Pourbaix-Guided Mineralization and Site-Selective Photoluminescence Properties of Rare Earth Substituted B-Type Carbonated Hydroxyapatite Nanocrystals
Abstract
1. Introduction
2. Results and Discussion
2.1. Pourbaix Diagram of the Chemical Balance in the Calcium, Phosphates, Cabonates in Aqueous System
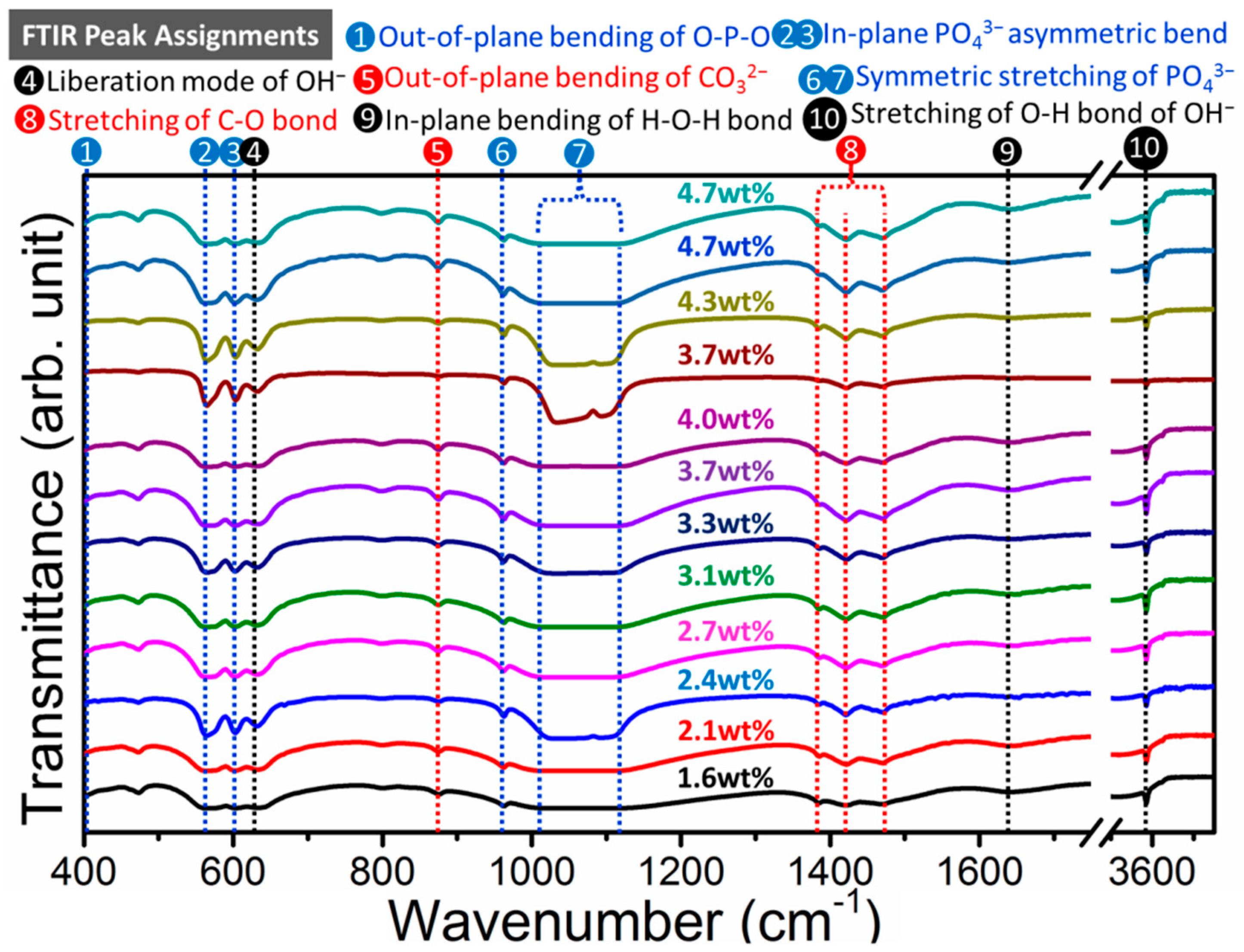
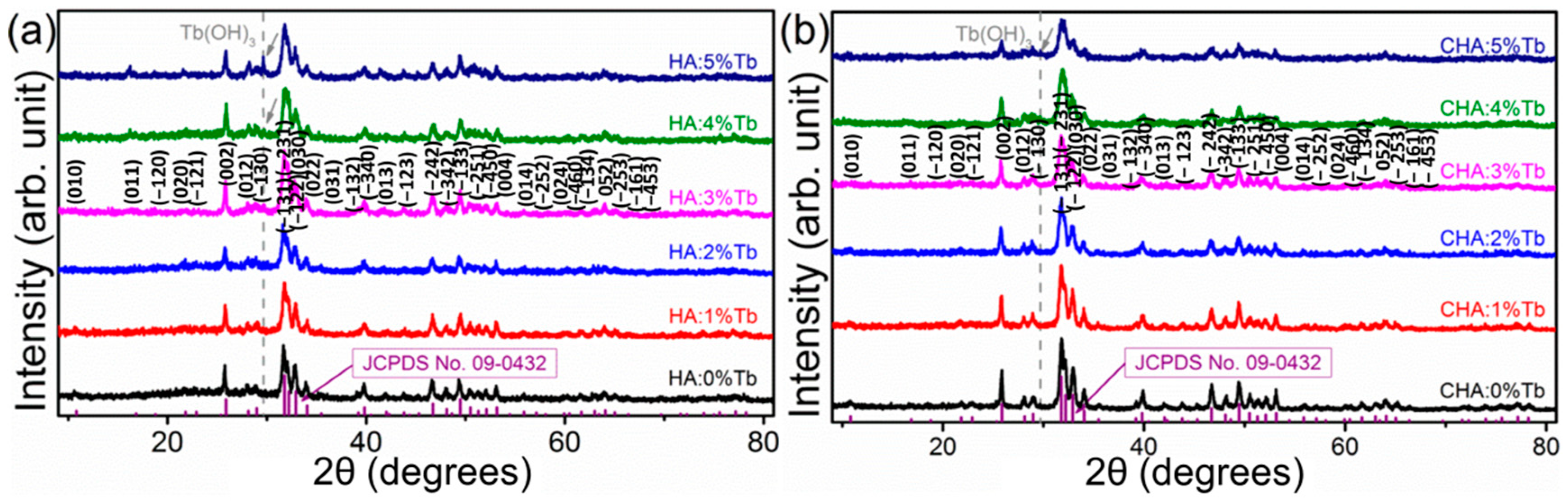
2.2. Effect of Carbonate Source on the Structure of Carbonated Hydroxyapatite
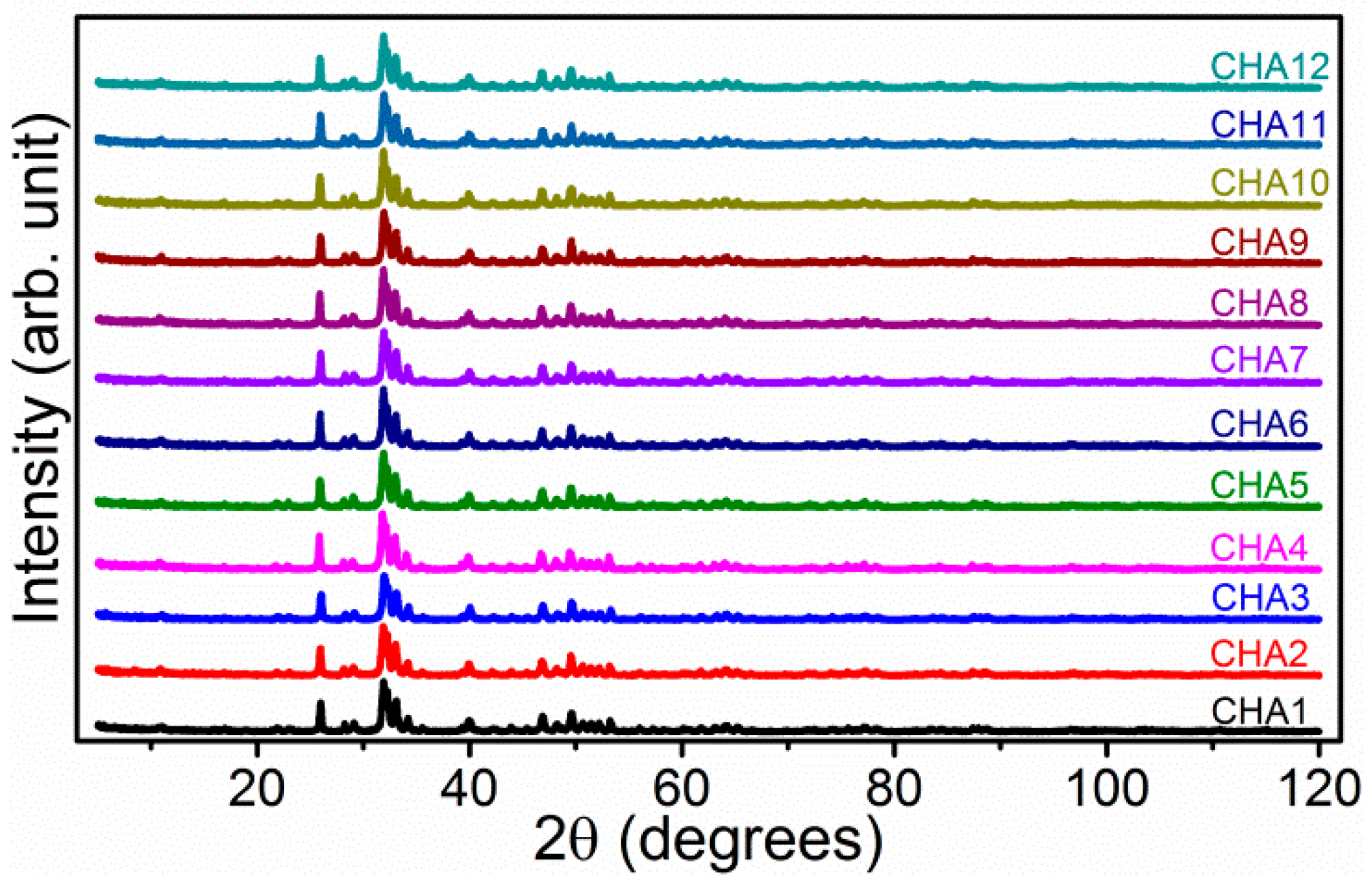
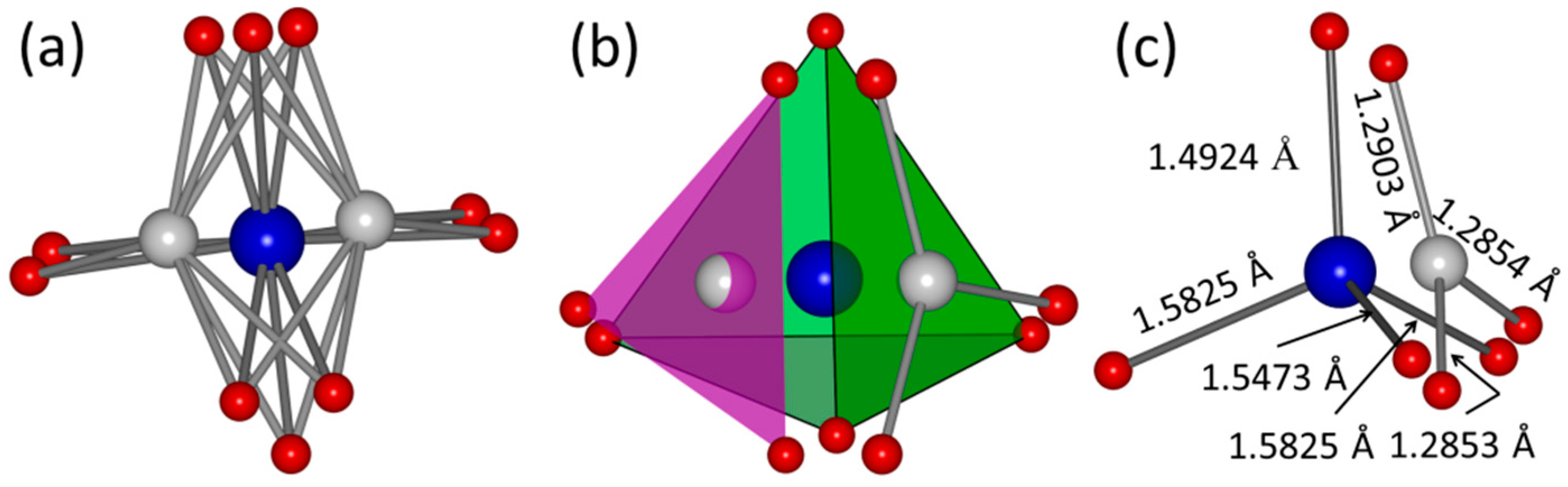
2.3. Structure Characteristics of B-Type Carbonated Hydroxyapatite
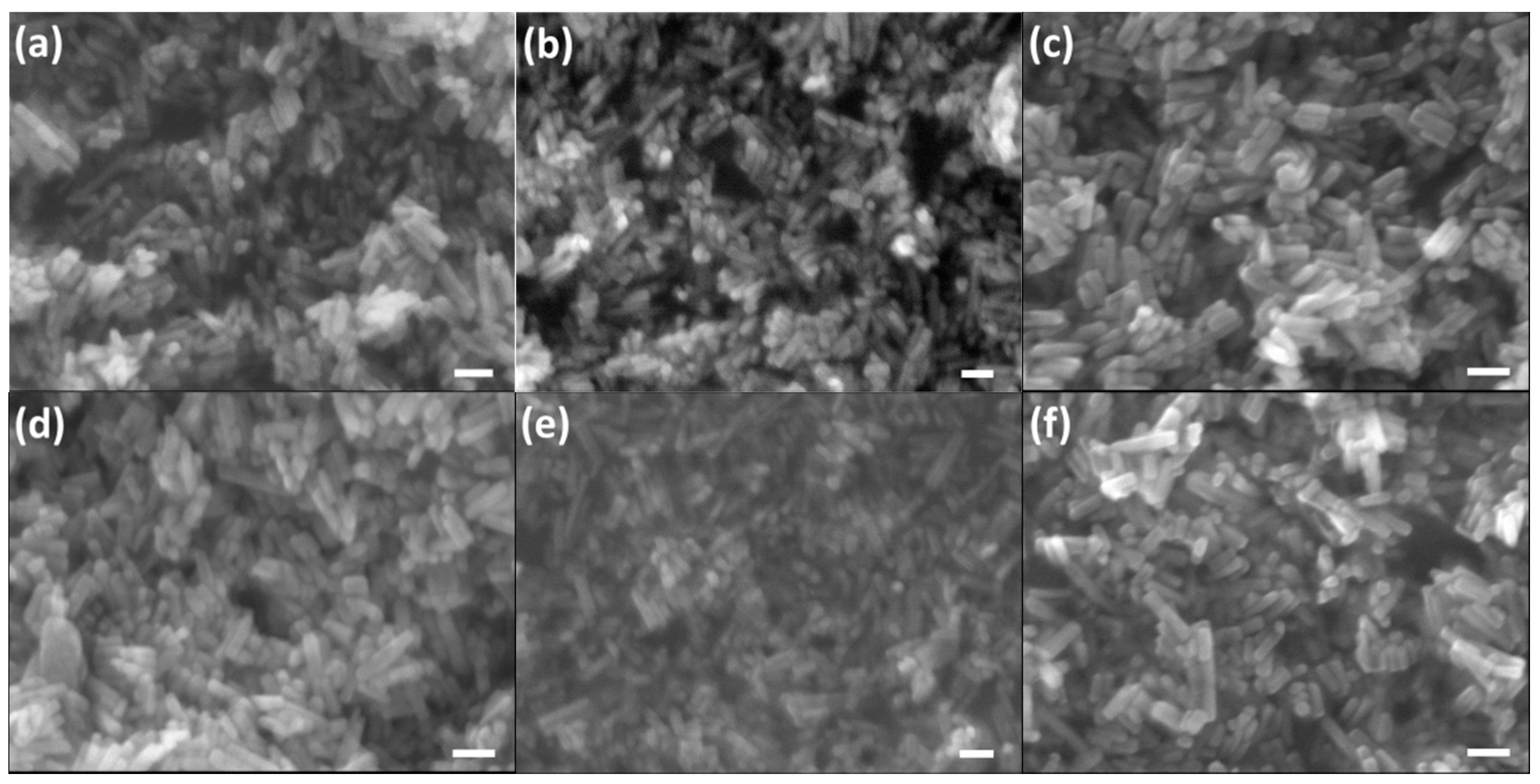
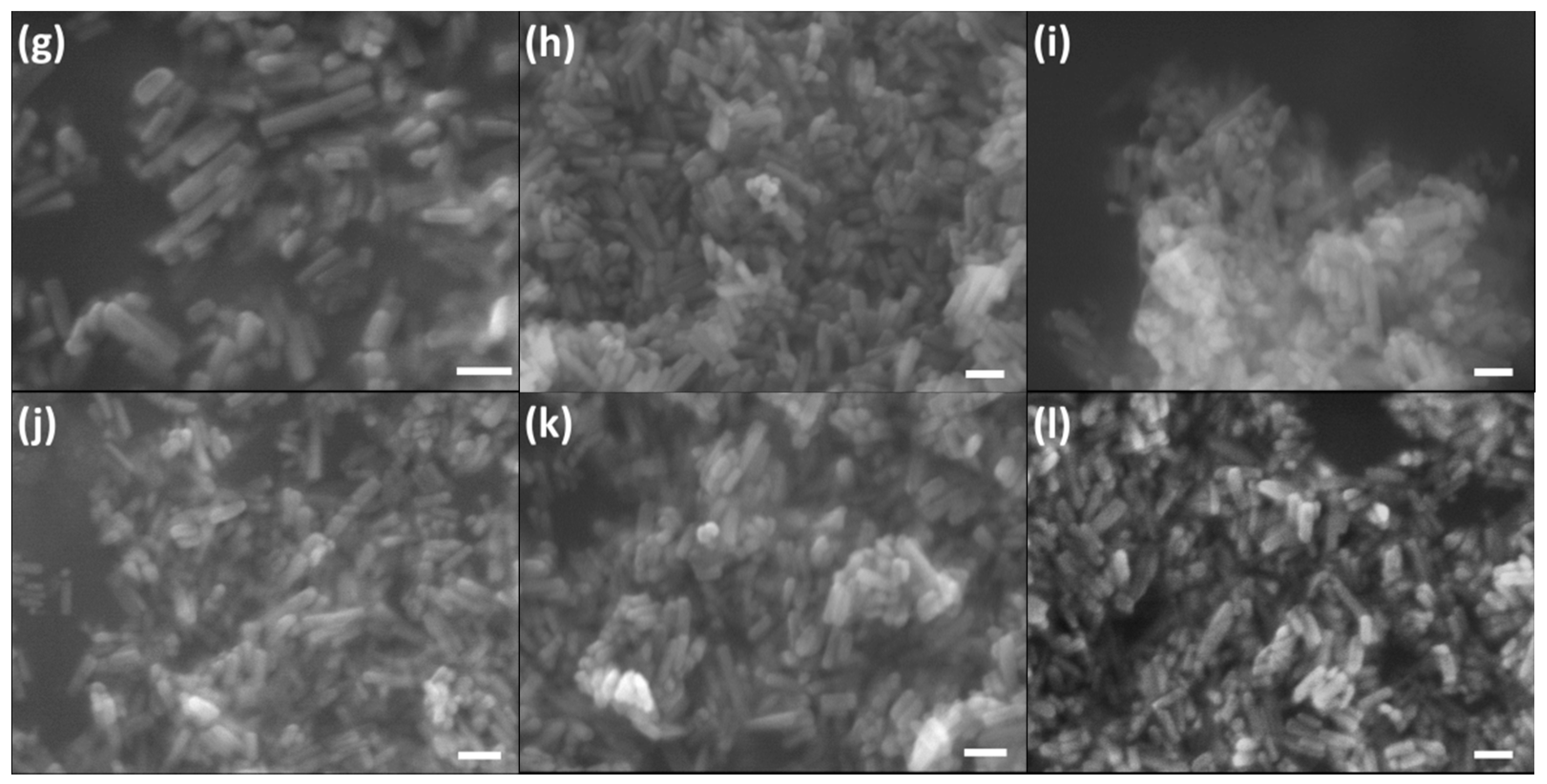
2.4. Morphology Evolution from Hydroxyapatite (HA) to Carbonated Hydroxyapatite (CHA)
2.5. Rare-Earth Ions Substitution in HA and CHA Materials
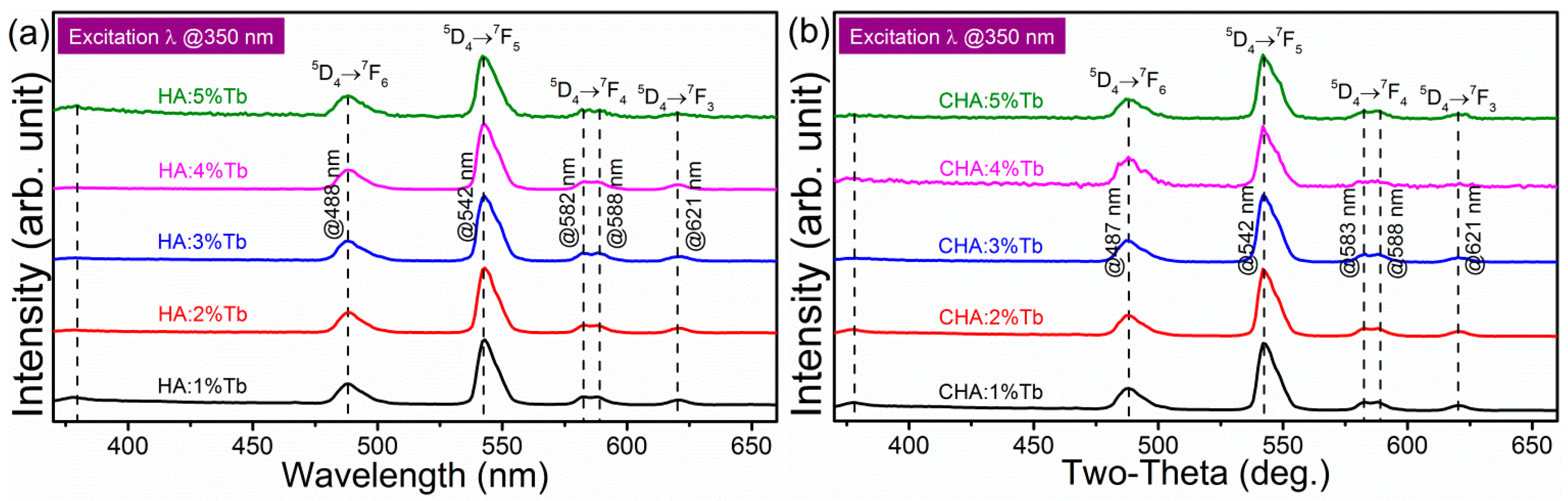
2.6. Photoluminescence of Tb3+ in the Site-Selective Occupancy of Hydrothermally Crystalized HA Nanomaterials
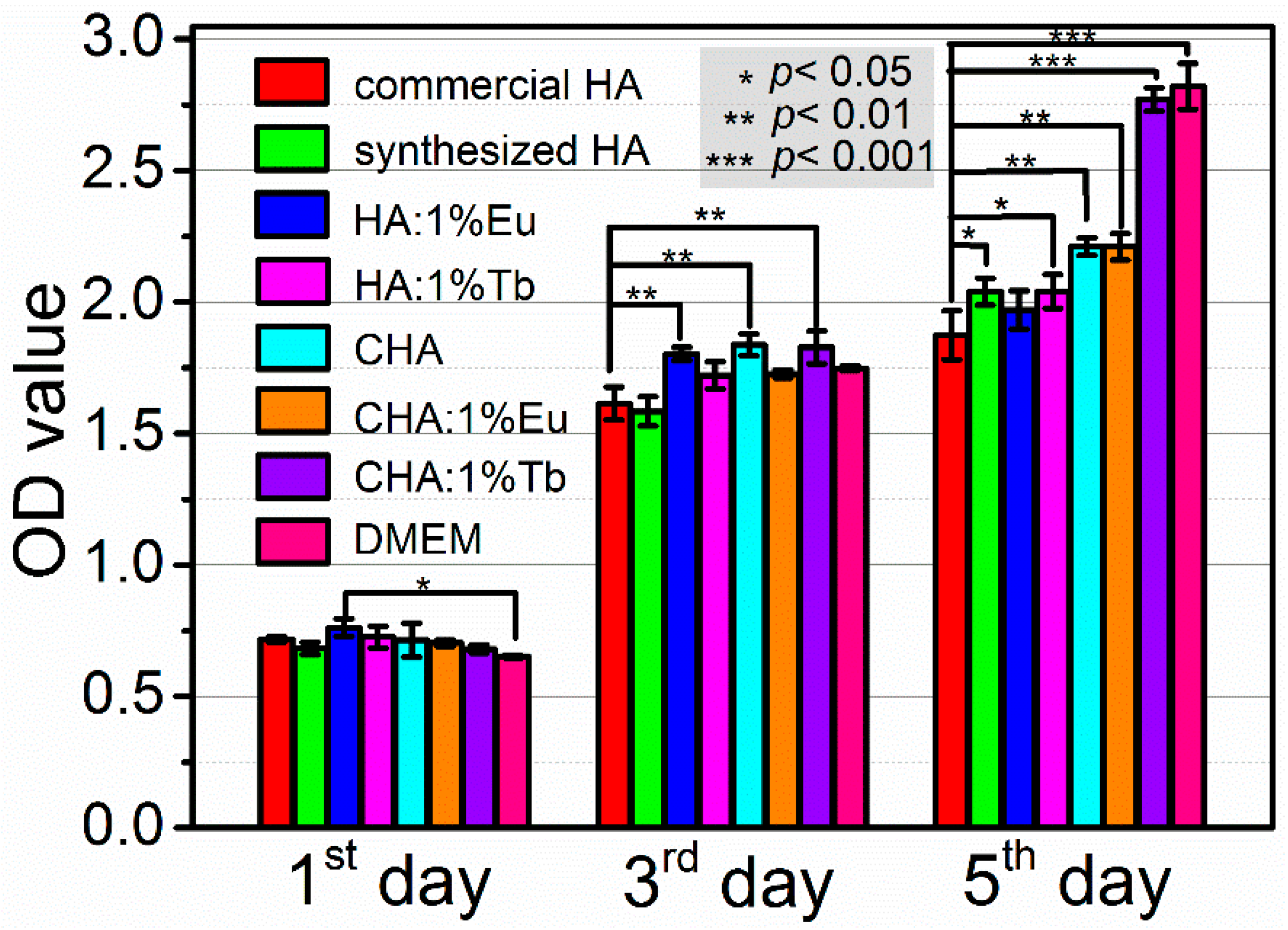
2.7. Cytotoxicity Assessment of As-Synthesized HA, CHA, and RE-Doped HA, CHA Nanomaterials
3. Materials and Methods
3.1. Chemicals
3.2. Hydrothermal Syntheses of the Samples
3.2.1. Synthesis of Hydroxyapatite Nanocrystals.
3.2.2. Synthesis of Biomimetic Carbonated-Hydroxyapatite
Ca10−(x/2)(PO4)6−(2/3)x(CO3)x(OH)2 + (20 + (4/3)x)K+ + (20 + (4/3)x)NO3− + 12H2O
3.2.3. Synthesis of Rare-Earth Doped Hydroxyapatite and Carbonated-Hydroxyapatite Nanocrystals
3.3. Characterizations
3.4. Cell Culture and Vialability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef] [PubMed]
- Reznikov, N.; Bilton, M.; Lari, L.; Stevens, M.M.; Kröger, R. Fractal-like hierarchical organization of bone begins at the nanoscale. Science 2018, 507, eaao2189. [Google Scholar] [CrossRef] [PubMed]
- Reznikov, N.; Hoac, B.; Buss, D.J.; Addison, W.N.; Barros, N.M.T.; McKee, M.D. Biological stenciling of mineralization in the skeleton: Local enzymatic removal of inhibitors in the extracellular matrix. Bone 2020, 138, 115447. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V.; Epple, M. Biological and Medical Significance of Calcium Phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Roschger, A.; Wagermaier, W.; Gamsjaeger, S.; Hassler, N.; Schmidt, I.; Blouin, S.; Berzlanovich, A.; Gruber, G.M.; Weinkamer, R.; Roschger, P.; et al. Newly formed and remodeled human bone exhibits differences in the mineralization process. Acta Biomater. 2020, 104, 221–230. [Google Scholar] [CrossRef]
- Xu, Y.; Nudelman, F.; Eren, E.D.; Wirix, M.J.; Cantaert, B.; Nijhuis, W.H.; Hermida-Merino, D.; Portale, G.; Bomans, P.H.; Ottmann, C.; et al. Intermolecular channels direct crystal orientation in mineralized collagen. Nat. Commun. 2020, 11, 5068. [Google Scholar] [CrossRef]
- Liu, X.; Shieh, S.R.; Fleet, M.E.; Zhang, L.; He, Q. Equation of state of carbonated hydroxylapatite at ambient temperature up to 10 GPa: Signifcance of carbonate. Am. Miner. 2011, 96, 74–80. [Google Scholar] [CrossRef]
- Uskoković, V. Ion-doped hydroxyapatite: An impasse or the road to follow? Ceram. Int. 2020, 46, 11443–11465. [Google Scholar] [CrossRef]
- Sato, N.; Handa, K.; Venkataiah, V.S.; Hasegawa, T.; Njuguna, M.M.; Yahata, Y.; Saito, M. Comparison of the vertical bone defect healing abilities of carbonate apatite, β-tricalcium phosphate, hydroxyapatite and bovine-derived heterogeneous bone. Dent. Mater. J. 2020, 39, 309–318. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Nanosized and nanocrystalline calcium orthophosphates. Acta Biomater. 2010, 6, 715–734. [Google Scholar] [CrossRef]
- Shao, C.; Jin, B.; Mu, Z.; Lu, H.; Zhao, Y.; Wu, Z.; Yan, L.; Zhang, Z.; Zhou, Y.; Pan, H.; et al. Repair of tooth enamel by a biomimetic mineralization frontier ensuring epitaxial growth. Sci. Adv. 2019, 5, eaaw9569. [Google Scholar] [CrossRef] [PubMed]
- DeRocher, K.A.; Smeets, P.J.M.; Goodge, B.H.; Zachman, M.J.; Balachandran, P.V.; Stegbauer, L.; Cohen, M.J.; Gordon, L.M.; Rondinelli, J.M.; Kourkoutis, L.F.; et al. Chemical gradients in human enamel crystallites. Nature 2020, 583, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Akita, K.; Fukuda, N.; Kamada, K.; Kudoh, K.; Kurio, N.; Tsuru, K.; Ishikawa, K.; Miyamoto, Y. Fabrication of porous carbonate apatite granules using microfiber and its histological evaluations in rabbit calvarial bone defects. J. Biomed. Mater. Res. 2020, 108A, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Ulian, G.; Valdrè, G.; Corno, M.; Ugliengo, P. The vibrational features of hydroxylapatite and type A carbonated apatite: A first principle contribution. Am. Miner. 2013, 98, 752–759. [Google Scholar] [CrossRef]
- Ulian, G.; Valdrè, G.; Corno, M.; Ugliengo, P. DFT investigation of structural and vibrational properties of type B and mixed A-B carbonated hydroxylapatite. Am. Miner. 2014, 99, 117–127. [Google Scholar] [CrossRef]
- Peroos, S.; Du, Z.; de Leeuw, N.H. A computer modelling study of the uptake, structure and distribution of carbonate defects in hydroxy-apatite. Biomaterials 2006, 27, 2150–2161. [Google Scholar] [CrossRef]
- Elliott, J.C. On the interpretation of the carbonate bands in the infra-red spectrum of dental enamel. J. Dent. Res. 1963, 42, 1081. [Google Scholar]
- Ito, A.; Maekawa, K.; Tsutsumi, S.; Ikazaki, F.; Tateishi, T. Solubility product of OH-carbonated hydroxyapatite. J. Biomed. Mater. Res. 1997, 36, 522–528. [Google Scholar] [CrossRef]
- Peccati, F.; Corno, M.; Piane, M.D.; Ulian, G.; Ugliengo, P.; Valdrè, G. CO32− Mobility in Carbonate Apatite as Revealed by Density Functional Modeling. J. Phys. Chem. C 2014, 118, 1364–1369. [Google Scholar] [CrossRef]
- Nordström, E.G.; Karlsson, K.H. Carbonate-doped hydroxyapatite. J. Mater. Sci. Mater. Med. 1990, 1, 182–184. [Google Scholar] [CrossRef]
- Astala, R.; Stott, M.J. First Principles Investigation of Mineral Component of Bone: CO3 Substitutions in Hydroxyapatite. Chem. Mater. 2005, 17, 4125–4133. [Google Scholar] [CrossRef]
- Liu, Q.; Matinlinna, J.P.; Chen, Z.; Ning, C.; Ni, G.; Pan, H.; Darvell, B.W. Effect of thermal treatment on carbonated hydroxyapatite: Morphology, composition, crystal characteristics and solubility. Ceram. Int. 2015, 41, 6149–6157. [Google Scholar] [CrossRef]
- Wu, Y.-S.; Lee, Y.-H.; Chang, H.-C. Preparation and characteristics of nanosized carbonated apatite by urea addition with coprecipitation method. Mater. Sci. Eng. C 2009, 29, 237–241. [Google Scholar] [CrossRef]
- Fleet, M. Carbonated Hydroxyapatite: Materials, Synthesis, and Applications; Taylor & Francis Group: Abingdon, UK; CRC Press: Boca Raton, FL, USA, 2015; ISBN-13: 978-981-4463-68-3. [Google Scholar]
- Lovón-Quintana, J.J.; Rodriguez-Guerrero, J.K.; Valença, P.G. Carbonate hydroxyapatite as a catalyst for ethanol conversion to hydrocarbon fuels. Appl. Catal. A General 2017, 542, 136–145. [Google Scholar] [CrossRef]
- Liu, M.; Shu, M.; Xu, W.; Liu, X.; Hou, Z.; Xing, B.; Lin, J. BMP-2-Loaded HAp:Ln3+ (Ln = Yb, Er, Gd) Nanorods with Dual-Mode Imaging for Efficient MC3t3-E1 Cell Differentiation Regulation. Langmuir 2019, 35, 15287–15294. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, H. Yb3+/Ho3+ Co-Doped Apatite Upconversion Nanoparticles to Distinguish Implanted Material from Bone Tissue. ACS Appl. Mater. Interfaces 2016, 8, 27458–27464. [Google Scholar] [CrossRef]
- Cawthray, J.F.; Creagh, A.L.; Haynes, C.A.; Orvig, C. Ion Exchange in Hydroxyapatite with Lanthanides. Inorg. Chem. 2015, 54, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Sambito, M.A.; Aslani, A.; Kalkhoran, N.M.; Slamovich, E.B.; Webster, T.J. Increased osteoblast functions on undoped and yttrium-doped nanocrystalline hydroxyapatite coatings on titanium. Biomaterials 2006, 27, 2358–2369. [Google Scholar] [CrossRef]
- Morais, D.S.; Coelho, J.; Ferraz, M.P.; Gomes, P.S.; Fernandes, M.H.; Hussain, N.S.; Santos, J.D.; Lopes, M.A. Samarium doped glass-reinforced hydroxyapatite with enhanced osteoblastic performance and antibacterial properties for bone tissue regeneration. J. Mater. Chem. B 2014, 2, 5872–5881. [Google Scholar] [CrossRef]
- Chen, F.; Huang, P.; Zhu, Y.-J.; Wu, J.; Zhang, C.-L.; Cui, D.-X. The photoluminescence, drug delivery and imaging properties of multifunctional Eu3+/Gd3+ dual-doped hydroxyapatite nanorods. Biomaterials 2011, 32, 9031–9039. [Google Scholar] [CrossRef]
- Ignjatović, N.L.; Mančić, L.; Vuković, M.; Stojanović, Z.; Nikolić, M.G.; Škapin, S.; Jovanović, S.; Veselinović, L.; Uskoković, V.; Lazić, S.; et al. Rare-earth (Gd3+, Yb3+/Tm3+, Eu3+) co-doped hydroxyapatite as magnetic, up-conversion and down-conversion materials for multimodal imaging. Sci. Rep. 2019, 9, 16305. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xia, Z.; Molokeev, M.S.; Lin, C.C.; Su, C.; Chuang, Y.-C.; Liu, Q. Probing Eu2+ Luminescence from Different Crystallographic Sites in Ca10M(PO4)7:Eu2+ (M = Li, Na, and K) with β-Ca3(PO4)2-Type Structure. Chem. Mater. 2017, 29, 7563–7570. [Google Scholar] [CrossRef]
- Li, X.; Zou, Q.; Li, W.; Chen, H. Intracellular Interaction of Hydroxyapatite-Based Nanocrystals with Uniform Shape and Traceable Fluorescence. Inorg. Chem. 2018, 57, 13739–13748. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zou, Q.; Chen, H.; Li, W. In vivo changes of nanoapatite crystals during bone reconstruction and the differences with native bone apatite. Sci. Adv. 2019, 5, eaay6484. [Google Scholar] [CrossRef]
- Li, X.; Ma, B.; Li, J.; Shang, L.; Liu, H.; Ge, S. A method to visually observe the degradation-diffusion-reconstruction behavior of hydroxyapatite in the bone repair process. Acta Biomater. 2020, 101, 554–564. [Google Scholar] [CrossRef]
- Procopio, A.; Malucelli, E.; Pacureanu, A.; Cappadone, C.; Farruggia, G.; Sargenti, A.; Castiglioni, S.; Altamura, D.; Sorrentino, A.; Giannini, C.; et al. Chemical Fingerprint of Zn−Hydroxyapatite in the Early Stages of Osteogenic Differentiation. ACS Cent. Sci. 2019, 5, 1449–1460. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, M.; Yan, J.; Wang, S.; Yuan, L.; Zhou, Y. Morphology, Structure Evolution and Site-Selective Occupancy of Eu3+ in Ca10(PO4)6(OH)2 Nanorods Synthesized via Subcritical Hydrothermal Method. Chem. Select 2018, 3, 7749–7756. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, W.; Schmelzer, E.; Gerlach, J.; Liu, C.; Nettleship, I. The degradation behavior of calcium-rich hydroxyapatite foams in vitro. J. Biomed. Mater. Res. 2020. [Google Scholar] [CrossRef]
- Resende, R.F.; Sartoretto, S.C.; Uzeda, M.J.; Alves, A.T.; Calasans-Maia, J.A.; Rossi, A.M.; Granjeiro, J.M.; Calasans-Maia, M.D. Randomized Controlled Clinical Trial of Nanostructured Carbonated Hydroxyapatite for Alveolar Bone Repair. Materials 2019, 12, 3645. [Google Scholar] [CrossRef]
- Mondal, S.; Park, S.; Choi, J.; Tran, L.H.; Yi, M.; Shin, J.H.; Lee, C.Y.; Oh, J. Bioactive, luminescent erbium-doped hydroxyapatite nanocrystals for biomedical applications. Ceram. Int. 2020, 46, 16020–16031. [Google Scholar] [CrossRef]
- Marieb, E.N.; Hoehn, K. Human Anatomy & Physiology, 9th ed.; Pearson Education, Inc.: Hong Kong, China, 2013; ISBN-13: 978-0-321-74326-8. [Google Scholar]
- Elliott, J.C. Structure and Chemistry of the Apatites and Other Calcium Orthophosphates; Elsevier Science: Amsterdam, The Netherlands, 1994; ISBN 0-444-81582-1. [Google Scholar]
- von Euw, S.; Wang, Y.; Laurent, G.; Drouet, C.; Babonneau, F.; Nassif, N.; Azaïs, T. Bone Mineral: New Insights into Its Chemical Composition. Sci. Rep. 2019, 9, 8456. [Google Scholar] [CrossRef] [PubMed]
- Lotsari, A.; Rajasekharan, A.K.; Halvarsson, M.; Andersson, M. Transformation of Amorphous Calcium Phosphate to Bone-like Apatite. Nat. Commun. 2018, 9, 4170. [Google Scholar] [CrossRef] [PubMed]
- Minh, D.P.; Tran, N.D.; Nzihou, A.; Sharrock, P. Carbonate-containing apatite (CAP) synthesis under moderate conditions starting from calcium carbonate and orthophosphoric acid. Mater. Sci. Eng. C 2013, 33, 2971–2980. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Nancollas, G.H. Calcium Orthophosphates: Crystallization and Dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef]
- Dhanaraj, G.; Byrappa, K.; Prasad, V.; Dudley, M. Springer Handbook of Crystal Growth; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-540-74182-4. [Google Scholar]
- Kim, H.-W.; Kim, H.-E.; Salih, V. Stimulation of osteoblast responses to biomimetic nanocomposites of gelatin-hydroxyapatite for tissue engineering scaffolds. Biomaterials 2005, 26, 5221–5230. [Google Scholar] [CrossRef]
- Chernov, A.A. Modern Crystallography III—Crystal Growth; Springer: New York, NY, USA, 1984. [Google Scholar]
- Wang, Z.; Ma, G.; Liu, X.Y. Will Fluoride Toughen or Weaken Our Teeth? Understandings Based on Nucleation, Morphology, and Structural Assembly. J. Phys. Chem. B 2009, 113, 16393–16399. [Google Scholar] [CrossRef]
- Andersson, J.; Areva, S.; Spliethoff, B.; Lindén, M. Sol–gel synthesis of a multifunctional, hierarchically porous silica/apatite composite. Biomaterials 2005, 26, 6827–6835. [Google Scholar] [CrossRef]
- Sun, R.; Yang, L.; Zhang, Y.; Chu, F.; Wang, G.; Lv, Y.; Chen, K. Novel synthesis of AB-type carbonated hydroxyapatite hierarchical microstructures with sustained drug delivery properties. CrystEngComm 2016, 18, 8030–8037. [Google Scholar] [CrossRef]
- Qi, M.-L.; Yao, S.; Liu, X.-C.; Wang, X.; Cui, F. Nanosheet-assembled carbonated hydroxyapatite microspheres prepared by an EDTA-assisted hydrothermal homogeneous precipitation route. CrystEngComm 2020, 22, 2884–2888. [Google Scholar] [CrossRef]
- Titorenkova, R.; Dyulgerova, E.; Petkova, V.; Ilieva, R. Carbonation and dehydroxylation of apatite during high energy milling of biphasic Ca-phosphate ceramics. Ceram. Int. 2019, 45, 7025–7033. [Google Scholar] [CrossRef]
- Clasen, A.B.S.; Ruyter, I.E. Quantitative Determination of Type A and Type B Carbonate in Human Deciduous and Permanent Enamel by Means of Fourier Transform Infrared Spectrometry. Adv. Dent. Res. 1997, 11, 523–527. [Google Scholar] [CrossRef] [PubMed]
- El Feki, H.; Savariault, J.M.; Salah, A.B.; Jemal, M. Sodium and carbonate distribution in substituted calcium hydroxyapatite. Solid State Sci. 2000, 2, 577–586. [Google Scholar] [CrossRef]
- Kubota, T.; Nakamura, A.; Toyoura, K.; Matsunaga, K. The effect of chemical potential on the thermodynamic stability of carbonate ions in hydroxyapatite. Acta Biomater. 2014, 10, 3716–3722. [Google Scholar] [CrossRef] [PubMed]
- Housecroft, C.E.; Sharpe, A.G. Inorganic Chemistry, 2nd ed.; Pearson Education Limited: London, UK, 2004; ISBN 0130-39913-2. [Google Scholar]
- Ren, F.; Leng, Y.; Ding, Y.; Wang, K. Hydrothermal growth of biomimetic carbonated apatite nanoparticles with tunable size, morphology and ultrastructure. CrystEngComm 2013, 15, 2137–2146. [Google Scholar] [CrossRef]
- Bian, T.; Zhao, K.; Meng, Q.; Tang, Y.; Jiao, H.; Luo, J.; Liu, X. Synthesis of plate-like single-crystal hydroxyapatite rods with c-axis orientation by biotemplate small intestinal submucosa. Ceram. Int. 2017, 43, 11807–11814. [Google Scholar] [CrossRef]
- Zhang, H.; Darvell, B.W. Synthesis and characterization of hydroxyapatite whiskers by hydrothermal homogeneous precipitation using acetamide. Acta Biomater. 2010, 6, 3216–3222. [Google Scholar] [CrossRef]
- Wei, X.; Fu, C.; Savino, K.; Yates, M.Z. Carbonated Hydroxyapatite Coatings with Aligned Crystal Domains. Cryst. Growth Des. 2012, 12, 3474–3480. [Google Scholar] [CrossRef]
- Aneem, T.H.; Saha, S.K.; Jahan, R.A.; Wong, S.Y.; Li, X.; Arafat, M.T. Effects of organic modifiers and temperature on the synthesis of biomimetic carbonated hydroxyapatite. Ceram. Int. 2019, 45, 24717–24726. [Google Scholar] [CrossRef]
- Stojanović, Z.S.; Ignjatović, N.; Wu, V.; Žunič, V.; Veselinović, L.; Škapin, S.; Miljković, M.; Uskoković, V.; Uskoković, D. Hydrothermally processed 1D hydroxyapatite: Mechanism of formation and biocompatibility studies. Mater. Sci. Eng. C 2016, 68, 746–757. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Birkbak, M.E.; Birkedal, H. Spatial Organization of Hydroxyapatite Nanorods on a Substrate via a Biomimetic Approach. Cryst. Growth Des. 2013, 13, 4213–4219. [Google Scholar] [CrossRef]
- Zhuang, Z.; Yamamoto, H.; Aizawa, M. Synthesis of plate-shaped hydroxyapatite via an enzyme reaction of urea with urease and its characterization. Power Technol. 2012, 222, 193–200. [Google Scholar] [CrossRef]
- Neira, I.S.; Guitián, F.; Taniguchi, T.; Watanabe, T.; Yoshimura, M. Hydrothermal synthesis of hydroxyapatite whiskers with sharp faceted hexagonal morphology. J. Mater. Sci. 2008, 43, 2171–2178. [Google Scholar] [CrossRef]
- Lin, K.; Chang, J.; Zhu, Y.; Wu, W.; Cheng, G.; Zeng, Y.; Ruan, M. A Facile One-Step Surfactant-Free and Low-Temperature Hydrothermal Method to Prepare Uniform 3D Structured Carbonated Apatite Flowers. Cryst. Growth Des. 2009, 9, 177–181. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Q.; Wang, M.; Long, J.; Mao, Z.; Chen, X. Hydrothermal Synthesis of Hydroxyapatite with Different Morphologies: Influence of Supersaturation of the Reaction System. Cryst. Growth Des. 2014, 14, 4864–4871. [Google Scholar] [CrossRef]
- Jiang, S.-D.; Yao, Q.-Z.; Zhou, G.-T.; Fu, S.-Q. Fabrication of Hydroxyapatite Hierarchical Hollow Microspheres and Potential Application in Water Treatment. J. Phys. Chem. C 2012, 116, 4484–4492. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Christenson, H.K.; Meldrum, F.C. Confinement Increases the Lifetimes of Hydroxyapatite Precursors. Chem. Mater. 2014, 26, 5830–5838. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Zhang, W.; Putnis, C.V.; Putnis, A. Direct Observation of Spiral Growth, Particle Attachment, and Morphology Evolution of Hydroxyapatite. Cryst. Growth Des. 2016, 16, 4509–4518. [Google Scholar] [CrossRef]
- Yuan, L.; Huang, K.; Feng, W.; Li, B.; Hou, C.; Ye, K.; Wu, X.; Shi, Z.; Wang, S.; Feng, S. Design and synthesis of metal hydroxide three-dimensional inorganic cationic frameworks. Dalton Trans. 2018, 47, 3339–3345. [Google Scholar] [CrossRef]
- Wang, S.; Wu, X.; Wang, T.; Zhang, J.; Zhang, C.; Yuan, L.; Cui, X.; Lu, D. Mild Hydrothermal Crystallization of Heavy Rare-Earth Chromite RECrO3 (RE = Er, Tm, Yb, Lu) Perovskites and Magnetic Properties. Inorg. Chem. 2019, 58, 2315–2329. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Apatites in Biological Systems. Prog. Crystal Growth Charact. 1981, 4, 1–45. [Google Scholar] [CrossRef]
- Saito, T.; Yokoi, T.; Nakamura, A.; Matsunaga, K. Formation energies and site preference of substitutional divalent cations in carbonated apatite. J. Am. Ceram. Soc. 2020, 103, 5354–5364. [Google Scholar] [CrossRef]
- Yuan, L.; Yu, J.; Huang, K.; Ren, X.; Sun, Y.; Wu, X.; Feng, S. UV–vis absorption shift of mixed valance state tungstate oxide: Ca0.72La0.28WO4. Mater. Lett. 2015, 143, 212–214. [Google Scholar] [CrossRef]
- Fleet, M.E.; Pan, Y. Rare earth elements in apatite: Uptake from H2O-bearing phosphate-fluoride melts and the role of volatile components. Geochim. Cosmochim. Acta 1997, 61, 4745–4760. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta. Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Sharma, S.; Jana, S. Luminescence properties of Tb3+ embedded zinc lead phosphate glasses. Mater. Chem. Phys. 2020, 251, 122968. [Google Scholar] [CrossRef]
- Sontakke, A.D.; Biswas, K.; Annapurna, K. Concentration-dependent luminescence of Tb3+ ions in high calcium aluminosilicate glasses. J. Lumin. 2009, 129, 1347–1355. [Google Scholar] [CrossRef]
- Tuyen, V.P.; Quang, V.X.; Khaidukov, N.M.; Thanh, L.D.; Ca, N.X.; van Hao, N.; van Nghia, N.; van Do, P. K2YF5:Tb3+ single crystal: An in-depth study of spectroscopic properties, energy transfer and quantum cutting. Opt. Mater. 2020, 106, 109939. [Google Scholar] [CrossRef]
- Lisiecki, R.; Macalik, B.; Kowalski, R.; Strzep, A.; Glowacki, M.; Berkowski, M.; Ryba-Romanowski, W. Effect of Tb3+ concentration and co-doping with Ce3+ ions on luminescence characteristics of terbium-doped (Lu0.25Gd0.75)2SiO5 single crystals. Opt. Mater. 2020, 107, 110155. [Google Scholar] [CrossRef]
- Atwood, D.A. The Rare Earth Elements: Fundamentals and Applications; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2012; ISBN-13: 978-1-119-95097-4. [Google Scholar]
- Wang, Z.; Li, P.; Yang, Z.; Guo, Q. Luminescence and concentration quenching of Tb3+ in Sr2ZnMoO6. Opt. Commun. 2014, 321, 100–103. [Google Scholar] [CrossRef]
- Fan, H.; Chen, Y.; Yan, T.; Lin, J.; Peng, G.; Wang, J.; Boughton, R.I.; Ye, N. Crystal growth, spectral properties and Judd-Ofelt analysis of Pr3+:LaMgAl11O19. J. Alloy Compd. 2018, 767, 938–943. [Google Scholar] [CrossRef]
- Wright, A.O.; Seltzer, M.D.; Gruber, J.B.; Zandi, B.; Merkle, L.D.; Chai, B.H.T. Spectroscopic Investigation of Pr3+ in Fluorapatite Crystals. J. Phys. Chem. Solids 1996, 57, 1337–1350. [Google Scholar] [CrossRef]
- Thomas, S.; George, R.; Qamhieh, N.; Gopchandran, K.G.; Mahmoud, S.T.; Quatela, A. Sm3+-doped strontium barium borate phosphor for white light emission: Spectroscopic properties and Judd–Ofelt analysis. Spectrochim. Acta Part A 2021, 248, 119187. [Google Scholar] [CrossRef] [PubMed]
- Deopa, N.; Rao, A.S. Spectroscopic studies of single near ultraviolet pumped Tb3+ doped Lithium Lead Alumino Borate glasses for green lasers and tricolour w-LEDs. J. Lumin. 2018, 194, 56–63. [Google Scholar] [CrossRef]
- Lide, D.R.; Haynes, W.M.M. CRC Handbook of Chemistry and Physics, 90th ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 13: 9781420090840. [Google Scholar]
- Madhavasarma, P.; Veeraragavan, P.; Kumaravel, S.; Sridevi, M. Studies on physiochemical modifcations on biologically important hydroxyapatite materials and their characterization for medical applications. Biophys. Chem. 2020, 267, 106474. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hao, Y.; Zhen, L.; Liu, H.; Shao, M.; Xu, X.; Liang, K.; Gao, Y.; Yuan, H.; Li, J.; et al. Biomineralization of dentin. J. Struct. Biol. 2019, 207, 115–122. [Google Scholar] [CrossRef]
- Yun, F.; Swain, M.V.; Chen, H.; Gairney, J.; Qu, J.; Sha, G.; Liu, H.; Ringer, S.P.; Han, Y.; Liu, L.; et al. Nanoscale Pathways for Human Tooth Decay—Central Planar Defect, Organic-Rich Precipitate and High-Angle Grain Boundary. Biomaterials 2020, 235, 119748. [Google Scholar] [CrossRef]

| Cations | Radii of Cations in Coordinated Number | |
|---|---|---|
| VI | VII | |
| Ca2+ | 100 pm | 106 pm |
| Pr3+ | 99 pm | - b |
| Sm3+ | 95.8 pm | 102 pm |
| Eu3+ | 94.7 pm | 101 pm |
| Tb3+ | 92.3 pm | 98 pm |
| Ho3+ | 90.1 pm | - |
| Sample | EDS Results | ICP-AES Results |
|---|---|---|
| 0% Tb-HA | Ca:Tb = 1:0 | Ca:Tb = 1:0.0001 |
| 1% Tb-HA | Ca:Tb = 1:0.0089 | Ca:Tb = 1:0.0096 |
| 2% Tb-HA | Ca:Tb = 1:0.0166 | Ca:Tb = 1:0.0182 |
| 3% Tb-HA | Ca:Tb = 1:0.0245 | Ca:Tb = 1:0.0275 |
| 4% Tb-HA | Ca:Tb = 1:0.0312 | Ca:Tb = 1:0.0367 |
| 5% Tb-HA | Ca:Tb = 1:0.0378 | Ca:Tb = 1:0.0445 |
| 0% Tb-CHA | Ca:Tb = 1:0 | Ca:Tb = 1:0.0000 |
| 1% Tb-CHA | Ca:Tb = 1:0.0093 | Ca:Tb = 1:0.0096 |
| 2% Tb-CHA | Ca:Tb = 1:0.0176 | Ca:Tb = 1:0.0185 |
| 3% Tb-CHA | Ca:Tb = 1:0.0261 | Ca:Tb = 1:0.0280 |
| 4% Tb-CHA | Ca:Tb = 1:0.0331 | Ca:Tb = 1:0.0373 |
| 5% Tb-CHA | Ca:Tb = 1:0.0389 | Ca:Tb = 1:0.0452 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Li, Z.; Yuan, L.; Sun, X.; Zhou, Y. Pourbaix-Guided Mineralization and Site-Selective Photoluminescence Properties of Rare Earth Substituted B-Type Carbonated Hydroxyapatite Nanocrystals. Molecules 2021, 26, 540. https://doi.org/10.3390/molecules26030540
Liu P, Li Z, Yuan L, Sun X, Zhou Y. Pourbaix-Guided Mineralization and Site-Selective Photoluminescence Properties of Rare Earth Substituted B-Type Carbonated Hydroxyapatite Nanocrystals. Molecules. 2021; 26(3):540. https://doi.org/10.3390/molecules26030540
Chicago/Turabian StyleLiu, Peng, Zhengqiang Li, Long Yuan, Xiaolin Sun, and Yanmin Zhou. 2021. "Pourbaix-Guided Mineralization and Site-Selective Photoluminescence Properties of Rare Earth Substituted B-Type Carbonated Hydroxyapatite Nanocrystals" Molecules 26, no. 3: 540. https://doi.org/10.3390/molecules26030540
APA StyleLiu, P., Li, Z., Yuan, L., Sun, X., & Zhou, Y. (2021). Pourbaix-Guided Mineralization and Site-Selective Photoluminescence Properties of Rare Earth Substituted B-Type Carbonated Hydroxyapatite Nanocrystals. Molecules, 26(3), 540. https://doi.org/10.3390/molecules26030540






