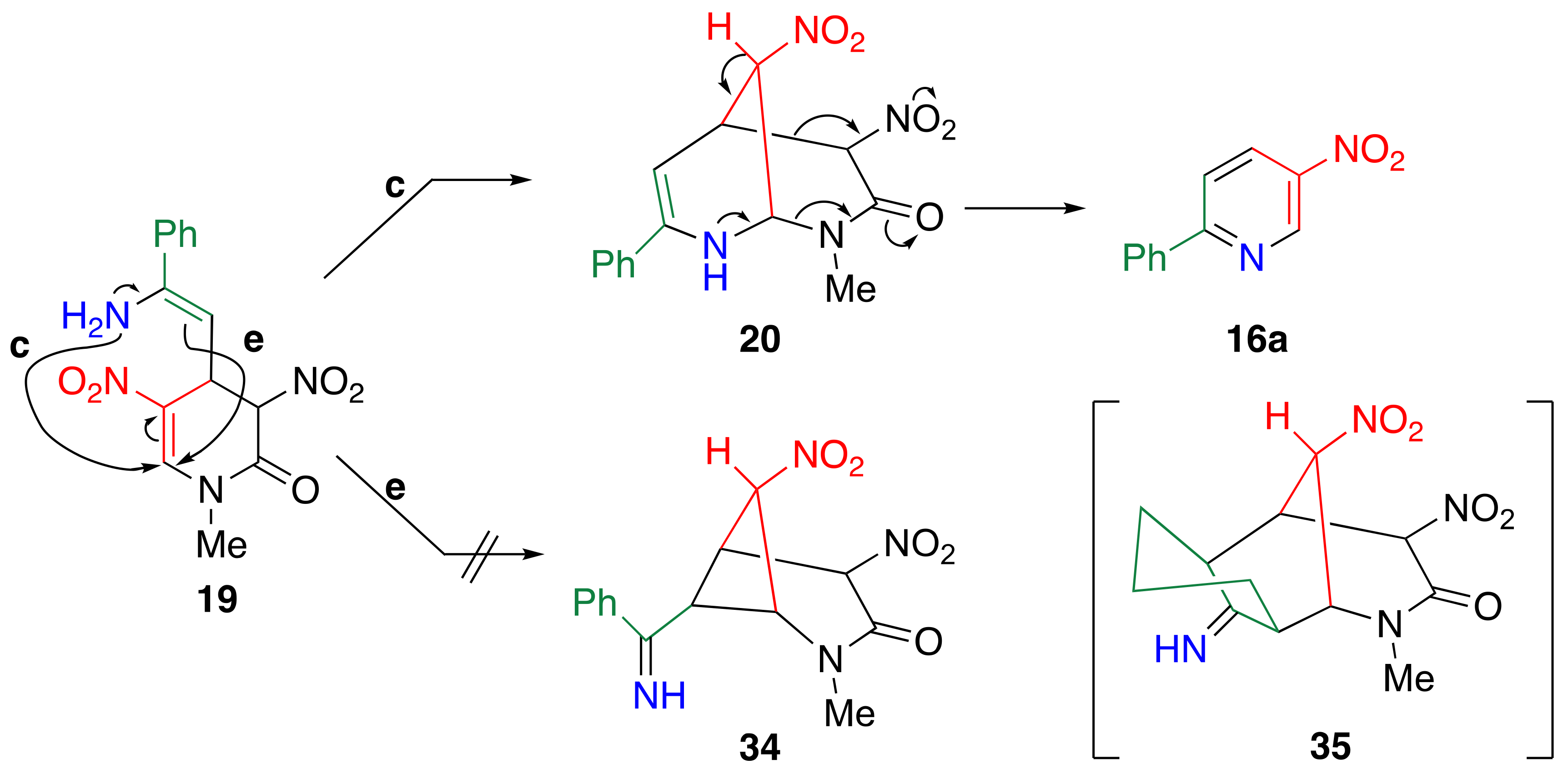
3.6. TCRT Using Cyclic Ketones 13
3.7. Reconsideration about the Reaction Mechanism of TCRT
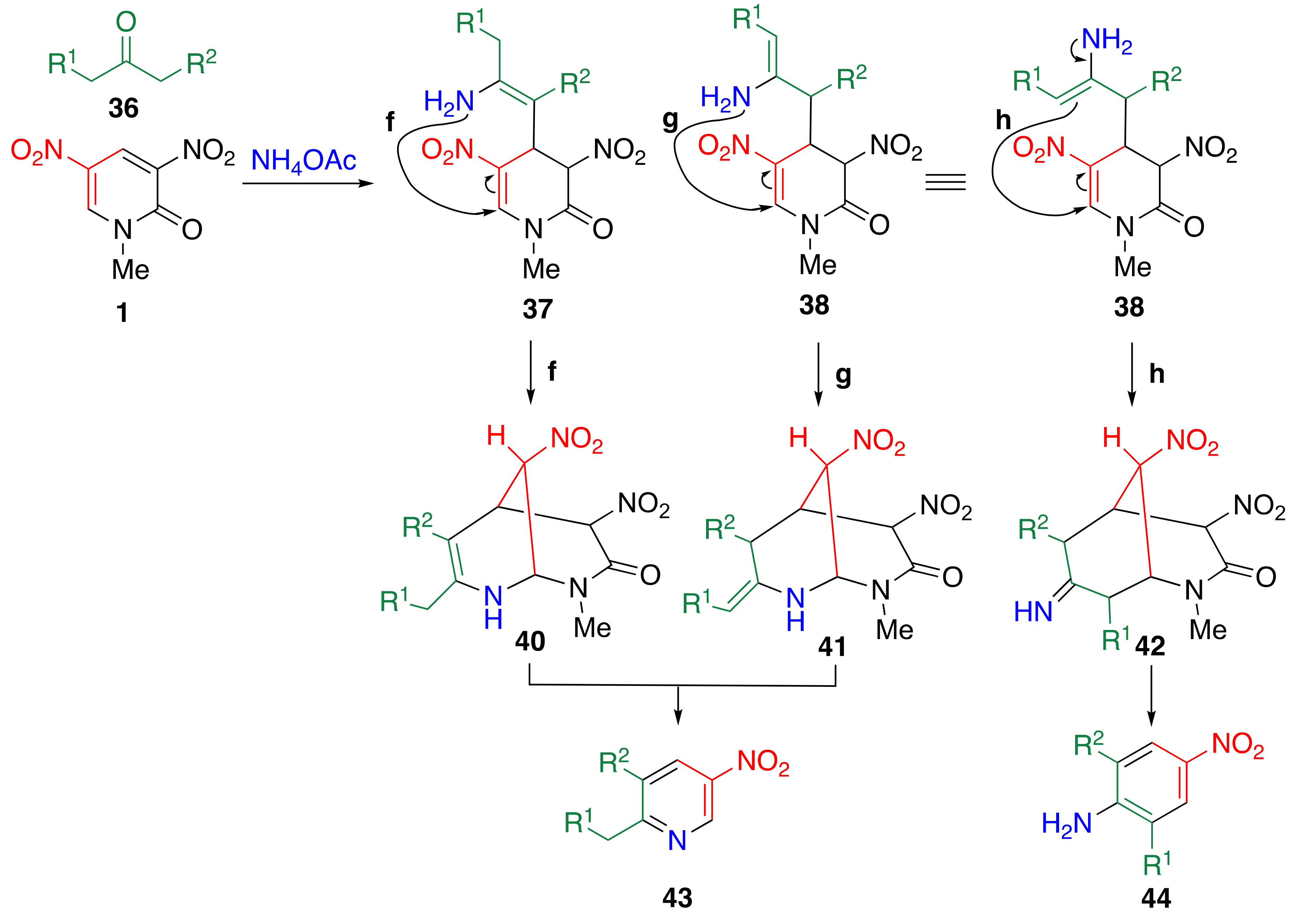
3.8. TCRT Using Aliphatic Ketones 36
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xue, J.; Gao, E.; Wang, X.-N.; Chang, J. Metal-Free Formal Inverse-Electron-Demand Diels–Alder Reaction of 1,2-Diazines with Ynamides. Org. Lett. 2018, 20, 6055–6058. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.K.; Medellin, D.C.; Kornienko, A. C-H Functionalization Directed by Transformable Nitrogen Heterocycles: Synthesis of ortho-Oxygenated Arylnaphthalenes from Arylphthalazines. Org. Biomol. Chem. 2014, 12, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Huntley, R.J.; Gurram, M.; Walker, J.R.; Jenkins, D.M.; Robe, E.J.; Ahmed, F. Synthesis of Isoindolinones via Inverse-Electron Demand Diels–Alder Cycloadditions. Tetrahedron Lett. 2014, 55, 2286–2289. [Google Scholar] [CrossRef]
- Firsov, A.; Bakulina, O.; Dar’in, D.; Guranova, N.; Krasavin, M. Further Insight into the Castagnoli−Cushman-type Synthesis of 1,4,6-Trisubstituted 1,6-Dihydropyridin-2-(3H)-ones from 3-Arylglutaconic Acid Anhydrides. J. Org. Chem. 2020, 85, 6822–6829. [Google Scholar] [CrossRef]
- Khalifa, M.M.; Philkhana, S.C.; Golden, J.E. Synthesis of Ring-Fused, N-Substituted 4-Quinolinones Using pKa-Guided, Base-Promoted Annulations with Isatoic Anhydrides: Total Synthesis of Penicinotam. J. Org. Chem. 2020, 85, 464–481. [Google Scholar] [CrossRef]
- Laws, S.W.; Moore, L.C.; Di Maso, M.J.; Nguyen, Q.N.N.; Tantillo, D.J.; Shaw, J.T. Diastereoselective Base-Catalyzed Formal [4 + 2] Cycloadditions of N-Sulfonyl Imines and Cyclic Anhydrides. Org. Lett. 2017, 19, 2466–2469. [Google Scholar] [CrossRef] [Green Version]
- Van der Plas, H.C. Degenerate Ring Transformations in Heterocyclic Systems. J. Heterocycl. Chem. 2000, 37, 427–438. [Google Scholar] [CrossRef]
- Glinkerman, C.M.; Boger, D.L. Synthesis, Characterization, and Rapid Cycloadditions of 5-Nitro-1,2,3-triazine. Org. Lett. 2018, 18, 2628–2631. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki, N.; Sugimoto, R.; Saigo, K.; Kobiro, K. Mechanistic Aspect of Ring Transformations in the Reaction of 5-Nitro-4-pyrimidinone with Acetophenone Derivatives and Cycloalkanones Depending on the Electron Density/Ring Size of the Ketone. Tetrahedron Lett. 2013, 54, 956–959. [Google Scholar] [CrossRef] [Green Version]
- Nishiwaki, N.; Yamashita, K.; Azuma, M.; Adachi, T.; Tamura, M.; Ariga, M. Novel Synthesis of Bihetaryl Compounds. Synthesis 2004, 1996–2000. [Google Scholar] [CrossRef]
- Nishiwaki, N.; Azuma, M.; Tamura, M.; Hori, K.; Tohda, Y.; Ariga, M. Facile Synthesis of Functionalized 4-Aminopyridines. Chem. Commun. 2002, 2170–2171. [Google Scholar] [CrossRef] [PubMed]
- Gromov, S.P. Ring Transformation of Pyridines and Benzo Derivatives under the Action of C-Nucleophiles. Heterocycles 1995, 40, 441–475. [Google Scholar] [CrossRef]
- Rusinov, V.L.; Chupakhin, O.N. Transformations of Nitropyrimidines by Action of C-Nucleophiles. Heterocycles 2000, 53, 1607–1630. [Google Scholar]
- Fanta, P.E.; Stein, R.A. The Chemistry of Sodium Nitromalonaldehyde. Chem. Rev. 1960, 60, 261–266. [Google Scholar] [CrossRef]
- Nishiwaki, N.; Hirao, S.; Sawayama, J.; Saigo, K. Practically Usable C3 Building Blocks for the Syntheses of Nitro Heterocycles. Heterocycles 2012, 84, 115–134. [Google Scholar] [CrossRef] [Green Version]
- Tohda, Y.; Ariga, M.; Kawashima, T.; Matsumura, E. The Nucleophilic Reaction upon Electron-Deficient Pyridone Derivatives. VIII. Novel Fragmentation of 3,5-Dinitro-2-pyridone by Primary Amine. Bull. Chem. Soc. Jpn. 1987, 60, 201–204. [Google Scholar] [CrossRef] [Green Version]
- Takaichi, J.; Ohkubo, K.; Sugimoto, H.; Nakano, M.; Usa, D.; Maekawa, H.; Fujieda, N.; Nishiwaki, N.; Seki, S.; Fukuzumi, S.; et al. Copper Complexes of the Non-Innocent β-Diketiminate Ligand Containing Phenol Groups. Dalton Trans. 2013, 42, 2438–2444. [Google Scholar] [CrossRef]
- Shimokawa, C.; Tachi, Y.; Nishiwaki, N.; Ariga, M.; Itoh, S. Structural Characterization of Copper(I) Complexes Supported by β-Diketiminate Ligands with Different Substitution Pattern. Bull. Chem. Soc. Jpn. 2006, 79, 118–125. [Google Scholar] [CrossRef]
- Shimokawa, C.; Yokota, S.; Tachi, Y.; Nishiwaki, N.; Ariga, M.; Itoh, S. Substituent Effects of β-Diketiminate Ligands on the Structure and Physicochemical Properties of Copper(II) Complexes. Inorg. Chem. 2003, 42, 8395–8405. [Google Scholar] [CrossRef]
- Nishiwaki, N.; Tohda, Y.; Ariga, M. Facile Synthesis of Functionalized Nitroenamines. III. Aminolysis of 1-Methyl-5-nitropyrimidin-2(1H)-one. Bull. Chem. Soc. Jpn. 1996, 69, 1997–2002. [Google Scholar] [CrossRef]
- Matsumura, E.; Ariga, M.; Tohda, Y. The Nucleophilic Reaction of Electron-Deficient Pyridone Derivatives. I. The Ring Transformation of 1-Substituted 3,5-Dinitro-2-pyridones with Sodio β-Keto Esters. Bull. Chem. Soc. Jpn. 1979, 52, 2413–2419. [Google Scholar] [CrossRef]
- Ariga, M.; Tohda, Y.; Matsumura, E. Ring Transformation of 1,4 (or 1,6)-Disubstituted 3,5-Dinitro-2-pyridones with Sodio β-Keto Esters. Bull. Chem. Soc. Jpn. 1985, 58, 393–394. [Google Scholar] [CrossRef]
- Nishiwaki, N.; Tohda, Y.; Ariga, M. Nitropyrimidinones; Synthetic Equivalents of Diformylamine and Nitromalonaldehyde. Synthesis 1997, 1277–1280. [Google Scholar] [CrossRef]
- Tohda, Y.; Eiraku, M.; Nakagawa, T.; Usami, Y.; Ariga, M.; Kawashima, T.; Tani, K.; Watanabe, H.; Mori, Y. The Nucleophilic Reaction upon Electron-Deficient Pyridone Derivatives. X. One-Pot Synthesis of 3-Nitropyridines by Ring Transformation of 1-Methyl-3,5-dinitro-2-pyridone with Ketones or Aldehydes in the Presence of Ammonia. Bull. Chem. Soc. Jpn. 1990, 63, 2820–2827. [Google Scholar] [CrossRef] [Green Version]
- Drescher, K.; Haupt, A.; Unger, L.; Turner, S.C.; Braje, W.; Grandel, R.; Henry, C. Preparation of Aminotetrahydronaphtalenylbenzenesulfonamides and Related Compounds as Modulators of the Dopamine D3 Receptor. WO 2006040178, 20 April 2006. [Google Scholar]
- Yuan, J.; Han, N.; Yi, H.; Wang, Y.; Yang, S.; Wong, J.C. Preparation of Potent Small molecule inhibitors of Autophagy Useful in Treatment of Cancers and Acute Pancreatitis. WO 2014145512,, 18 September 2014. [Google Scholar]
- Ge, M.; Yang, L.; Zhou, C.; Lin, S.; Cline, E. Preparation of Fused Pyridines as Antidiabetics. WO 2006083612, 10 August 2006. [Google Scholar]
- Bauta, W.E.; Cantrell, W.R.; Tidwell, M.W. Preparation of Hydroxyimino Tetrahydroquinoline Compounds as Reactivators of Organophosphorous-Inhibited Acetylcholinesterase. US 20140350262, 27 November 2014. [Google Scholar]
- Kelly, M.G.; Kaub, C.J.; Kincaid, J.; Janagani, S.; Wu, G.; Wei, Z.-L.; Sahasrabudhe, K.; Duncton, M.; Upasani, R.B.; Fang, Y.; et al. Preparation of Amide Derivatives as Ion-Channel Ligands. WO 2007100758, 7 September 2007. [Google Scholar]
- Ananthan, S.; Kezar, H.S., III; Saini, S.K.; Khare, N.K.; Davis, P.; Dersch, C.M.; Porreca, F.; Rothman, R.B. Synthesis, Opioid Receptor Binding, and Functional Activity of 5’-Substituted 17-Cyclopropylmethylpyrido[2’,3’:6,7]morphinas. Bioorg. Med. Chem. Lett. 2003, 13, 529–532. [Google Scholar] [CrossRef]
- Coulton, S.; Harling, J.D.; Porter, R.A.; Thompson, M. Preparation of Substituted Isoquinolines as Anticonvulsants. WO 200008020, 17 February 2020. [Google Scholar]
- Aicher, T.D.; Skalitzky, D.J.; Toogood, P.L.; Vanhuis, C.A. Preparation of Dihydroisoquinoline-2(1H)-carboxamides and Related Compounds and Their Use in Treating Medical Conditions. WO 2019200120, 17 October 2019. [Google Scholar]
- Coulton, S.; Novelli, R.; Porter, R.; Thompson, M.; Ward, R.W. Preparation of Pyrido[2,1-a]isoquinolines and Pyrrolo[2,1-a]isoquinolines as Anticonsuvulsants. WO 200008020, 17 February 2020. [Google Scholar]
- Guiadeen, D.; Kothandaraman, S.; Yang, L.; Mills, S.G.; MacCoss, M. An Expeditious Synthesis of 3-(Difluoromethoxy)- and 3-(Trifluoromethoxy)-5,6,7,8-tetrahydro-1,6-naphthyridines. Tetrahedron Lett. 2008, 49, 6368–6370. [Google Scholar] [CrossRef]
- DeMartino, J.; Akiyama, T.; Struthers, M.; Yang, L.; Berger, J.P.; Morriello, G.; Pastemak, A.; Zhou, C.; Mills, S.G.; Kothandaraman, S.; et al. Preparation of Tetrahydropyranylaminocyclopentylcarbonyltetrahydropyridopyridines as Modulators of CCR2 Chemokine Receptor Activity. WO 20060030582, 9 February 2006. [Google Scholar]
- Harling, J.D.; Harrington, F.P.; Thompson, M. A Facile Synthesis of the 3-Amino-5,6,7,8-tetrahydro[1,6]naphthyridine System and Some Alkylated and Polycyclic Homologues. Synth. Commun. 2001, 31, 787–797. [Google Scholar] [CrossRef]
- Berger, R.; Blizzard, T.A.; Campbell, B.T.; Chen, H.Y.; Debenham, J.S.; Dewnani, S.V.; Dubois, B.; Guo, Z.; Harper, B. Preparation of Isoquinoline Derivatives as MGAT2 Inhibitors. WO 2015112465, 30 July 2015. [Google Scholar]
- Aicher, T.D.; van Huis, C.A.; Thomas, W.D.; Maclean, J.K.; Andersen, B.M.; Barr, K.J.; Bienstock, C.E.; Anthony, N.J.; Daniels, M.; Liu, K.; et al. Preparation of Tetrahydronaphthyridines, Benzoxazines, Azabenzoxazines, and Related Bicyclic Compounds for Inhibition of RORgamma Activity and the Treatment of Disease. WO 2015095795, 25 June 2015. [Google Scholar]
- Stansfield, I.; Querolle, O.A.G.; Ligny, Y.A.E.; Gross, G.M.; Jacoby, E.; Meerpoel, L.; Green, S.R.; Hynd, G.; Kulagowski, J.J.; Macleod, C.; et al. Cyanoindoline Derivatives as NIK Inhibitors and Their Preparation. WO 2018002219, 4 January 2018. [Google Scholar]
- Takada, S.; Sasatani, T.; Chomei, N.; Adachi, M.; Fujishita, T.; Eigyo, M.; Murata, S.; Kawasaki, K.; Matsushita, A. Synthesis and Structure–Activity Relationship of Fused Imidazopyridines: A New Series of Benzodiazepine Receptor Ligands. J. Med. Chem. 1996, 39, 2844–2851. [Google Scholar] [CrossRef]
- Barfoot, C.; Davies, D.T.; Miles, T.; Pearson, N.D. Bicyclic Nitrogen-Containing Compounds as Mycobacterium Tuberculosis H37Rv Inhibitors and Their Preparation, Pharmaceutical Compositions and Use in the Treatment of Bacterial Infections. WO 2008128961, 30 October 2008. [Google Scholar]
- Frank, R.; Christoph, T.; Lesch, B.; Lee, J. Preparation of Substituted Bicyclic Aromatic Carboxamide and Urea Derivatives as Vanilloid Receptor Ligands. WO 2013013816, 31 January 2013. [Google Scholar]
- Bell, I.M.; Fraley, M.; Biftu, T.; Zhu, C.; Nair, A. Heterocyclic CGRP Receptor Antagonists for Migraine Therapy. WO 2013169565, 14 November 2013. [Google Scholar]
- Henry, C.; Haupt, A.; Turner, S.C. Microwave-Assisted Synthesis of Novel (5-Nitropyridin-2-yl)alkyl and (5-Nitropyridin-3-yl)alkyl Carbamates. J. Org. Chem. 2009, 74, 1932–1938. [Google Scholar] [CrossRef]
- Vara Prasad, J.V.N.; Boyer, F.E.; Chupak, L.; Dermyer, M.; Ding, Q.; Gavardinas, K.; Hagen, S.E.; Huband, M.D.; Jiao, W.; Kaneko, T.; et al. Synthesis and Structure–Acitivity Studies of Novel Benzocycloheptanone Oxazolidinone Antibacterial Agents. Bioorg. Med. Chem. Lett. 2006, 16, 5392–5397. [Google Scholar] [CrossRef] [PubMed]
- Chupak, L.S.; Kaneko, T.; Josyula, V.P.V.N.; Kim, J.-Y.; Choy, A.L.; Hagen, S.E.; Boyer, F.E., Jr. Preparation of Tricyclyl-Substituted Oxazolidinones and Related Compounds as Antibacterial Agents. WO 2004069832, 19 August 2004. [Google Scholar]
- Schultz, T.; Turner, S.C.; Braje, W.M. Microwave-Assisted Synthesis of Nitro-Substituted Tetrahydropyridoazepines. Synthesis 2010, 1339–1343. [Google Scholar]
- Goff, D.; Zhang, J.; Singh, R.; Holland, S.; Yu, J.; Heckrodt, T.; Ding, P.; Litvak, J. Preparation of Polycyclic Aryl and Heteroaryl Substituted Triazoles as AXL Receptor Tyrosine Kinase Inhibitors. WO 2009054864, 30 April 2009. [Google Scholar]
- Sagitullina, G.P.; Garkushenko, A.K.; Vinokurova, Y.O.; Nyrkova, V.A.; Atavin, E.G.; Sagitullin, R.S. Nitropyridines: VI. Synthesis of 2-aryl(hetaryl)- and 2,3-polymethylene-5-nitropyridines. Russ. J. Org. Chem. 2009, 45, 1045–1049. [Google Scholar]
- Kato, K.; Terauchi, J.; Mori, M.; Suzuki, N.; Shimomura, Y.; Takekawa, S.; Ishihara, Y. Preparation of N-Tetrahydronaphthalenyl Carboxamides as Melamin Concentrating Hormone Antagonists. WO 0121577, 29 March 2001. [Google Scholar]
- Le, T.S.; Asahara, H.; Kobiro, K.; Sugimoto, R.; Saigo, K.; Nishiwaki, N. Synthesis of 2-Aryl-5-nitropyridines by Three-Component Ring Transformation of 3,5-Dinitro-2-pyridone. Asian J. Org. Chem. 2014, 3, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Le, T.S.; Asahara, H.; Nishiwaki, N. Metal-Free Synthesis of 2-Alkenyl/Alkynyl-5-nitropyridines Using Three-Component Ring Transformation. Chem. Lett. 2015, 44, 776–778. [Google Scholar] [CrossRef]
- Furuyama, H.; Kurihara, H.; Terao, T.; Nakagawa, D.; Tanabe, S.; Kato, T.; Yamamoto, M.; Sekine, S.; Mashiko, T.; Inuki, S.; et al. Preparation of Nitrogen-Containing Heterocyclic Compounds as PI3K and ERK Inhibitors. WO 2014109414, 17 July 2014. [Google Scholar]
- Le, T.S.; Asahara, H.; Nishiwaki, N. An Alternative Synthetic Approach to 3-Alkylated/Arylated 5-Nitropyridines. J. Org. Chem. 2015, 80, 8856–8858. [Google Scholar] [CrossRef]
- Le, T.S.; Asahara, H.; Nishiwaki, N. An Efficient Synthesis of Nitrated Cycloalka[b]pyridines. Synthesis. 2014, 46, 2175–2178. [Google Scholar]
- Le, T.S.; Asahara, H.; Nishiwaki, N. Tailor-Made Synthesis of N,N,2,6-Tetrasubstituted 4-Nitroanilines by Three-Component Ring Transformation of Dinitropyridone. Eur. J. Org. Chem. 2015, 2015, 1203–1206. [Google Scholar] [CrossRef] [Green Version]
- Carver, F.J.; Hunter, C.A.; Livingstone, D.J.; McCabe, J.F.; Seward, E.M. Substituent Effects on Edge-to-Face Aromatic Interactions. Chem. Eur. J. 2002, 8, 2847–2859. [Google Scholar] [CrossRef]
- Naito, S.; Yokoyama, S.; Asahara, H.; Nishiwaki, N. Synthesis of Functionalized 4-Nitroanilines by Ring Transformation of Dinitropyridone with Enaminones. Tetrahedron Lett. 2017, 58, 4699–4702. [Google Scholar] [CrossRef]

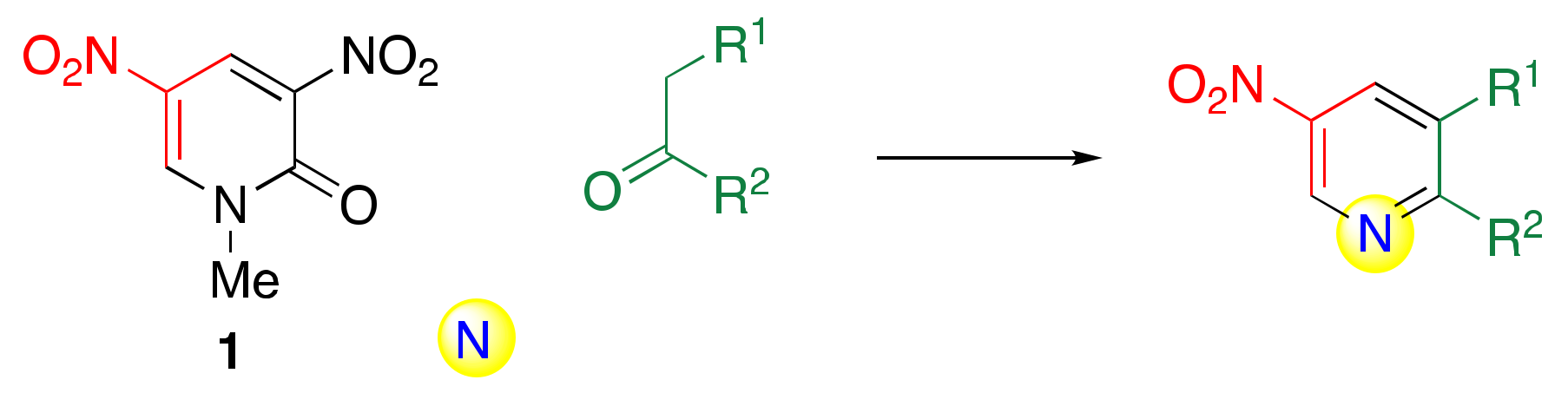
| R1 | R2 | Solv. | Temp./°C | Yield/% | |
|---|---|---|---|---|---|
| OEt | COOEt | a | pyridine | 50 | 91 |
| OEt | H | b | pyridine | 70 | 61 |
| Me | H | c | DMF | 70 | 53 |
| COOEt | H | d | pyridine | 110 | 42 |
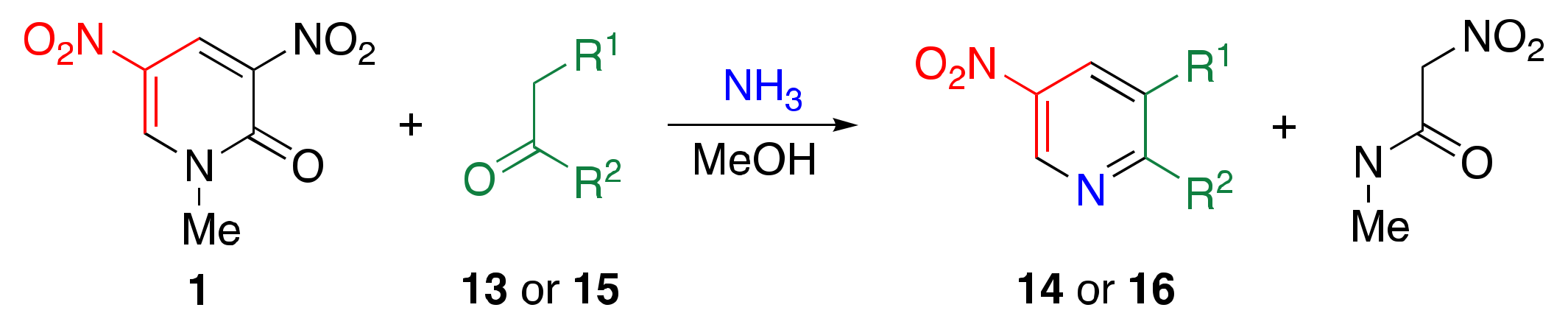
| Ketone | Condition 1 | Product | |||
|---|---|---|---|---|---|
| R1 | R2 | Yield/% | |||
| –(CH2)4– | 13a | A | 14a | 83 | |
| –(CH2)3– | 13b | A | 14b | 27 | |
| H | Ph | 15a | A | 16a | 44 |
| H | Ph | 15a | B | 16a | 81 |
| H | 4-NH2C6H4 | 15b | B | 16b | 44 |
| H | 4-MeOC6H4 | 15c | B | 16c | 64 |
| H | 4-MeC6H4 | 15d | B | 16d | 30 |
| H | 4-NO2C6H4 | 15e | B | 16e | 27 |
| H | 2-pyridyl | 15f | B | 16f | 72 |
| H | 2-furyl | 15g | B | 16g | 62 |
| H | 2-thienyl | 15h | B | 16h | 56 |
| Me | Ph | 15i | A | 16i | 10 |
| Me | Ph | 15i | B | 16i | 37 |
| H | i-Pr | 15j | A | 16j | 36 |
| H | i-Pr | 15j | B | 16j | 21 |
| H | tert-Bu | 15k | B | 16k | 69 |

| NH4OAc /equiv. | Time/h | Yield/% | Ratio of 16a/21a | Ratio of exo-21a/endo-21a | |
|---|---|---|---|---|---|
| 16a | 21 | ||||
| 3 | 24 | 19 | 61 | 24/76 | 56/44 |
| 5 | 24 | 43 | 46 | 48/52 | 59/41 |
| 10 | 24 | 64 | 25 | 72/28 | 70/30 |
| 15 | 24 | 79 | 0 | 100/0 | — |
| 5 1 | 7 | 92 | 5 | 95/5 | 60/40 |
| 15 1 | 5 | 90 | 0 | 100/0 | — |

| Ketone | NH4OAc/ equiv. | Yield/% | ||||
|---|---|---|---|---|---|---|
| Ar | R | 16 | 21 | 16 + 21 | ||
| Ph | H | a | 15 | 79 | 0 | 79 |
| 4-MeOC6H4 | H | c | 5 1,2 | 95 | 0 | 95 |
| 3-MeOC6H4 | H | l | 10 | 97 | 0 | 97 |
| 2-MeOC6H4 | H | m | 5 | 94 | 0 | 94 |
| 4-MeC6H4 | H | d | 5 | 88 | 0 | 88 |
| 4-ClC6H4 | H | n | 10 | 96 | 0 | 96 |
| 4-NO2C6H4 | H | e | 15 | 93 | 2 | 95 |
| 4-pyridyl | H | o | 15 | 66 | 33 | 99 |
| 3-pyridyl | H | p | 15 | 97 | 0 | 97 |
| 2-pyridyl | H | f | 15 | 80 | 12 | 92 |
| 2-furyl | H | g | 5 | 87 | 0 | 87 |
| 2-thienyl | H | h | 10 | 85 | 0 | 95 |
| Ph | Me | i | 15 1,3 | 98 | 0 | 98 |
| Ph | Pr | q | 15 1,3 | 97 | 0 | 97 |
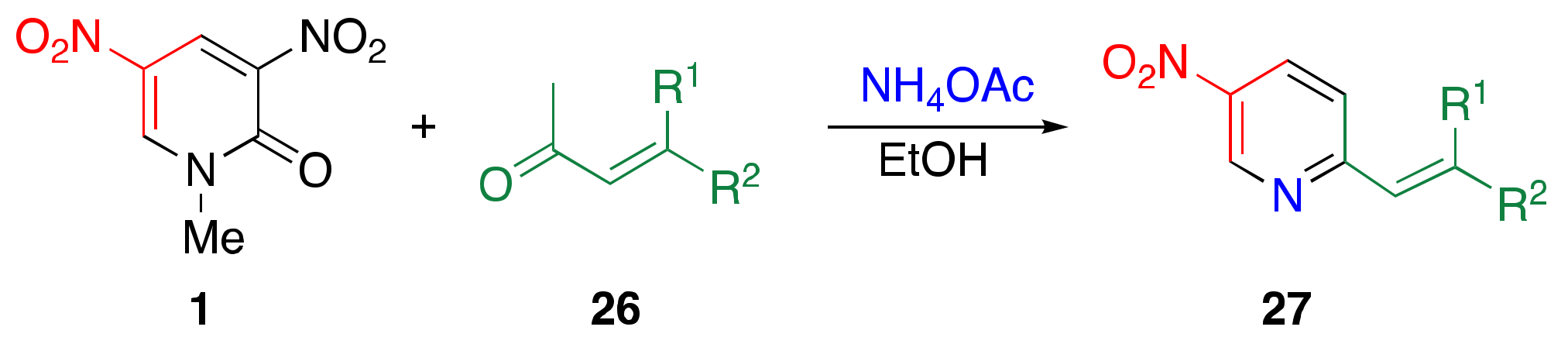
| Ketone | NH4OAc/ equiv. | Temp./°C | Time/h | Yield/% | ||
|---|---|---|---|---|---|---|
| R1 | R2 | |||||
| H | Ph | a | 15 | 80 1 | 4 | 82 |
| H | 4-MeOC6H4 | b | 30 | 65 | 24 | 94 |
| H | 4-ClC6H4 | c | 30 | 80 1 | 4 | 75 |
| H | H | d | 30 | 65 | 24 | 0 |
| Me | Me | e | 15 | 80 1 | 2 | 25 |
| H | 2,4,6-trimethylcyclohexyl | f | 30 | 80 1 | 6 | 79 |
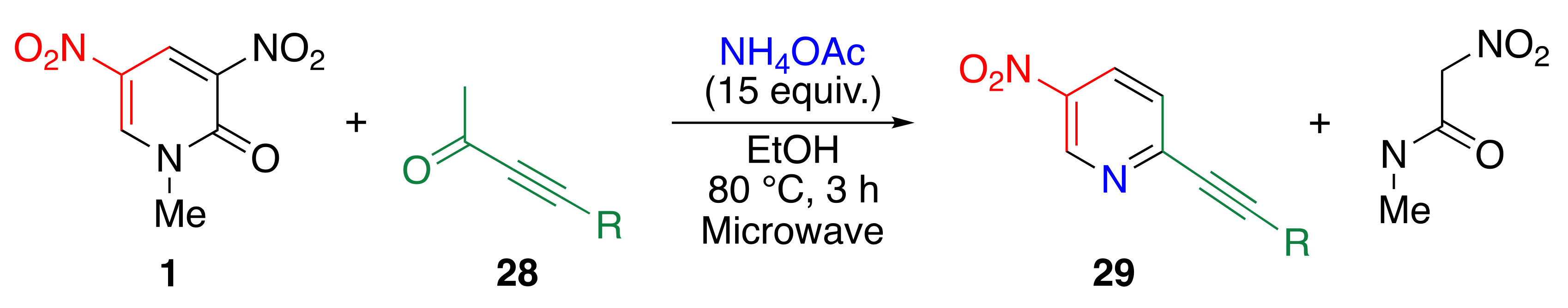
| R | Yield/% | |
|---|---|---|
| Ph | a | 87 |
| Et | b | 80 |
| Me3Si | c | 29c 24/29d 60 1 |
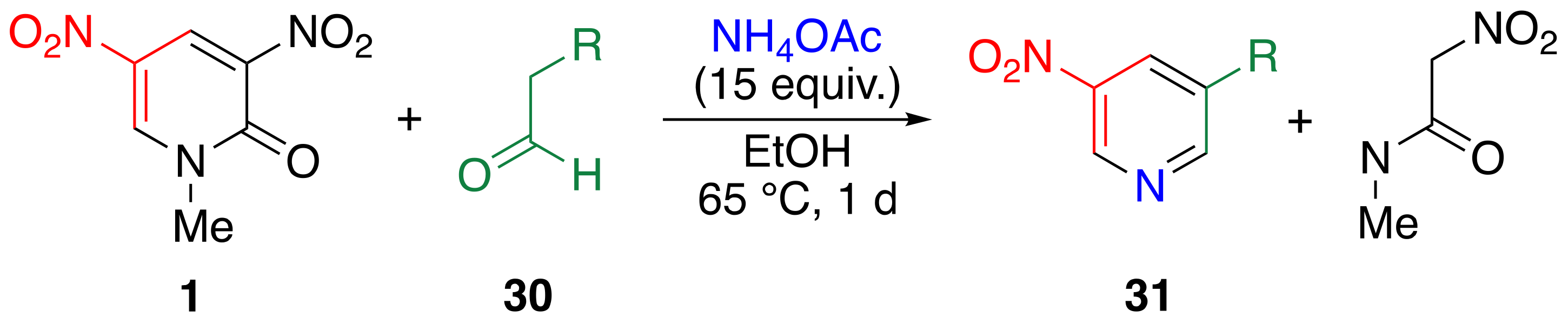
| R | Yield/% | |
|---|---|---|
| Me | a | 52 |
| Et | b | 86 |
| i-Pr | c | 71 |
| t-Bu | d | 68 1 |
| PhCH2 | e | 34 |
| Ph | f | 75 1 |
| Substrate | Product | Condition C | Condition D | |||
|---|---|---|---|---|---|---|
| Time/h | Yield/% | Time/h | Yield/% | |||
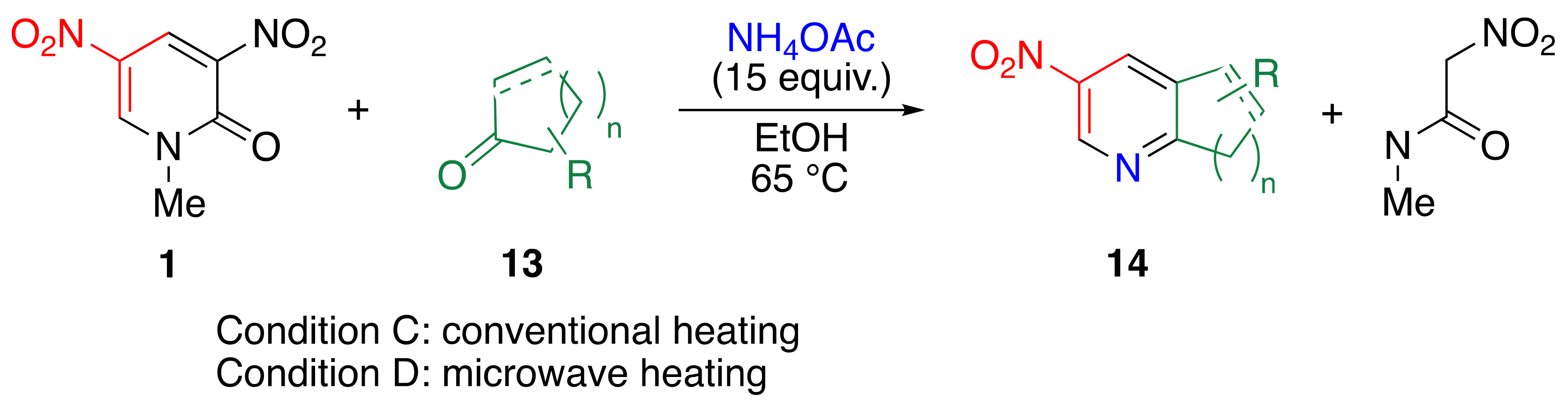
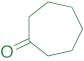
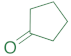 |  | 14b | 24 | 67 | 2 | 87 |
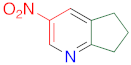
 | 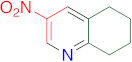 | 14a | 24 | 95 | 1 | 97 |
 | 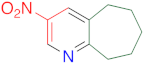 | 14s | 24 | 94 | 1 | 91 |
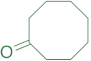 | 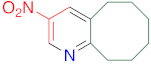 | 14w | 24 | 85 | 1 | 95 |
 | 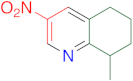 | 14aa | 24 | 83 | 2 | 86 |
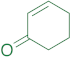 | 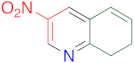 | 14ab | 24 | 59 | 3 | 89 |
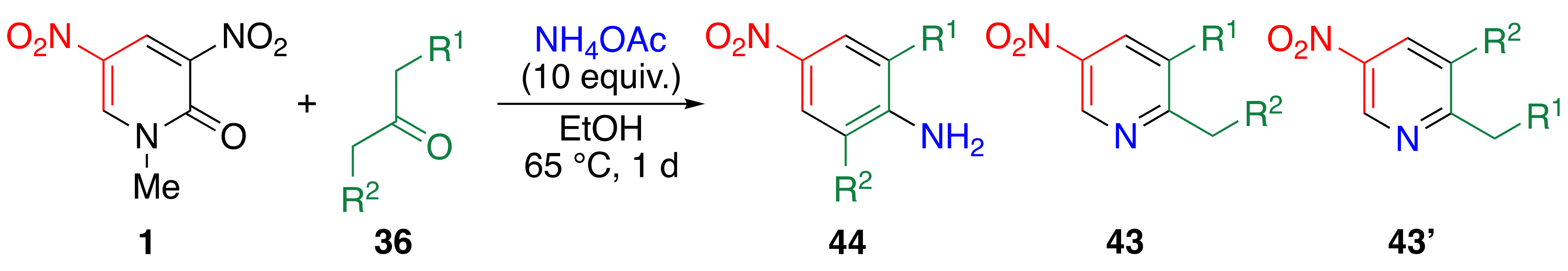
| Ketone | Yield/% | ||||
|---|---|---|---|---|---|
| R1 | R2 | 44 | 43 | 43’ | |
| Me | Me | a1 | 50 | 44 | — |
| Me | Me | a | 83 | 13 | — |
| H | H | b | 51 | 47 | — |
| Et | H | c | 66 | 10 | 8 |
| i-Pr | H | d | 58 | 0 | 31 |
| Pr | H | e | 83 | 9 | 6 |
| Et | Et | f | 67 | 24 | — |
| Pr | Pr | g | 74 | 22 | — |
| C6H5 | Pr | h | 62 | 24 | 13 |
| C6H5 | C6H5 | i | 8 | 81 | — |
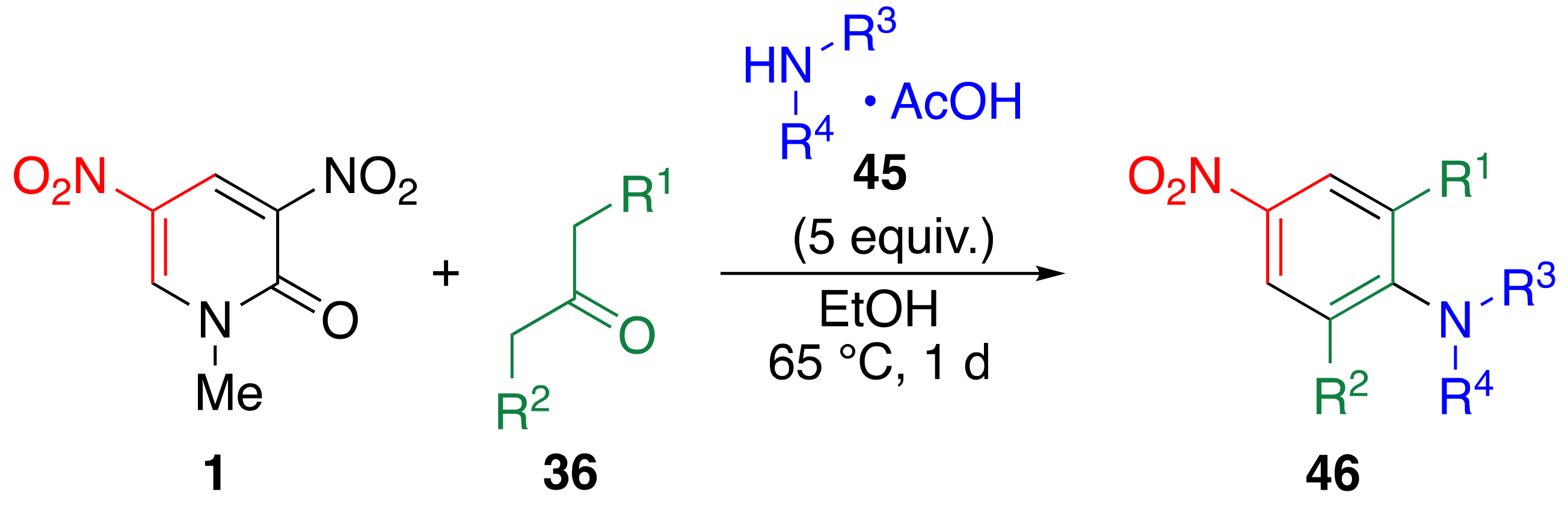
| Ketone | Amine | Product | Yield/% | ||||
|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | ||||
| Me | Me | 36a | Pr | H | 45A | 46Aa | 99 |
| Me | Me | 36a | –(CH2)4– | 45B | 46Ba | 98 | |
| Me | Me | 36a | Et | Et | 45C | 46Ca | 98 |
| Et | H | 36c | Pr | H | 45A | 46Ac | 83 |
| Et | H | 36c | –(CH2)4– | 45B | 46Bc | 68 | |
| Pr | H | 36e | Pr | H | 45A | 46Ae | 77 |
| Pr | H | 36e | –(CH2)4– | 45B | 46Be | 87 | |
| Pr | H | 36e | Et | Et | 45C | 46Ce | 51 |
| i-Pr | H | 36d | Pr | H | 45A | 46Ad | 83 |
| Et | Et | 36f | Pr | H | 45A | 46Af | 69 |
| Et | Et | 36f | –(CH2)4– | 45B | 46Bf | 68 | |
| Pr | Pr | 36g | Pr | H | 45A | 46Ag | 81 |
| Pr | Pr | 36g | –(CH2)4– | 45B | 46Bg | 59 | |
| C6H5 | Pr | 36h | Pr | H | 45A | 46Ah | 80 |
| C6H5 | C6H5 | 36i | Pr | H | 45A | 46Ai | 32 |
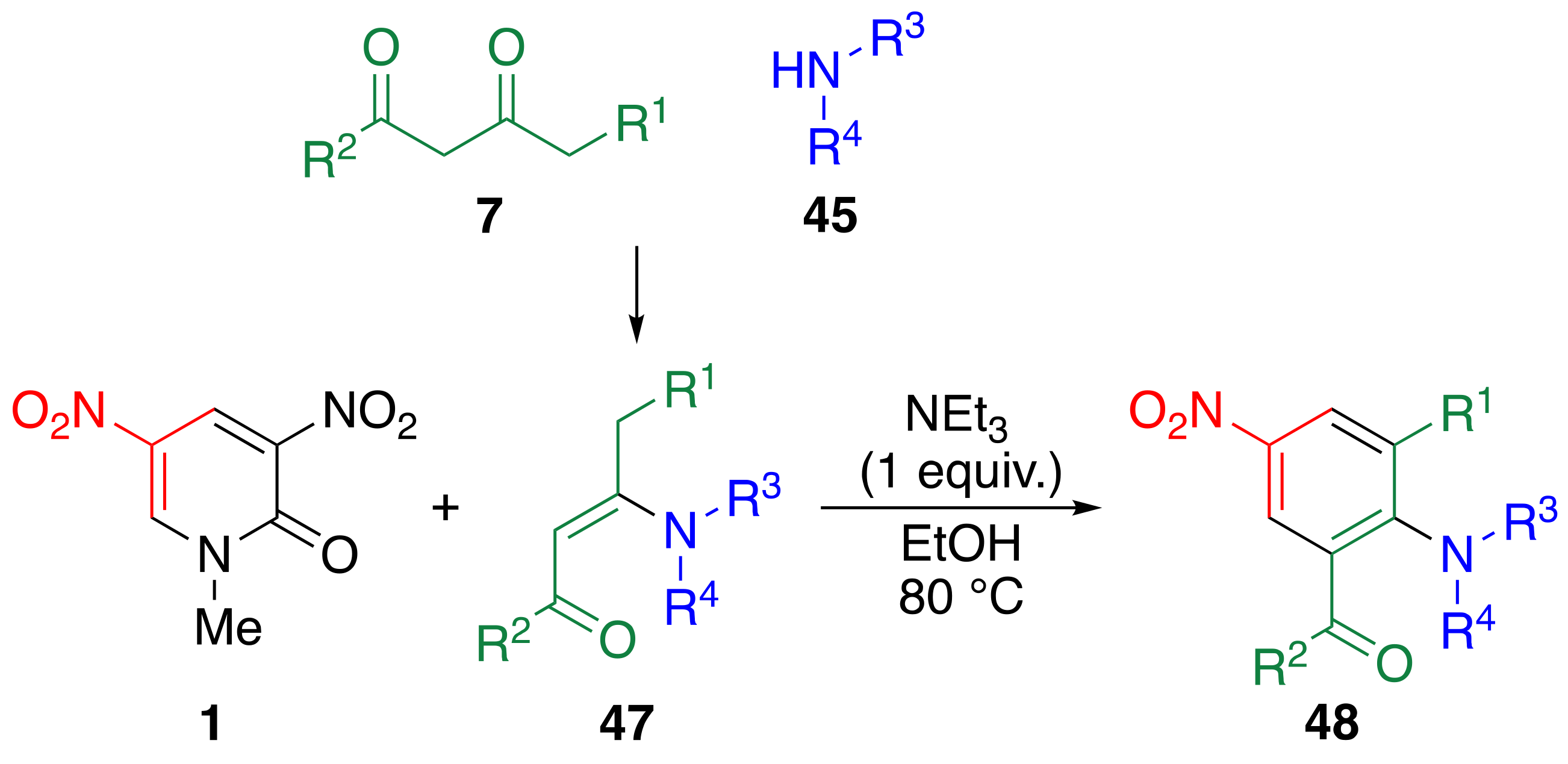
| 1,3-Dicarbonyl Compound | Amine | Time/d | Product | Yield/% | ||||
|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | |||||
| H | Me | 7c | Pr | H | 45A | 2 | 48Ac | 57 |
| H | Me | 7c | sec-Bu | H | 45D | 2 | 48Ca | 64 |
| H | Me | 7c | tert-Bu | H | 45E | 2 | 48Ca | 39 |
| H | Me | 7c | C6H5 | H | 45F | 2 | 48Ac | 23 |
| H | Me | 7c | 4-MeC6H4 | H | 45G | 2 | 48Gc | 36 |
| H | Me | 7c | –(CH2)4– | 45B | 2 | 48Bc | 87 | |
| H | C6H5 | 7e | Pr | H | 45A | 4 | 48Ae | 33 |
| H | C6H5 | 7e | Et | Et | 45C | 2 | 48Ce | 45 |
| H | OEt | 7b | Pr | H | 45A | 1 | 48Ab | 61 |
| H | OEt | 7b | HOCH2CH2 | H | 45H | 1 | 48Hb | 45 |
| H | OEt | 7b | Et | Et | 45C | 1 | 48Cb | 57 |
| Et | OEt | 7f | Pr | H | 45A | 2 | 48Af | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, S.T.; Asahara, H.; Nishiwaki, N. Synthesis of Nitroaromatic Compounds via Three-Component Ring Transformations. Molecules 2021, 26, 639. https://doi.org/10.3390/molecules26030639
Le ST, Asahara H, Nishiwaki N. Synthesis of Nitroaromatic Compounds via Three-Component Ring Transformations. Molecules. 2021; 26(3):639. https://doi.org/10.3390/molecules26030639
Chicago/Turabian StyleLe, Song Thi, Haruyasu Asahara, and Nagatoshi Nishiwaki. 2021. "Synthesis of Nitroaromatic Compounds via Three-Component Ring Transformations" Molecules 26, no. 3: 639. https://doi.org/10.3390/molecules26030639
APA StyleLe, S. T., Asahara, H., & Nishiwaki, N. (2021). Synthesis of Nitroaromatic Compounds via Three-Component Ring Transformations. Molecules, 26(3), 639. https://doi.org/10.3390/molecules26030639








