Carpachromene Ameliorates Insulin Resistance in HepG2 Cells via Modulating IR/IRS1/PI3k/Akt/GSK3/FoxO1 Pathway
Abstract
:1. Introduction
2. Results
2.1. Insulin Resistant Model (IRM) Development
2.2. Cell Viability Assay
2.3. Effect of Carpachromene on Glucose Concentration
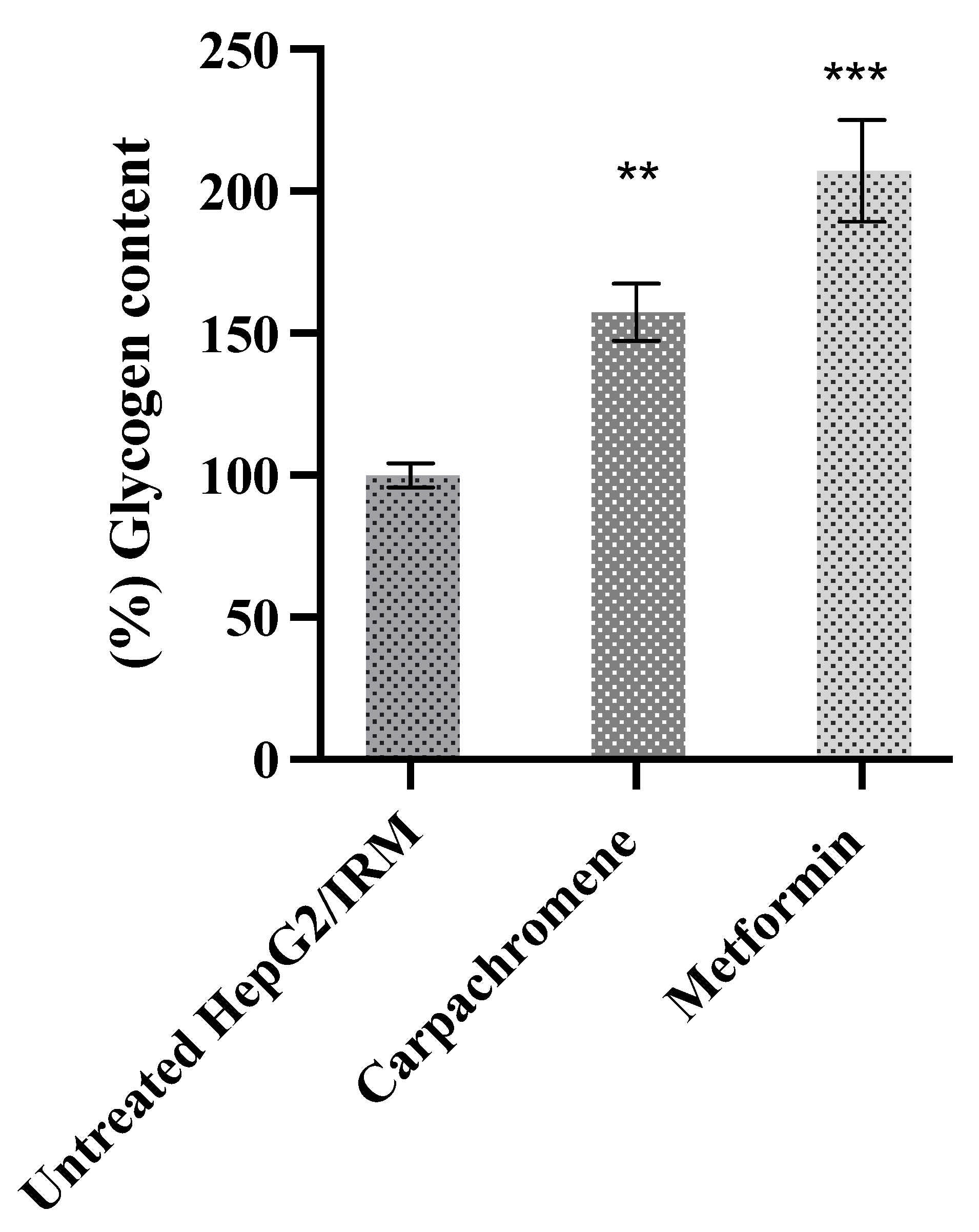
2.4. Carpachromene Improved Cellular Glycogen Synthesis in HepG2/IRM Cells
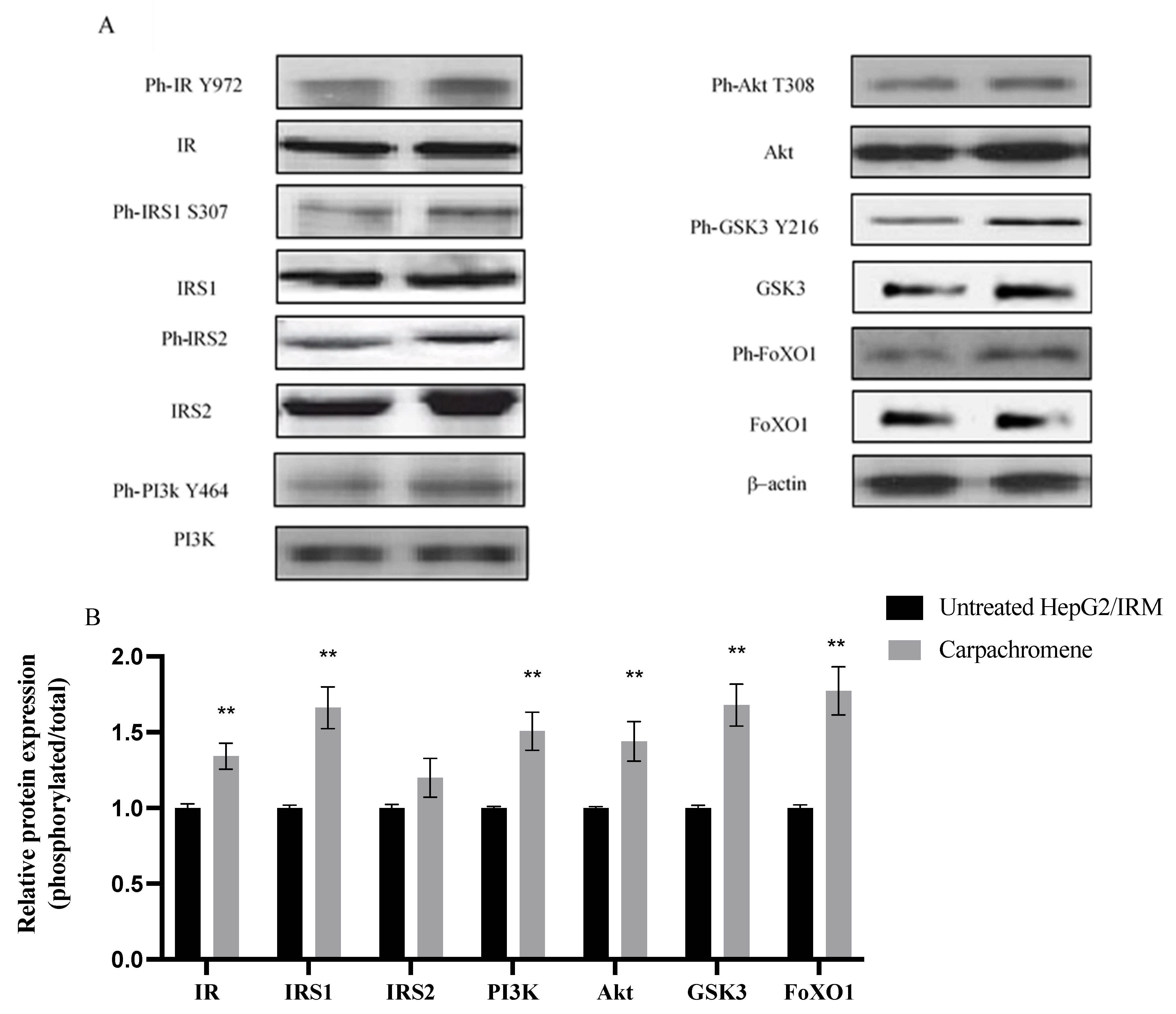
2.5. Carpachromene Modulated the Expression of Proteins Involved in Insulin Signaling
2.6. Carpachromene Modulated Hepatic Enzymes Activity
3. Discussion
4. Materials and Methods
4.1. Isolation of Carpachromene
4.2. Cell Culture and Induction of Insulin Resistant HepG2 Cell Model
4.3. Cell Viability Assay
4.4. Carpachromene Effect on Glucose Concentration in HepG2/IRM
4.5. Determination of Intracellular Glycogen Content
4.6. Analysis of Protein Expression
4.7. Effect of Carpachromene on Hepatic Enzymes
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability Statement
References
- James, D.E.; Stöckli, J.; Birnbaum, M.J. The aetiology and molecular landscape of insulin resistance. Nat. Rev. Mol. Cell Biol. 2021, 22, 751–771. [Google Scholar] [CrossRef]
- Abdel-Hamid, N.; Fathy, M.; Amgad, S.W. Glycoregulatory Enzymes as Early Diagnostic Markers during Premalignant Stage in Hepatocellular Carcinoma. Am. J. Cancer Prev. 2013, 1, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Hammarstedt, A.; Gogg, S.; Hedjazifar, S.; Nerstedt, A.; Smith, U. Impaired adipogenesis and dysfunctional adipose tissue in human hypertrophic obesity. Physiol. Rev. 2018, 98, 1911–1941. [Google Scholar] [CrossRef]
- Boucher, J.; Kleinridders, A.; Kahn, C. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.C.; Copps, K.D.; Guo, S.; Li, Y.; Kollipara, R.; DePinho, R.A.; White, M.F. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008, 8, 65–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fathy, M.; Awale, S.; Nikaido, T. Phosphorylated Akt Protein at Ser473 Enables HeLa Cells to Tolerate Nutrient-Deprived Conditions. Asian Pac. J. Cancer Prev. 2017, 18, 3255–3260. [Google Scholar] [CrossRef] [PubMed]
- Melvin, A.; O’Rahilly, S.; Savage, D. Genetic syndromes of severe insulin resistance. Curr. Opin. Genet. Dev. 2018, 50, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Teng, W.; Yin, W.; Zhao, L.; Ma, C.; Huang, J.; Ren, F. Resveratrol metabolites ameliorate insulin resistance in HepG2 hepatocytes by modulating IRS-1/AMPK. RSC Adv. 2018, 8, 36034–36042. [Google Scholar] [CrossRef] [Green Version]
- Axelsson, A.S.; Tubbs, E.; Mecham, B.; Chacko, S.; Nenonen, H.A.; Tang, Y.; Fahey, J.W.; Derry, J.M.; Wollheim, C.B.; Wierup, N. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, H.A.; Allam, A.E.; Abu-Baih, D.H.; Mohamed, M.F.A.; Abdelmohsen, U.R.; Shimizu, K.; Desoukey, S.Y.; Hayallah, A.M.; Elrehany, M.A.; Mohamed, K.M.; et al. Isolation and characterization of novel acetylcholinesterase inhibitors from Ficus benghalensis L. leaves. RSC Adv. 2020, 10, 36920–36929. [Google Scholar] [CrossRef]
- Tajuddeen, N.; Van Heerden, F.R. Antiplasmodial natural products: An update. Malar. J. 2019, 18, 404. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Wang, J.; Zhang, C.; Yu, Y.; Cai, H. Proapoptotic Protein Smac Mediates Apoptosis in Ovarian Cancer Cells When Treated with the Carpachromene. Arch. Med. Sci. 2021. [Google Scholar] [CrossRef]
- Chen, L.W.; Cheng, M.J.; Peng, C.F.; Chen, I.S. Secondary metabolites and antimycobacterial activities from the roots of Ficus nervosa. Chem. Biodivers. 2010, 7, 1814–1821. [Google Scholar] [CrossRef]
- Zheng, Z.P.; Cheng, K.W.; To, J.T.; Li, H.; Wang, M. Isolation of tyrosinase inhibitors from Artocarpus heterophyllus and use of its extract as antibrowning agent. Mol. Nutr. Food Res. 2008, 52, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Alaaeldin, R.; Abuo-Rahma, G.E.A.; Zhao, Q.L.; Fathy, M. Modulation of Apoptosis and Epithelial-Mesenchymal Transition E-cadherin/TGF-beta/Snail/TWIST Pathways by a New Ciprofloxacin Chalcone in Breast Cancer Cells. Anticancer Res. 2021, 41, 2383–2395. [Google Scholar] [CrossRef]
- Alaaeldin, R.; Mustafa, M.; Abuo-Rahma, G.E.A.; Fathy, M. In vitro inhibition and molecular docking of a new ciprofloxacin chalcone against SARS-CoV-2 main protease. Fundam. Clin. Pharmacol. 2021, 1–11. [Google Scholar] [CrossRef]
- Alaaeldin, R.; Nazmy, M.H.; Abdel-Aziz, M.; Abuo-Rahma, G.E.A.; Fathy, M. Cell Cycle Arrest and Apoptotic Effect of 7-(4-(N-substituted carbamoylmethyl) piperazin-1-yl) Ciprofloxacin-derivative on HCT 116 and A549 Cancer Cells. Anticancer Res. 2020, 40, 2739–2749. [Google Scholar] [CrossRef]
- Eisa, M.A.; Fathy, M.; Abuo-Rahma, G.; Abdel-Aziz, M.; Nazmy, M.H. Anti-Proliferative and Pro-Apoptotic Activities of Synthesized 3,4,5 Tri-Methoxy Ciprofloxacin Chalcone Hybrid, through p53 Up-Regulation in HepG2 and MCF7 Cell Lines. Asian Pac. J. Cancer Prev. 2021, 22, 3393–3404. [Google Scholar] [CrossRef]
- Fathy, M.; Sun, S.; Zhao, Q.-L.; Abdel-Aziz, M.; Abuo-Rahma, G.E.-D.A.; Awale, S.; Nikaido, T. A New Ciprofloxacin-derivative Inhibits Proliferation and Suppresses the Migration Ability of HeLa Cells. Anticancer Res. 2020, 40, 5025–5033. [Google Scholar] [CrossRef]
- Oba, J.; Okabe, M.; Yoshida, T.; Soko, C.; Fathy, M.; Amano, K.; Kobashi, D.; Wakasugi, M.; Okudera, H. Hyperdry human amniotic membrane application as a wound dressing for a full-thickness skin excision after a third-degree burn injury. Burn. Trauma 2020, 8, tkaa014. [Google Scholar] [CrossRef]
- Okabe, M.; Yoshida, T.; Suzuki, M.; Goto, M.; Omori, M.; Taguchi, M.; Toda, A.; Suzuki, T.; Nakagawa, K.; Hiramoto, F.; et al. Hyperdry Human Amniotic Membrane (HD-AM) is Supporting Aciclovir Included Device of Poly-N-p-Vinylbenzyl-D-Lactonamide (PVLA) Sphere for Treatment of HSV-1 Infected Rabbit Keratitis Model. J. Biotechnol. Biomater. 2017, 7, 251. [Google Scholar] [CrossRef] [Green Version]
- Fawzy, M.A.; Maher, S.A.; Bakkar, S.M.; El-Rehany, M.A.; Fathy, M. Pantoprazole Attenuates MAPK (ERK1/2, JNK, p38)-NF-kappaB and Apoptosis Signaling Pathways after Renal Ischemia/Reperfusion Injury in Rats. Int. J. Mol. Sci. 2021, 22, 10669. [Google Scholar] [CrossRef]
- Aminudin, N.I.; Ahmad, F.; Taher, M.; Zulkifli, R.M. alpha-Glucosidase and 15-Lipoxygenase Inhibitory Activities of Phytochemicals from Calophyllum symingtonianum. Nat. Prod. Commun. 2015, 10, 1585–1587. [Google Scholar]
- Dewi, K.; Widyarto, B.; Erawijantari, P.P.; Widowati, W. In vitro study of Myristica fragrans seed (Nutmeg) ethanolic extract and quercetin compound as anti-inflammatory agent. Int. J. Res. Med. Sci. 2015, 3, 2303–2310. [Google Scholar] [CrossRef] [Green Version]
- Molinaro, A.; Becattini, B.; Mazzoli, A.; Bleve, A.; Radici, L.; Maxvall, I.; Sopasakis, V.R.; Molinaro, A.; Bäckhed, F.; Solinas, G. Insulin-driven PI3K-AKT signaling in the hepatocyte is mediated by redundant PI3Kα and PI3Kβ activities and is promoted by RAS. Cell Metab. 2019, 29, 1400–1409.e1405. [Google Scholar] [CrossRef] [PubMed]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, L.; Teng, H.; Song, H.; Wu, X.; Xu, M. Phenolic compounds ameliorate the glucose uptake in HepG2 cells’ insulin resistance via activating AMPK: Anti-diabetic effect of phenolic compounds in HepG2 cells. J. Funct. Foods 2015, 19, 487–494. [Google Scholar] [CrossRef]
- Sagbo, I.J.; van de Venter, M.; Koekemoer, T.; Bradley, G. In vitro antidiabetic activity and mechanism of action of Brachylaena elliptica (Thunb.) DC. Evid.-Based Complement. Altern. Med. 2018, 2018, 4170372. [Google Scholar] [CrossRef] [Green Version]
- Sefried, S.; Häring, H.-U.; Weigert, C.; Eckstein, S.S. Suitability of hepatocyte cell lines HepG2, AML12 and THLE-2 for investigation of insulin signalling and hepatokine gene expression. R. Soc. Open Biol. 2018, 8, 180147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Li, L.; Li, J.; Cheng, Y.; Chen, J.; Shen, M.; Zhang, S.; Wei, H. Insulin resistance contributes to multidrug resistance in HepG2 cells via activation of the PERK signaling pathway and upregulation of Bcl-2 and P-gp. Oncol. Rep. 2016, 35, 3018–3024. [Google Scholar] [CrossRef] [Green Version]
- Fathy, M.; Fawzy, M.A.; Hintzsche, H.; Nikaido, T.; Dandekar, T.; Othman, E.M. Eugenol exerts apoptotic effect and modulates the sensitivity of HeLa cells to cisplatin and radiation. Molecules 2019, 24, 3979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naseem, M.; Othman, E.M.; Fathy, M.; Iqbal, J.; Howari, F.M.; AlRemeithi, F.A.; Kodandaraman, G.; Stopper, H.; Bencurova, E.; Vlachakis, D.; et al. Integrated structural and functional analysis of the protective effects of kinetin against oxidative stress in mammalian cellular systems. Sci. Rep. 2020, 10, 13330. [Google Scholar] [CrossRef]
- Fathy, M.; Khalifa, E.; Fawzy, M.A. Modulation of inducible nitric oxide synthase pathway by eugenol and telmisartan in carbon tetrachloride-induced liver injury in rats. Life Sci. 2019, 216, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Fathy, M.; Nikaido, T. In vivo modulation of iNOS pathway in hepatocellular carcinoma by Nigella sativa. Environ. Health Prev. Med. 2013, 18, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Abdellatef, A.A.; Fathy, M.; Mohammed, A.E.I.; Bakr, M.S.A.; Ahmed, A.H.; Abbass, H.S.; El-Desoky, A.H.; Morita, H.; Nikaido, T.; Hayakawa, Y. Inhibition of cell-intrinsic NF-kappaB activity and metastatic abilities of breast cancer by aloe-emodin and emodic-acid isolated from Asphodelus microcarpus. J. Nat. Med. 2021, 75, 840–853. [Google Scholar] [CrossRef] [PubMed]
- Fathy, M.; Okabe, M.; Othman, E.M.; Saad Eldien, H.M.; Yoshida, T. Preconditioning of Adipose-Derived Mesenchymal Stem-Like Cells with Eugenol Potentiates Their Migration and Proliferation In Vitro and Therapeutic Abilities in Rat Hepatic Fibrosis. Molecules 2020, 25, 2020. [Google Scholar] [CrossRef]
- Fathy, M.; Okabe, M.; Saad Eldien, H.M.; Yoshida, T. AT-MSCs Antifibrotic Activity is Improved by Eugenol through Modulation of TGF-beta/Smad Signaling Pathway in Rats. Molecules 2020, 25, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fathy, M.; Nikaido, T. In vivo attenuation of angiogenesis in hepatocellular carcinoma by Nigella sativa. Turk. J. Med. Sci. 2018, 48, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Othman, E.M.; Fathy, M.; Bekhit, A.A.; Abdel-Razik, A.H.; Jamal, A.; Nazzal, Y.; Shams, S.; Dandekar, T.; Naseem, M. Modulatory and Toxicological Perspectives on the Effects of the Small Molecule Kinetin. Molecules 2021, 26, 670. [Google Scholar] [CrossRef]
- Bian, G.; Yang, J.; Elango, J.; Wu, W.; Bao, B.; Bao, C. Natural Triterpenoids Isolated from Akebia trifoliata Stem Explants Exert a Hypoglycemic Effect via α-Glucosidase Inhibition and Glucose Uptake Stimulation in Insulin-Resistant HepG2 Cells. Chem. Biodivers. 2021, 18, e2001030. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, W.; Zhang, J.; Xie, M.; Wang, X. Baicalein improves glucose metabolism in insulin resistant HepG2 cells. Eur. J. Pharmacol. 2019, 854, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ding, X.; Jian, T.; Lü, H.; Zhao, L.; Li, J.; Liu, Y.; Ren, B.; Chen, J. Four sesquiterpene glycosides from loquat (Eriobotrya japonica) leaf ameliorates palmitic acid-induced insulin resistance and lipid accumulation in HepG2 Cells via AMPK signaling pathway. PeerJ 2020, 8, e10413. [Google Scholar] [CrossRef] [PubMed]
- Sheu, Y.W.; Chiang, L.C.; Chen, I.S.; Chen, Y.C.; Tsai, I.L. Cytotoxic flavonoids and new chromenes from Ficus formosana f. formosana. Planta Med. 2005, 71, 1165–1167. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Monks, B.; Ge, Q.; Birnbaum, M.J. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1α transcription coactivator. Nature 2007, 447, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Prasad, A.K.; Parmar, V.S.; Ghosh, B.; Saini, N. Apoptogenic effect of 7,8-diacetoxy-4-methylcoumarin and 7,8-diacetoxy-4-methylthiocoumarin in human lung adenocarcinoma cell line: Role of NF-kappaB, Akt, ROS and MAP kinase pathway. Chem. Biol. Interact. 2009, 179, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Greenfield, E.A. Antibodies: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2013. [Google Scholar]
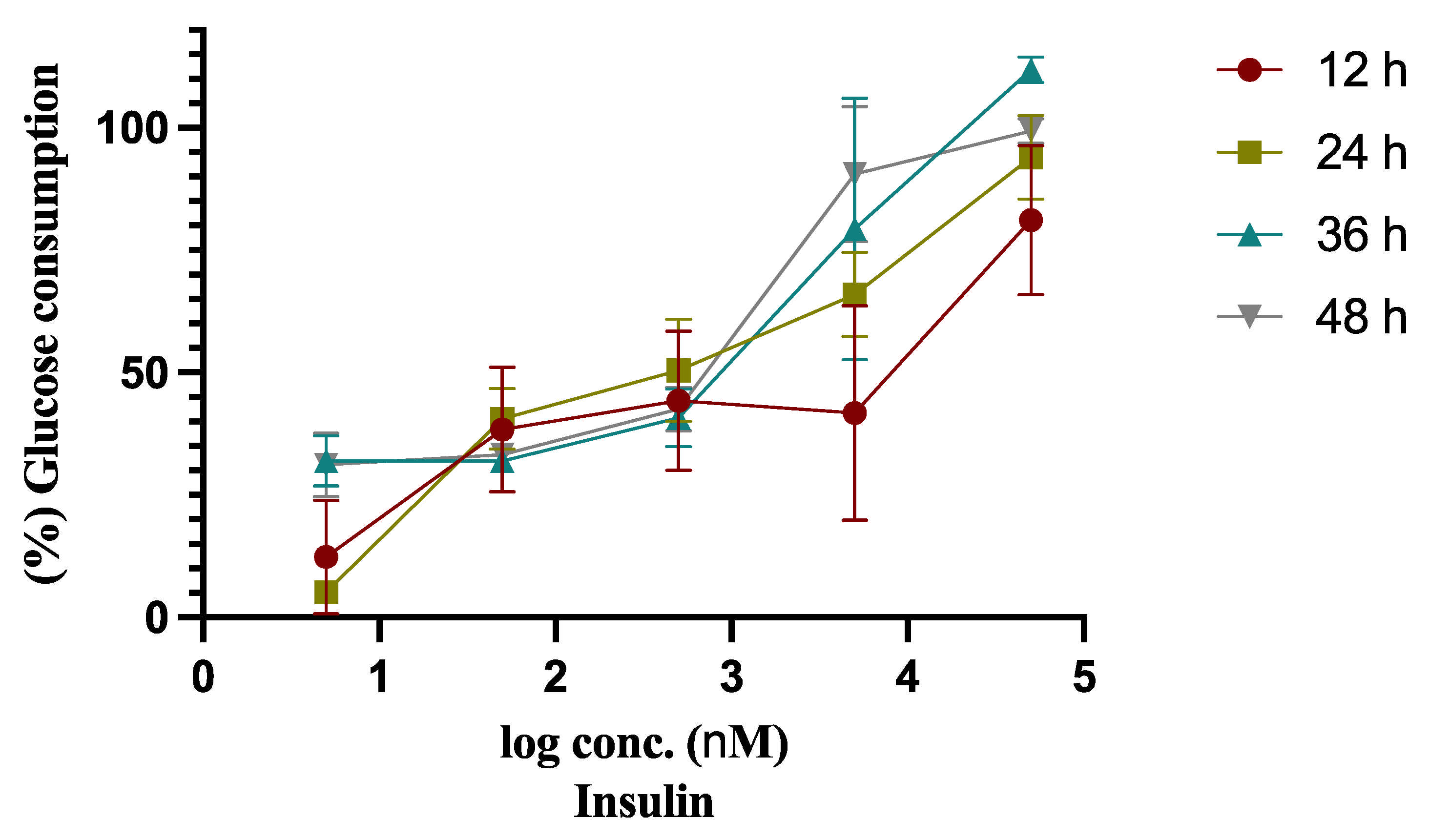




| Insulin Conc. (µM) | Glucose Consumption (mmol/L) | |||
|---|---|---|---|---|
| 12 h | 24 h | 36 h | 48 h | |
| - | 3.577 ± 0.199 | 4.441 ± 0.087 | 5.477 ± 0.045 | 6.200 ± 0.092 |
| 0.005 | 0.432 ± 0.406 *** | 0.229 ± 0.077 *** | 1.750 ± 0.270 *** | 1.932 ± 0.412 *** |
| 0.05 | 1.378 ± 0.477 *** | 1.803 ± 0.310 *** | 1.750 ± 0.026 *** | 2.062 ± 0.139 *** |
| 0.5 | 1.563 ± 0.445 *** | 2.248 ± 0.502 *** | 2.232 ± 0.310 *** | 2.636 ± 0.284 *** |
| 5 | 1.507 ± 0.841 *** | 2.933 ± 0.434 ** | 4.345 ± 1.462 * | 5.620 ± 0.923 |
| 50 | 2.916 ± 0.670 | 4.175 ± 0.430 | 6.124 ± 0.114 | 6.158 ± 0.094 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alaaeldin, R.; Abdel-Rahman, I.A.M.; Hassan, H.A.; Youssef, N.; Allam, A.E.; Abdelwahab, S.F.; Zhao, Q.-L.; Fathy, M. Carpachromene Ameliorates Insulin Resistance in HepG2 Cells via Modulating IR/IRS1/PI3k/Akt/GSK3/FoxO1 Pathway. Molecules 2021, 26, 7629. https://doi.org/10.3390/molecules26247629
Alaaeldin R, Abdel-Rahman IAM, Hassan HA, Youssef N, Allam AE, Abdelwahab SF, Zhao Q-L, Fathy M. Carpachromene Ameliorates Insulin Resistance in HepG2 Cells via Modulating IR/IRS1/PI3k/Akt/GSK3/FoxO1 Pathway. Molecules. 2021; 26(24):7629. https://doi.org/10.3390/molecules26247629
Chicago/Turabian StyleAlaaeldin, Rania, Iman A. M. Abdel-Rahman, Heba Ali Hassan, Nancy Youssef, Ahmed E. Allam, Sayed F. Abdelwahab, Qing-Li Zhao, and Moustafa Fathy. 2021. "Carpachromene Ameliorates Insulin Resistance in HepG2 Cells via Modulating IR/IRS1/PI3k/Akt/GSK3/FoxO1 Pathway" Molecules 26, no. 24: 7629. https://doi.org/10.3390/molecules26247629
APA StyleAlaaeldin, R., Abdel-Rahman, I. A. M., Hassan, H. A., Youssef, N., Allam, A. E., Abdelwahab, S. F., Zhao, Q.-L., & Fathy, M. (2021). Carpachromene Ameliorates Insulin Resistance in HepG2 Cells via Modulating IR/IRS1/PI3k/Akt/GSK3/FoxO1 Pathway. Molecules, 26(24), 7629. https://doi.org/10.3390/molecules26247629







