The Mechanisms Mediated by α7 Acetylcholine Nicotinic Receptors May Contribute to Peripheral Nerve Regeneration
Abstract
:1. Introduction
2. Results
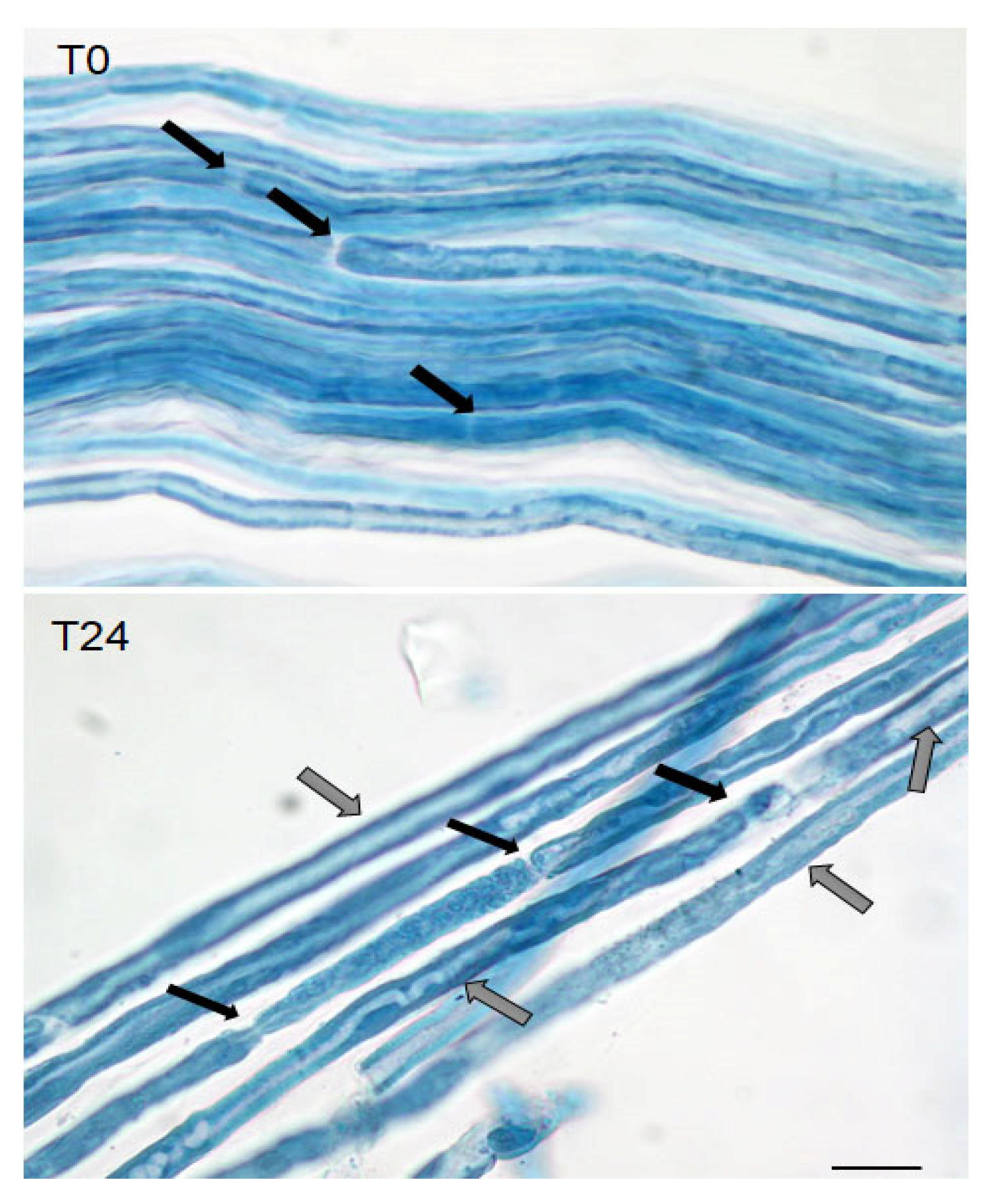
2.1. Expression of α7 Nicotinic Receptor in Schwann Cells after Sciatic Nerve Dissection
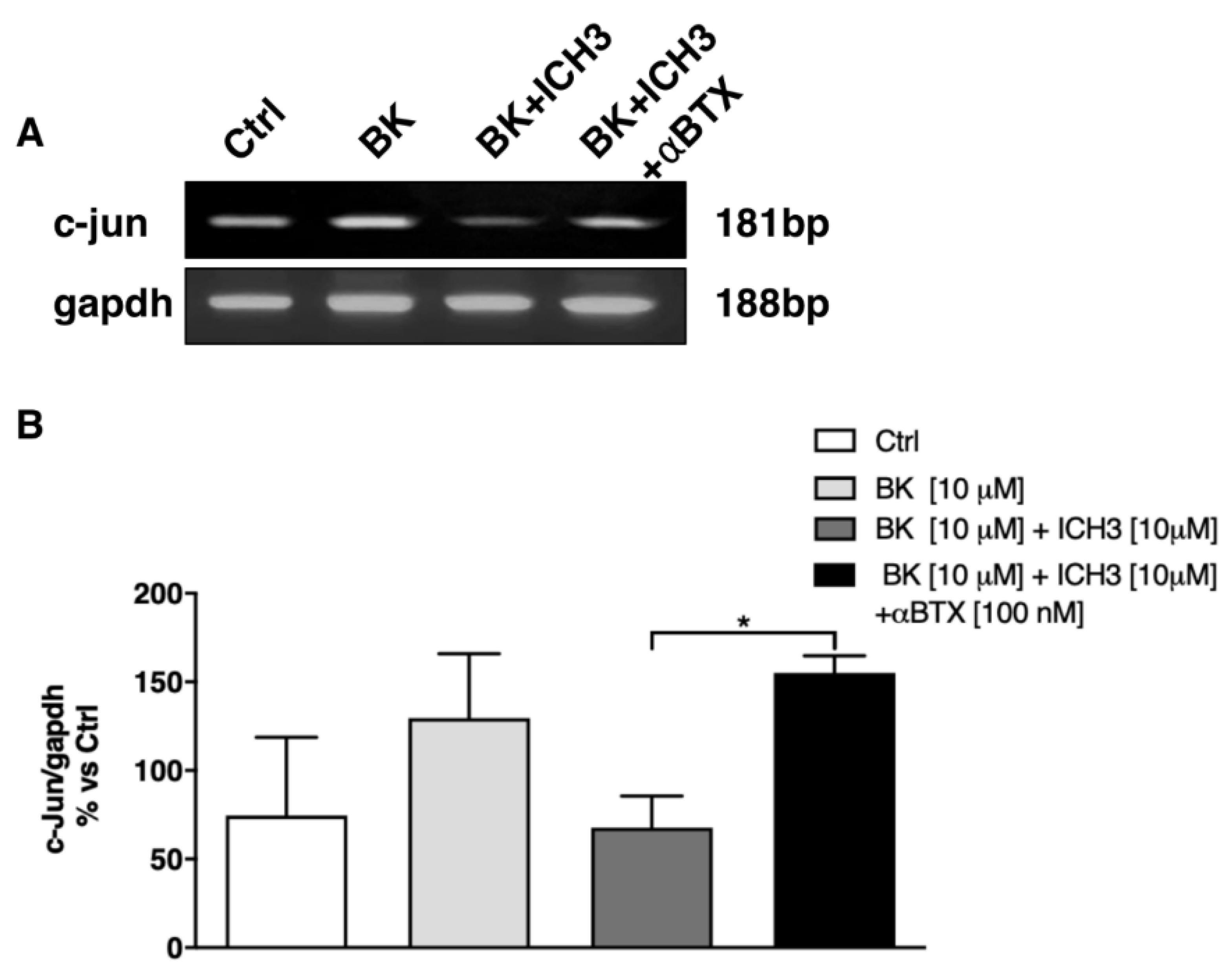
2.2. Analysis of the Expression of c-Jun Transcription Factor in Sciatic Nerves
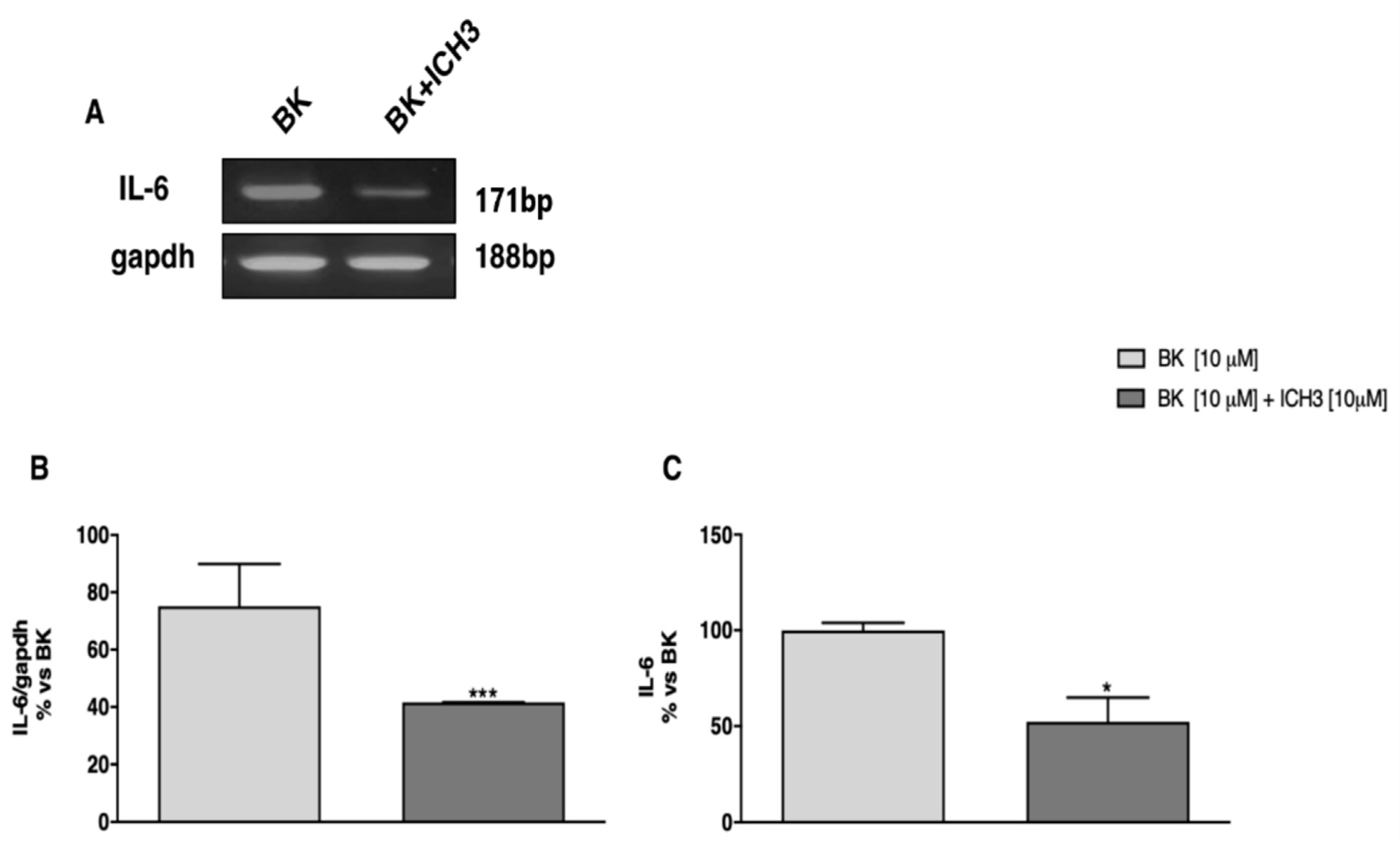
2.3. Analysis of the Expression and Release of IL-6
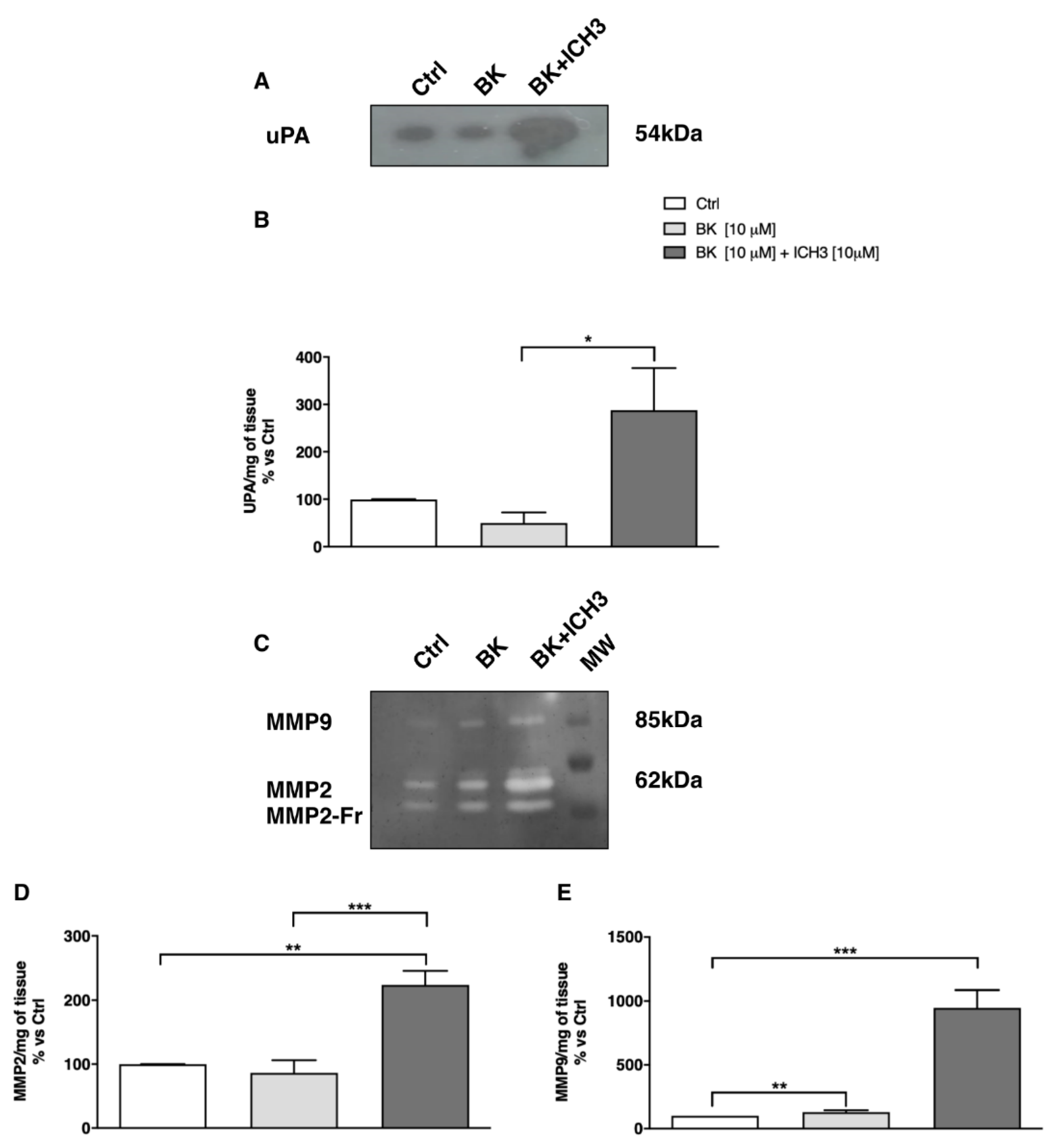
2.4. Analysis of Metalloproteinase Activity in Sciatic Nerve Culture Media
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Ex Vivo Sciatic Nerve Explants
4.3. Schwann Cell Cultures
4.4. Pharmacological Treatments
4.5. RNA Extraction and RT-PCR Analysis
- c-Jun: Forward 5′-GCGCGCCCTAGCTGAACTGC-3′
- Reverse 5′-AGTTGCTGAGGTTGGCGTAG-3′
- Il-6: Forward 5′-TGGTCTTCTGGAGTTCCGTT-3′
- Reverse: 5′-AGAGCATTGGAAGTTGGGGT -3′
- GAPDH: Forward 5′-TGGCATTGTGGAAGGGCTCATGAC-3′
- Reverse 5′-ATGCCAGTGAGCTTCCCGTTCAGC-3′
4.6. Western Blot
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Zymography
4.9. Teasing, Hystological Analysis, and Immunostaining of Sciatic Nerves
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomez-Sanchez, J.A.; Carty, L.; Iruarrizaga-Lejarreta, M.; Palomo-Irigoyen, M.; Varela-Rey, M.; Griffith, M.; Hantke, J.; Macias-Camara, N.; Azkargorta, M.; Aurrekoetxea, I.; et al. Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J. Cell. Biol. 2015, 210, 153–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessen, K.R.; Mirsky, R. The Success and Failure of the Schwann Cell Response to Nerve Injury. Front. Cell. Neurosci. 2019, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Piao, X.; Bonaldo, P. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol. 2015, 130, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Augusti-Tocco, G.; Biagioni, S.; Tata, A.M. Acetylcholine and regulation of gene expression in developing systems. J. Mol. Neurosci. 2006, 30, 45–48. [Google Scholar] [CrossRef]
- Magnaghi, V.; Procacci, P.; Tata, A.M. Chapter 15: Novel pharmacological approaches to Schwann cells as neuroprotective agents for peripheral nerve regeneration. Int. Rev. Neurobiol. 2009, 87, 295–315. [Google Scholar]
- Fields, R.D. Release of neurotransmitters from glia. Neuron Glia Biol. 2010, 6, 137–139. [Google Scholar] [CrossRef] [Green Version]
- Loreti, S.; Vilaró, M.T.; Visentin, S.; Rees, H.; Levey, A.I.; Tata, A.M. Rat Schwann cells express M1-M4 muscarinic receptor subtypes. J. Neurosci. Res. 2006, 84, 97–105. [Google Scholar] [CrossRef]
- Loreti, S.; Ricordy, R.; De Stefano, M.E.; Augusti-Tocco, G.; Tata, A.M. Acetylcholine inhibits cell cycle progression in rat Schwann cells by activation of the M2 receptor subtype. Neuron Glia Biol. 2007, 3, 269–279. [Google Scholar] [CrossRef]
- Uggenti, C.; De Stefano, M.E.; Costantino, M.; Loreti, S.; Pisano, A.; Avallone, B.; Talora, C.; Magnaghi, V.; Tata, A.M. M2 muscarinic receptor activation regulates Schwann cell differentiation and myelin organization. Dev. Neurobiol. 2014, 74, 676–691. [Google Scholar] [CrossRef]
- Piovesana, R.; Faroni, A.; Tata, A.M.; Reid, A.J. Functional Characterization of Muscarinic Receptors in Human Schwann Cells. Int. J. Mol. Sci. 2020, 21, 6666. [Google Scholar] [CrossRef]
- Piovesana, R.; Faroni, A.; Taggi, M.; Matera, A.; Soligo, M.; Canipari, R.; Manni, L.; Reid, A.J.; Tata, A.M. Muscarinic receptors modulate Nerve Growth Factor production in rat Schwann-like adipose-derived stem cells and in Schwann cells. Sci. Rep. 2020, 10, 7159. [Google Scholar] [CrossRef]
- Hoover, D.B. Cholinergic modulation of the immune system presents new approaches for treating inflammation. Pharmacol. Ther. 2017, 179, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Martelli, D.; McKinley, M.J.; McAllen, R.M. The cholinergic anti-inflammatory pathway: A critical review. Auton. Neurosci. 2014, 182, 65–69. [Google Scholar] [CrossRef]
- Piovesana, R.; Salazar Intriago, M.S.; Dini, L.; Tata, A.M. Cholinergic Modulation of Neuroinflammation: Focus on α7 Nicotinic Receptor. Int. J. Mol. Sci. 2021, 22, 4912. [Google Scholar] [CrossRef] [PubMed]
- Dallanoce, C.; Frigerio, F.; Martelli, G.; Grazioso, G.; Matera, C.; Pomè, D.Y.; Pucci, L.; Clementi, F.; Gotti, C.; De Amici, M. Novel tricyclic Δ2-isoxazoline and 3-oxo-2-methyl-isoxazolidine derivatives: Synthesis and binding affinity at neuronal nicotinic acetylcholine receptor subtypes. Bioorg. Med. Chem. 2010, 18, 4498–4508. [Google Scholar] [CrossRef]
- Dallanoce, C.; Magrone, P.; Matera, C.; Lo Presti, L.; De Amici, M.; Riganti, L.; Clementi, F.; Gotti, C.; De Micheli, C. Synthesis of novel chiral Δ2-isoxazoline derivatives related to ABT-418 and estimation of their affinity at neuronal nicotinic acetylcholine receptor subtypes. Eur. J. Med. Chem. 2010, 45, 5594–5601. [Google Scholar] [CrossRef] [PubMed]
- Dallanoce, C.; Frigerio, F.; Grazioso, G.; Matera, C.; Visconti, G.L.; De Amici, M.; Pucci, L.; Pistillo, F.; Fucile, S.; Gotti, C.; et al. New spirocyclic Δ2-isoxazoline derivatives related to selective agonists of α7 neuronal nicotinic acetylcholine receptors. Eur. J. Med. Chem. 2011, 46, 5790–5799. [Google Scholar] [CrossRef]
- Dallanoce, C.; Magrone, P.; Matera, C.; Frigerio, F.; Grazioso, G.; De Amici, M.; Fucile, S.; Piccari, V.; Frydenvang, K.; Pucci, L.; et al. Design, synthesis, and pharmacological characterization of novel spirocyclic quinuclidinyl-Δ2-isoxazoline derivatives as potent and selective agonists of α7 nicotinic acetylcholine receptors. ChemMedChem 2011, 6, 889–903. [Google Scholar] [CrossRef]
- Dallanoce, C.; Matera, C.; Pucci, L.; Gotti, C.; Clementi, F.; Amici, M.D.; Micheli, C.D. Synthesis and binding affinity at α4β2 and α7 nicotinic acetylcholine receptors of new analogs of epibatidine and epiboxidine containing the 7-azabicyclo[2.2.1]hept-2-ene ring system. Bioorg. Med. Chem. Lett. 2012, 22, 829–832. [Google Scholar] [CrossRef]
- Grazioso, G.; Sgrignani, J.; Capelli, R.; Matera, C.; Dallanoce, C.; De Amici, M.; Cavalli, A. Allosteric Modulation of Alpha7 Nicotinic Receptors: Mechanistic Insight through Metadynamics and Essential Dynamics. J. Chem. Inf. Model. 2015, 55, 2528–2539. [Google Scholar] [CrossRef]
- Quadri, M.; Matera, C.; Silnović, A.; Pismataro, M.C.; Horenstein, N.A.; Stokes, C.; Papke, R.L.; Dallanoce, C. Identification of α7 Nicotinic Acetylcholine Receptor Silent Agonists Based on the Spirocyclic Quinuclidine-Δ2-Isoxazoline Scaffold: Synthesis and Electrophysiological Evaluation. ChemMedChem 2017, 12, 1335–1348. [Google Scholar] [CrossRef] [PubMed]
- Quadri, M.; Silnović, A.; Matera, C.; Horenstein, N.A.; Stokes, C.; De Amici, M.; Papke, R.L.; Dallanoce, C. Novel 5-(quinuclidin-3-ylmethyl)-1,2,4-oxadiazoles to investigate the activation of the α7 nicotinic acetylcholine receptor subtype: Synthesis and electrophysiological evaluation. Eur. J. Med. Chem. 2018, 160, 207–228. [Google Scholar] [CrossRef]
- Pismataro, M.C.; Horenstein, N.A.; Stokes, C.; Quadri, M.; De Amici, M.; Papke, R.L.; Dallanoce, C. Design, synthesis, and electrophysiological evaluation of NS6740 derivatives: Exploration of the structure-activity relationship for alpha7 nicotinic acetylcholine receptor silent activation. Eur. J. Med. Chem. 2020, 205, 112669. [Google Scholar] [CrossRef] [PubMed]
- Pismataro, M.C.; Horenstein, N.A.; Stokes, C.; Dallanoce, C.; Thakur, G.A.; Papke, R.L. Stable desensitization of α7 nicotinic acetylcholine receptors by NS6740 requires interaction with S36 in the orthosteric agonist binding site. Eur. J. Pharmacol. 2021, 905, 174179. [Google Scholar] [CrossRef]
- De Jaco, A.; Bernardini, L.; Rosati, J.; Tata, A.M. Alpha-7 Nicotinic Receptors in Nervous System Disorders: From Function to Therapeutic Perspectives. Cent. Nerv. Syst. Agents Med. Chem. 2017, 17, 100–108. [Google Scholar] [CrossRef]
- Pernarella, M.; Piovesana, R.; Matera, C.; Faroni, A.; Fiore, M.; Dini, L.; Reid, A.J.; Dallanoce, C.; Tata, A.M. Effects mediated by the α7 nicotinic acetylcholine receptor on cell proliferation and migration in rat adipose-derived stem cells. Eur. J. Histochem. 2020, 64, 3159. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Pacini, A.; Matera, C.; Zanardelli, M.; Mello, T.; De Amici, M.; Dallanoce, C.; Ghelardini, C. Involvement of α7 nAChR subtype in rat oxaliplatin-induced neuropathy: Effects of selective activation. Neuropharmacology 2014, 79, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Matera, C.; Dondio, G.; Braida, D.; Ponzoni, L.; De Amici, M.; Sala, M.; Dallanoce, C. In vivo and in vitro ADMET profiling and in vivo pharmacodynamic investigations of a selective α7 nicotinic acetylcholine receptor agonist with a spirocyclic Δ2-isoxazoline molecular skeleton. Eur. J. Pharmacol. 2018, 820, 265–273. [Google Scholar] [CrossRef]
- Scabia, G.; Cancello, R.; Dallanoce, C.; Berger, S.; Matera, C.; Dattilo, A.; Zulian, A.; Barone, I.; Ceccarini, G.; Santini, F.; et al. ICH3, a selective alpha7 nicotinic acetylcholine receptor agonist, modulates adipocyte inflammation associated with obesity. J. Endocrinol. Investig. 2020, 43, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Hone, A.J.; McIntosh, J.M. Nicotinic acetylcholine receptors in neuropathic and inflammatory pain. FEBS Lett. 2018, 592, 1045–1062. [Google Scholar] [CrossRef] [Green Version]
- Arthur-Farraj, P.J.; Latouche, M.; Wilton, D.K.; Quintes, S.; Chabrol, E.; Banerjee, A.; Woodhoo, A.; Jenkins, B.; Rahman, M.; Turmaine, M.; et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 2012, 75, 633–647. [Google Scholar] [CrossRef] [Green Version]
- Martini, R.; Fischer, S.; López-Vales, R.; David, S. Interactions between Schwann cells and macrophages in injury and inherited demyelinating disease. Glia 2008, 56, 1566–1577. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R.; Lloyd, A.C. Schwann Cells: Development and Role in Nerve Repair. Cold Spring Harb. Perspect. Biol. 2015, 7, a020487. [Google Scholar] [CrossRef] [PubMed]
- Salani, M.; Anelli, T.; Tocco, G.A.; Lucarini, E.; Mozzetta, C.; Poiana, G.; Tata, A.M.; Biagioni, S. Acetylcholine-induced neuronal differentiation: Muscarinic receptor activation regulates EGR-1 and REST expression in neuroblastoma cells. J. Neurochem. 2009, 108, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Biagioni, S.; Tata, A.M.; De Jaco, A.; Augusti-Tocco, G. Acetylcholine synthesis and neuron differentiation. Int. J. Dev. Biol. 2000, 44, 689–697. [Google Scholar] [PubMed]
- Verdiyan, E.E.; Allakhverdiev, E.S.; Maksimov, G.V. Study of the Peripheral Nerve Fibers Myelin Structure Changes during Activation of Schwann Cell Acetylcholine Receptors. PLoS ONE 2016, 11, e0158083. [Google Scholar] [CrossRef] [Green Version]
- Corsetti, V.; Perrone-Capano, C.; Salazar Intriago, M.S.; Botticelli, E.; Poiana, G.; Augusti-Tocco, G.; Biagioni, S.; Tata, A.M. Expression of Cholinergic Markers and Characterization of Splice Variants during Ontogenesis of Rat Dorsal Root Ganglia Neurons. Int. J. Mol. Sci. 2021, 22, 5499. [Google Scholar] [CrossRef]
- Marinelli, S.; Vacca, V.; Ricordy, R.; Uggenti, C.; Tata, A.M.; Luvisetto, S.; Pavone, F. The analgesic effect on neuropathic pain of retrogradely transported botulinum neurotoxin A involves Schwann cells and astrocytes. PLoS ONE 2012, 7, e47977. [Google Scholar] [CrossRef] [Green Version]
- Fujii, T.; Mashimo, M.; Moriwaki, Y.; Misawa, H.; Ono, S.; Horiguchi, K.; Kawashima, K. Expression and Function of the Cholinergic System in Immune Cells. Front. Immunol. 2017, 8, 1085. [Google Scholar] [CrossRef] [Green Version]
- Nizri, E.; Irony-Tur-Sinai, M.; Lory, O.; Orr-Urtreger, A.; Lavi, E.; Brenner, T. Activation of the cholinergic anti-inflammatory system by nicotine attenuates neuroinflammation via suppression of Th1 and Th17 responses. J. Immunol. 2009, 183, 6681–6688. [Google Scholar] [CrossRef] [Green Version]
- Nizri, E.; Brenner, T. Modulation of inflammatory pathways by the immune cholinergic system. Amino Acids 2013, 45, 73–85. [Google Scholar] [CrossRef]
- Song, I.; Dityatev, A. Crosstalk between glia, extracellular matrix and neurons. Brain Res. Bull. 2018, 136, 101–108. [Google Scholar] [CrossRef]
- Pellegatta, M.; Taveggia, C. The Complex Work of Proteases and Secretases in Wallerian Degeneration: Beyond Neuregulin-1. Front. Cell. Neurosci. 2019, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Mizrachi, T.; Marsha, O.; Brusin, K.; Ben-David, Y.; Thakur, G.A.; Vaknin-Dembinsky, A.; Treinin, M.; Brenner, T. Suppression of neuroinflammation by an allosteric agonist and positive allosteric modulator of the α7 nicotinic acetylcholine receptor GAT107. J. Neuroinflamm. 2021, 18, 99. [Google Scholar] [CrossRef] [PubMed]
- Maroli, A.; Di Lascio, S.; Drufuca, L.; Cardani, S.; Setten, E.; Locati, M.; Fornasari, D.; Benfante, R. Effect of donepezil on the expression and responsiveness to LPS of CHRNA7 and CHRFAM7A in macrophages: A possible link to the cholinergic anti-inflammatory pathway. J. Neuroimmunol. 2019, 332, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-J.; Liu, Y.-F.; Wang, Q.-Y.; Tsuruoka, M.; Ohta, K.; Wu, S.-X.; Yakushiji, M.; Inoue, T. Functional expression of alpha 7 nicotinic acetylcholine receptors in human periodontal ligament fibroblasts and rat periodontal tissues. Cell Tissue Res. 2010, 340, 347–355. [Google Scholar] [CrossRef]
- Brockes, J.P. The nerve dependence of amphibian limb regeneration. J. Exp. Biol. 1987, 132, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.B.; Stroobant, P. Platelet-derived growth factors and fibroblast growth factors are mitogens for rat Schwann cells. J. Cell Biol. 1990, 110, 1353–1360. [Google Scholar] [CrossRef] [Green Version]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Pompili, E.; Ciraci, V.; Leone, S.; De Franchis, V.; Familiari, P.; Matassa, R.; Familiari, G.; Tata, A.M.; Fumagalli, L.; Fabrizi, C. Thrombin regulates the ability of Schwann cells to support neuritogenesis and to maintain the integrity of the nodes of Ranvier. Eur. J. Histochem. 2020, 64, 3109. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar Intriago, M.S.; Piovesana, R.; Matera, A.; Taggi, M.; Canipari, R.; Fabrizi, C.; Papotto, C.; Matera, C.; De Amici, M.; Dallanoce, C.; et al. The Mechanisms Mediated by α7 Acetylcholine Nicotinic Receptors May Contribute to Peripheral Nerve Regeneration. Molecules 2021, 26, 7668. https://doi.org/10.3390/molecules26247668
Salazar Intriago MS, Piovesana R, Matera A, Taggi M, Canipari R, Fabrizi C, Papotto C, Matera C, De Amici M, Dallanoce C, et al. The Mechanisms Mediated by α7 Acetylcholine Nicotinic Receptors May Contribute to Peripheral Nerve Regeneration. Molecules. 2021; 26(24):7668. https://doi.org/10.3390/molecules26247668
Chicago/Turabian StyleSalazar Intriago, Michael Sebastian, Roberta Piovesana, Alessandro Matera, Marilena Taggi, Rita Canipari, Cinzia Fabrizi, Claudio Papotto, Carlo Matera, Marco De Amici, Clelia Dallanoce, and et al. 2021. "The Mechanisms Mediated by α7 Acetylcholine Nicotinic Receptors May Contribute to Peripheral Nerve Regeneration" Molecules 26, no. 24: 7668. https://doi.org/10.3390/molecules26247668
APA StyleSalazar Intriago, M. S., Piovesana, R., Matera, A., Taggi, M., Canipari, R., Fabrizi, C., Papotto, C., Matera, C., De Amici, M., Dallanoce, C., & Tata, A. M. (2021). The Mechanisms Mediated by α7 Acetylcholine Nicotinic Receptors May Contribute to Peripheral Nerve Regeneration. Molecules, 26(24), 7668. https://doi.org/10.3390/molecules26247668








