Zirconium-Based Metal Organic Frameworks for the Capture of Carbon Dioxide and Ethanol Vapour. A Comparative Study
Abstract
:1. Introduction
2. Results and Discussion
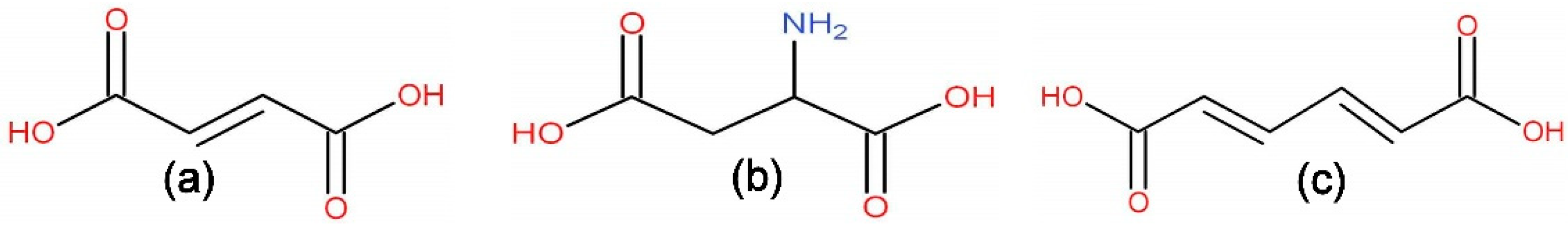
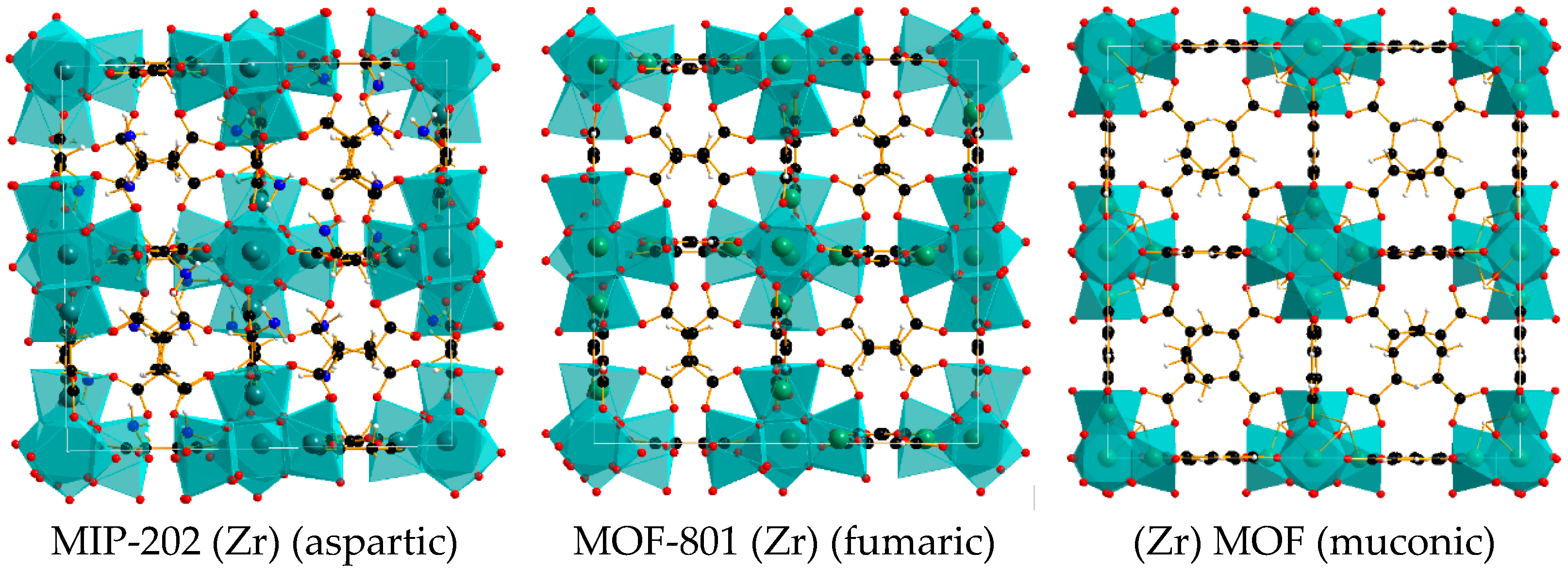
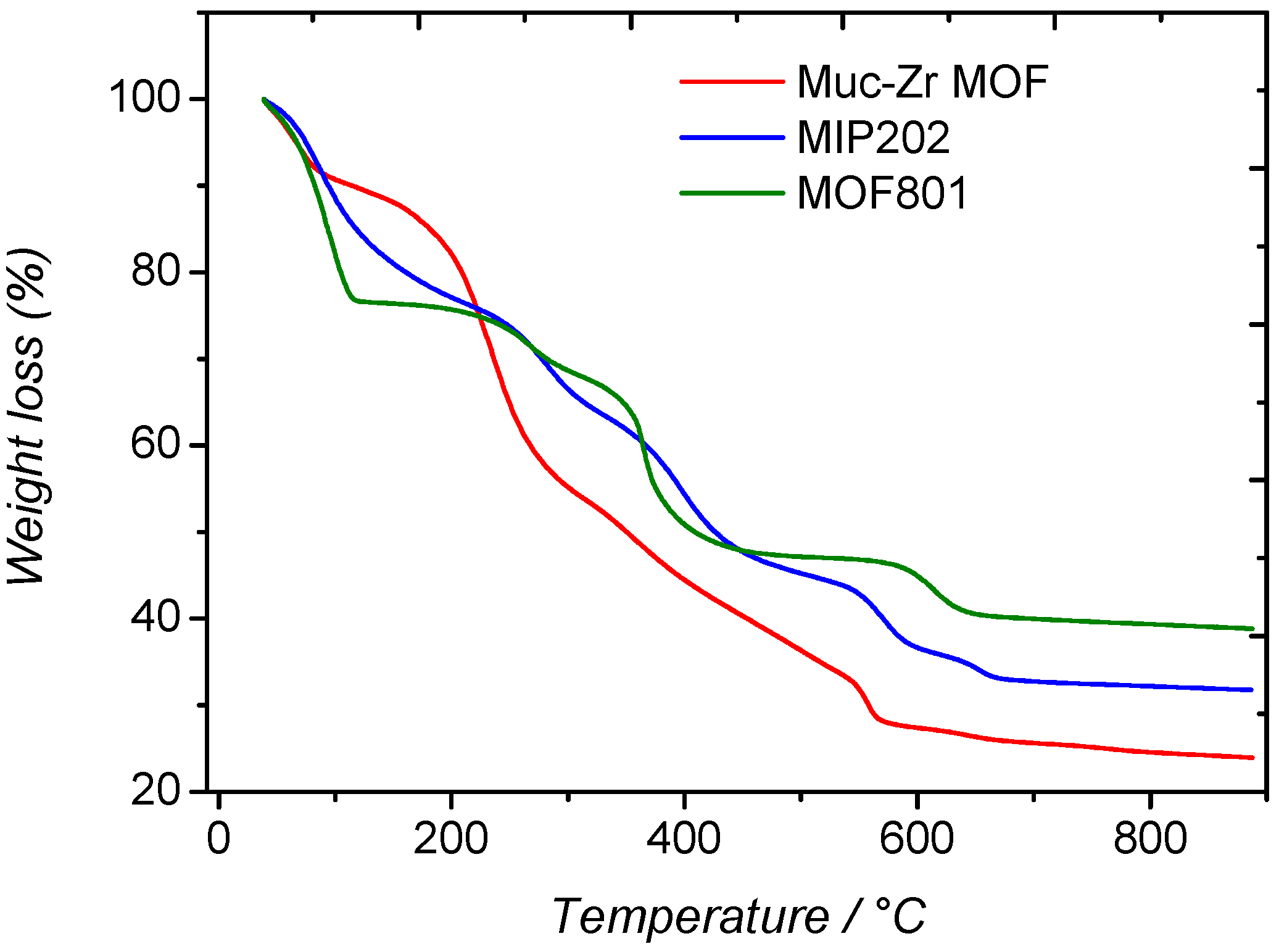
2.1. Characterization of MOFs
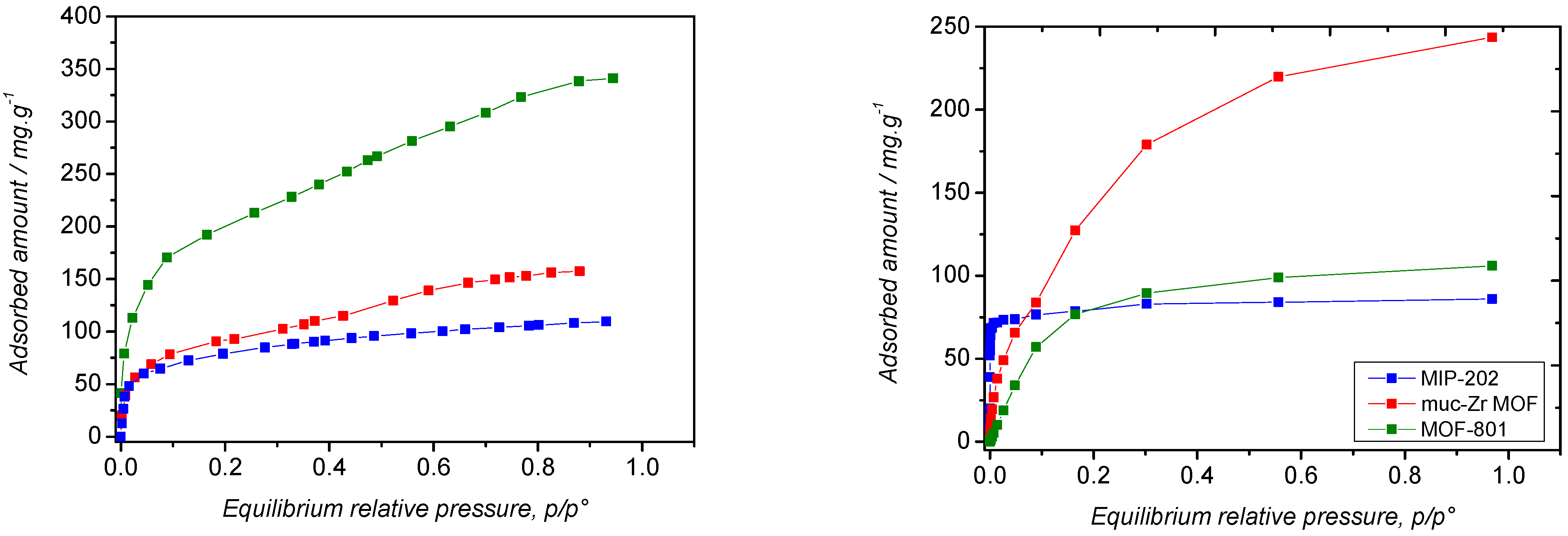
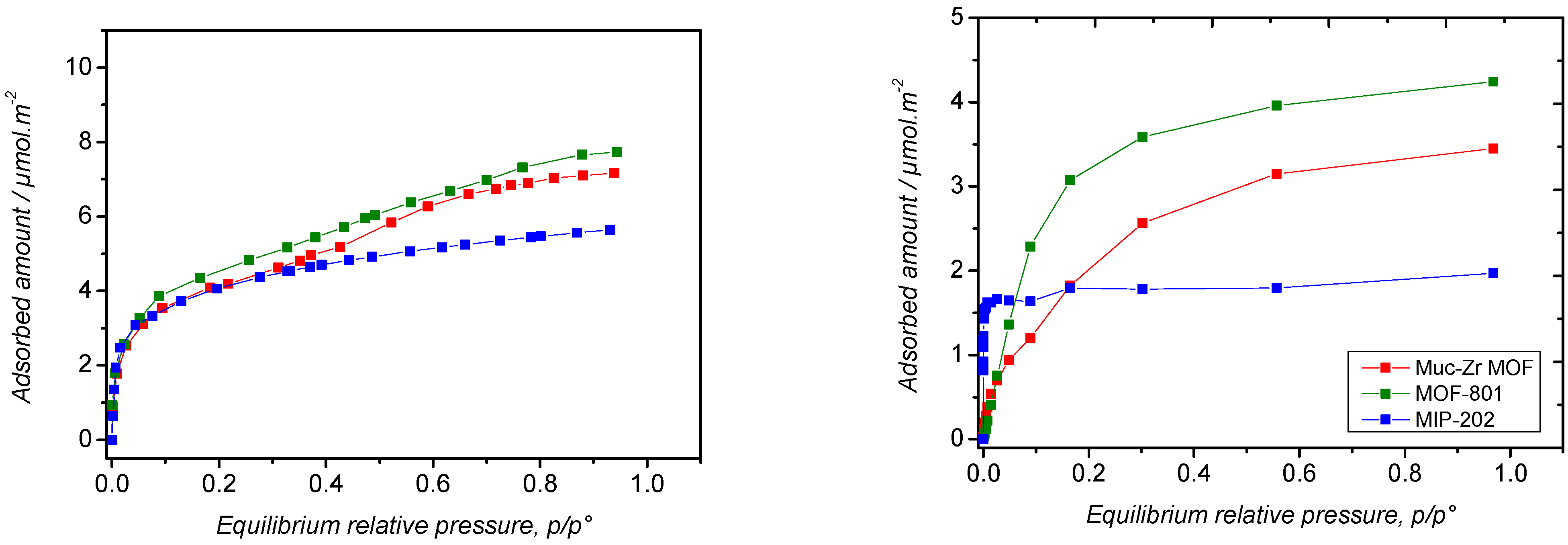
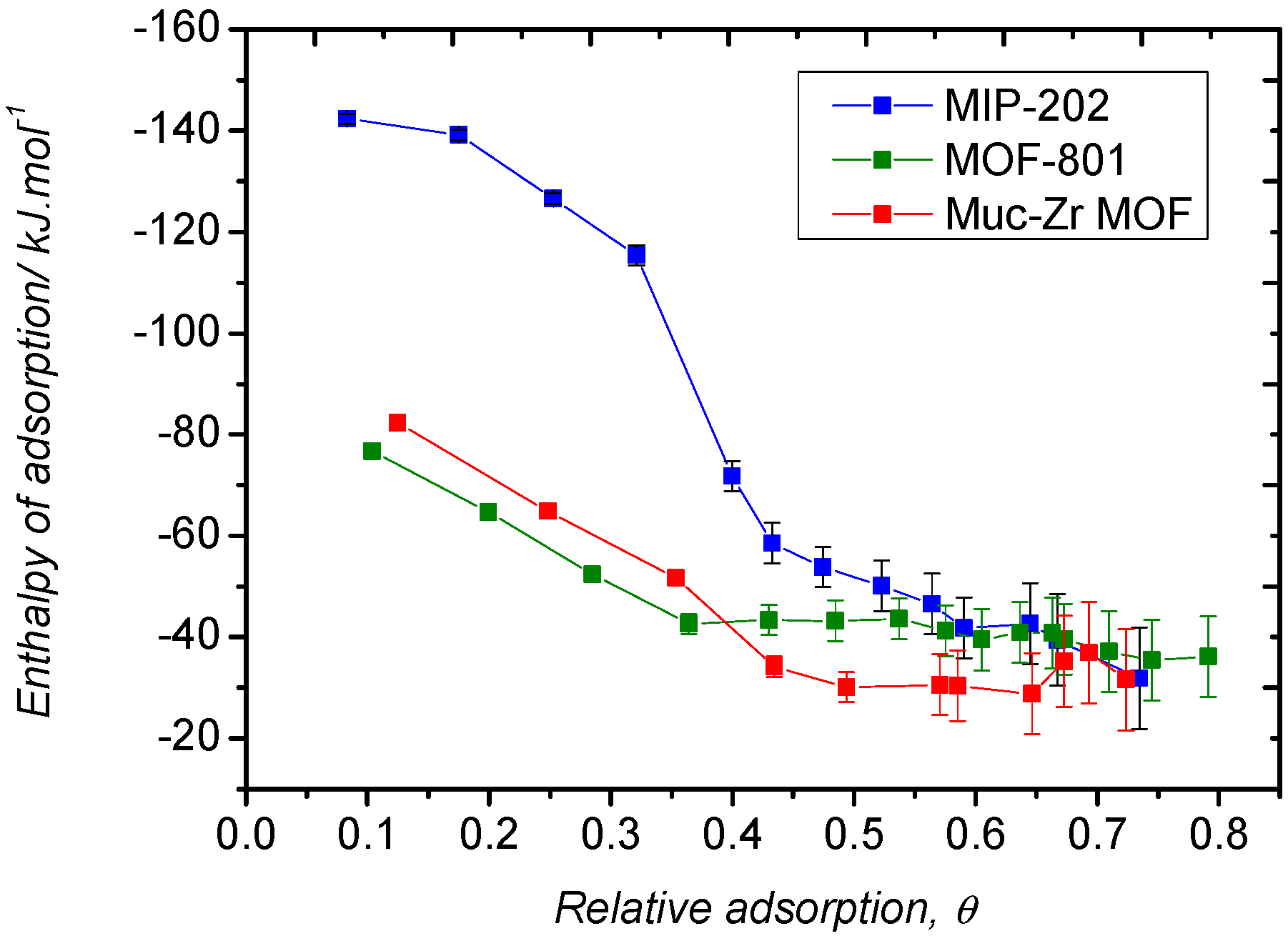
2.2. Carbon Dioxide Adsorption
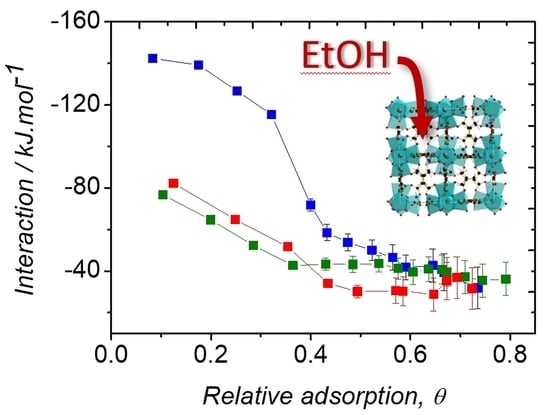
2.3. Ethanol Adsorption
3. Conclusions
4. Materials and Methods
4.1. Preparation of MOF-801
4.2. Preparation of MIP-202
4.3. Preparation of Muc-Zr MOF
4.4. Characterization and Analytical Procedures
4.5. Sorption Studies
4.6. Molecular Simulations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ghanbari, T.; Abnisa, F.; Wan Daud, W.M.A. A review on production of metal organic frameworks (MOF) for CO2 adsorption. Sci. Total Environ. 2020, 707, 135090. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review. Sep. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- Geng, C.; Yang, W.; Sun, X.; Wang, X.; Bai, Z.; Zhang, X. Emission factors, ozone and secondary organic aerosol formation potential of volatile organic compounds emitted from industrial biomass boilers. J. Environ. Sci. 2019, 83, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Wang, Y.; Duan, J.; Xie, X.; Liu, Y.; Peng, Y.; Qiao, L.; Cheng, T.; Lou, S.; Wang, H.; et al. Long-term aerosol size distributions and the potential role of volatile organic compounds (VOCs) in new particle formation events in Shanghai. Atmos. Environ. 2019, 202, 345–356. [Google Scholar] [CrossRef]
- Yang, W.; Li, J.; Wang, W.; Li, J.; Ge, M.; Sun, Y.; Chen, X.; Ge, B.; Tong, S.; Wang, Q.; et al. Investigating secondary organic aerosol formation pathways in China during 2014. Atmos. Environ. 2019, 213, 133–147. [Google Scholar] [CrossRef]
- Health Effects Institute. State of Global Air 2019; Health Effects Institute: Boston, MA, USA, 2019. [Google Scholar]
- Xu, G.; Meng, Z.; Guo, X.; Zhu, H.; Deng, K.; Xiao, C.; Liu, Y. Molecular simulations on CO2 adsorption and adsorptive separation in fullerene impregnated MOF-177, MOF-180 and MOF-200. Comput. Mater. Sci. 2019, 168, 58–64. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, D.; Luo, K.H. A critical review on VOCs adsorption by different porous materials: Species, mechanisms and modification methods. J. Hazard. Mater. 2020, 389, 122102. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Ghorbani-Shahna, F.; Bahrami, A.; Kazemian, H. Adsorptive removal of toluene and carbon tetrachloride from gas phase using Zeolitic Imidazolate Framework-8: Effects of synthesis method, particle size, and pretreatment of the adsorbent. Microporous Mesoporous Mater. 2018, 268, 58–68. [Google Scholar] [CrossRef]
- Vikrant, K.; Kim, K.H.; Kumar, V.; Giannakoudakis, D.A.; Boukhvalov, D.W. Adsorptive removal of an eight-component volatile organic compound mixture by Cu-, Co-, and Zr-metal-organic frameworks: Experimental and theoretical studies. Chem. Eng. J. 2020, 397, 125391. [Google Scholar] [CrossRef]
- Wang, Y.; Su, X.; Xu, Z.; Wen, K.; Zhang, P.; Zhu, J.; He, H. Preparation of surface-functionalized porous clay heterostructures via carbonization of soft-template and their adsorption performance for toluene. Appl. Surf. Sci. 2016, 363, 113–121. [Google Scholar] [CrossRef]
- Wang, H.; Tang, M.; Zhang, K.; Cai, D.; Huang, W.; Chen, R.; Yu, C. Functionalized hollow siliceous spheres for VOCs removal with high efficiency and stability. J. Hazard. Mater. 2014, 268, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Wang, Z.; Qin, L.; Fu, Y.; Li, B.; Zhang, M.; Liu, S.; Li, L.; Yi, H.; Liu, X.; et al. Metal-organic frameworks as burgeoning materials for the capture and sensing of indoor VOCs and radon gases. Coord. Chem. Rev. 2021, 427, 213565. [Google Scholar] [CrossRef]
- Tamon, H.; Ishizaka, H.; Yamamoto, T.; Suzuki, T. Preparation of mesoporous carbon by freeze drying. Carbon 1999, 37, 2049–2055. [Google Scholar] [CrossRef]
- Deng, L.; Yuan, P.; Liu, D.; Annabi-Bergaya, F.; Zhou, J.; Chen, F.; Liu, Z. Effects of microstructure of clay minerals, montmorillonite, kaolinite and halloysite, on their benzene adsorption behaviors. Appl. Clay Sci. 2017, 143, 184–191. [Google Scholar] [CrossRef]
- Hamon, L.; Serre, C.; Devic, T.; Loiseau, T.; Millange, F.; Férey, G.; De Weireld, G. Comparative study of hydrogen sulfide adsorption in the MIL-53(Al, Cr, Fe), MIL-47(V), MIL-100(Cr), and MIL-101(Cr) metal-organic frameworks at room temperature. J. Am. Chem. Soc. 2009, 131, 8775–8777. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Ma, Y.; McCarthy, M.C.; Sculley, J.; Yu, J.; Jeong, H.K.; Balbuena, P.B.; Zhou, H.C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- He, Y.; Wang, Z.; Wang, H.; Wang, Z.; Zeng, G.; Xu, P.; Huang, D.; Chen, M.; Song, B.; Qin, H.; et al. Metal-organic framework-derived nanomaterials in environment related fields: Fundamentals, properties and applications. Coord. Chem. Rev. 2021, 429, 213618. [Google Scholar] [CrossRef]
- Sun, X.; Gu, X.; Xu, W.; Chen, W.J.; Xia, Q.; Pan, X.; Zhao, X.; Li, Y.; Wu, Q.H. Novel Hierarchical Fe(III)-Doped Cu-MOFs With Enhanced Adsorption of Benzene Vapor. Front. Chem. 2019, 7, 652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghighi, E.; Zeinali, S. Nanoporous MIL-101(Cr) as a sensing layer coated on a quartz crystal microbalance (QCM) nanosensor to detect volatile organic compounds (VOCs). RSC Adv. 2019, 9, 24460–24470. [Google Scholar] [CrossRef] [Green Version]
- Shafiei, M.; Alivand, M.S.; Rashidi, A.; Samimi, A.; Mohebbi-Kalhori, D. Synthesis and adsorption performance of a modified micro-mesoporous MIL-101(Cr) for VOCs removal at ambient conditions. Chem. Eng. J. 2018, 341, 164–174. [Google Scholar] [CrossRef]
- Anbia, M.; Hoseini, V. Development of MWCNT@MIL-101 hybrid composite with enhanced adsorption capacity for carbon dioxide. Chem. Eng. J. 2012, 191, 326–330. [Google Scholar] [CrossRef]
- He, H.; Sun, F.; Aguila, B.; Perman, J.A.; Ma, S.; Zhu, G. A bifunctional metal-organic framework featuring the combination of open metal sites and Lewis basic sites for selective gas adsorption and heterogeneous cascade catalysis. J. Mater. Chem. A 2016, 4, 15240–15246. [Google Scholar] [CrossRef]
- Low, J.J.; Benin, A.I.; Jakubczak, P.; Abrahamian, J.F.; Faheem, S.A.; Willis, R.R. Virtual high throughput screening confirmed experimentally: Porous coordination polymer hydration. J. Am. Chem. Soc. 2009, 131, 15834–15842. [Google Scholar] [CrossRef] [PubMed]
- Boudjema, L.; Long, J.; Petitjean, H.; Larionova, J.; Guari, Y.; Trens, P.; Salles, F. Adsorption of volatile organic compounds by ZIF-8, Cu-BTC and a Prussian blue analogue: A comparative study. Inorg. Chim. Acta 2020, 501, 119316. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, X.; Bi, F.; Zheng, Z.; Sheng, L.; Xu, J.; Wang, Z.; Yang, Y. Effective toluene adsorption over defective UiO-66-NH2: An experimental and computational exploration. J. Mol. Liq. 2020, 316, 113812. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, Q.; Al-Enizi, A.M.; Nafady, A.; Ma, S. Recent advances in MOF-based photocatalysis: Environmental remediation under visible light. Inorg. Chem. Front. 2020, 7, 300–339. [Google Scholar] [CrossRef]
- Vellingiri, K.; Kumar, P.; Deep, A.; Kim, K.H. Metal-organic frameworks for the adsorption of gaseous toluene under ambient temperature and pressure. Chem. Eng. J. 2017, 307, 1116–1126. [Google Scholar] [CrossRef]
- Ma, X.; Li, L.; Chen, R.; Wang, C.; Li, H.; Wang, S. Heteroatom-doped nanoporous carbon derived from MOF-5 for CO2 capture. Appl. Surf. Sci. 2018, 435, 494–502. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, X.; Shi, X.; Yang, Y.; Yang, Y. Enhanced hydrophobic UiO-66 (University of Oslo 66) metal-organic framework with high capacity and selectivity for toluene capture from high humid air. J. Colloid Interface Sci. 2019, 539, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, Y.; Song, L.; Chen, J.; Yang, Y.; Wang, Y. Enhanced adsorption performance of gaseous toluene on defective UiO-66 metal organic framework: Equilibrium and kinetic studies. J. Hazard. Mater. 2019, 365, 597–605. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Lv, X.; Wang, Y.; Liu, N.; Chen, D.; Cui, L. Adsorption/desorption kinetics and breakthrough of gaseous toluene for modified microporous-mesoporous UiO-66 metal organic framework. J. Hazard. Mater. 2019, 366, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Ramsahye, N.A.; Trens, P.; Shepherd, C.; Gonzalez, P.; Trung, T.K.; Ragon, F.; Serre, C. The effect of pore shape on hydrocarbon selectivity on UiO-66(Zr), HKUST-1 and MIL-125(Ti) metal organic frameworks: Insights from molecular simulations and chromatography. Microporous Mesoporous Mater. 2014, 189, 222–231. [Google Scholar] [CrossRef]
- Caratelli, C.; Hajek, J.; Cirujano, F.G.; Waroquier, M.; Llabrés i Xamena, F.X.; Van Speybroeck, V. Nature of active sites on UiO-66 and beneficial influence of water in the catalysis of Fischer esterification. J. Catal. 2017, 352, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Rogge, S.M.J.; Wieme, J.; Vanduyfhuys, L.; Vandenbrande, S.; Maurin, G.; Verstraelen, T.; Waroquier, M.; Van Speybroeck, V. Thermodynamic insight in the high-pressure behavior of UiO-66: Effect of linker defects and linker expansion. Chem. Mater. 2016, 28, 5721–5732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trickett, C.A.; Gagnon, D.K.J.; Lee, S.; Gándara, D.F.; Bürgi, H.; Yaghi, O.M. Definitive Molecular Level Characterization of Defects in UiO-66 Crystals. Angew. Chem. 2015, 54, 11162–11167. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, Z.; Hou, L.; Yang, C.; Shen, H.; Yang, K.; Wang, Z. Metal-organic framework MOF-801/PIM-1 mixed-matrix membranes for enhanced CO2/N2 separation performance. Sep. Purif. Technol. 2020, 250, 117198. [Google Scholar] [CrossRef]
- Sun, J.; Li, Q.; Chen, G.; Duan, J.; Liu, G.; Jin, W. MOF-801 incorporated PEBA mixed-matrix composite membranes for CO2 capture. Sep. Purif. Technol. 2019, 217, 229–239. [Google Scholar] [CrossRef]
- Iacomi, P.; Formalik, F.; Marreiros, J.; Shang, J.; Rogacka, J.; Mohmeyer, A.; Behrens, P.; Ameloot, R.; Kuchta, B.; Lewellyn, P. Role of Structural Defects in the Adsorption and Separation of C3 Hydrocarbons in Zr-Fumarate-MOF (MOF-801). Chem. Mater. 2019, 31, 8413–8423. [Google Scholar] [CrossRef]
- Lv, D.; Chen, J.; Yang, K.; Wu, H.; Chen, Y.; Duan, C.; Wu, Y.; Xiao, J.; Xi, H.; Li, Z.; et al. Ultrahigh CO2/CH4 and CO2/N2 adsorption selectivities on a cost-effectively L-aspartic acid based metal-organic framework. Chem. Eng. J. 2019, 375, 122074. [Google Scholar] [CrossRef]
- Buragohain, A.; Biswas, S. Improved Synthesis of a Zirconium(IV) Muconate Metal–Organic Framework: Characterization, Stability and Gas Sorption Properties. Eur. J. Inorg. Chem. 2015, 2015, 2463–2468. [Google Scholar] [CrossRef]
- David, J.; Trolliard, G.; Volkringer, C.; Loiseau, T.; Masson, O.; Maître, A. Study of the reaction mechanisms involved in the formation of zirconium oxycarbide from Metal-Organic Frameworks (MOFs) precursors. J. Alloys Compd. 2016, 680, 571–585. [Google Scholar] [CrossRef]
- Furukawa, H.; Gándara, F.; Zhang, Y.B.; Jiang, J.; Queen, W.L.; Hudson, M.R.; Yaghi, O.M. Water adsorption in porous metal-organic frameworks and related materials. J. Am. Chem. Soc. 2014, 136, 4369–4381. [Google Scholar] [CrossRef]
- Wang, S.; Wahiduzzaman, M.; Davis, L.; Tissot, A.; Shepard, W.; Marrot, J.; Martineau-Corcos, C.; Hamdane, D.; Maurin, G.; Devautour-Vinot, S.; et al. A robust zirconium amino acid metal-organic framework for proton conduction. Nat. Commun. 2018, 9, 4937. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Kuppler, R.J.; Zhou, H.C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Xiong, L.; Hu, X.; Yan, Z.; Wang, L.; Xu, G. Remediation of ammonia-contaminated groundwater in landfill sites with electrochemical reactive barriers: A bench scale study. Waste Manag. 2018, 78, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Guillerm, V.; Gross, S.; Serre, C.; Devic, T.; Bauer, M.; Férey, G. A zirconium methacrylate oxocluster as precursor for the low-temperature synthesis of porous zirconium(iv) dicarboxylates. Chem. Commun. 2010, 46, 767–769. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Musyoka, N.M.; Langmi, H.W.; North, B.C.; Mathe, M.; Pang, W.; Wang, M.; Walker, J. In-situ IR monitoring of the formation of Zr-fumarate MOF. Appl. Surf. Sci. 2017, 404, 263–267. [Google Scholar] [CrossRef]
- Vetlitsyna-Novikova, K.S.; Butova, V.V.; Pankin, I.A.; Shapovalov, V.V.; Soldatov, A.V. Zirconium-Based Metal-Organic UiO-66, UiO-66-NDC and MOF-801 Frameworks. Influence of the Linker Effect on the Hydrogen Sorption Efficiency. J. Surf. Investig. 2019, 13, 787–792. [Google Scholar] [CrossRef]
- Trens, P.; Tanchoux, N.; Papineschi, P.M.; Maldonado, D.; Di Renzo, F.; Fajula, F. Confinements effects in MCM-41-type materials: Comparison of the energetics of n-hexane and 1-hexene adsorption. Microporous Mesoporous Mater. 2005, 86, 354–363. [Google Scholar] [CrossRef]
- Trens, P.; Tanchoux, N.; Maldonado, D.; Galarneau, A.; Di Renzo, F.; Fajula, F. Study of n-hexane adsorption in MCM-41 mesoporous materials: A scaling effect approach of capillary condensation processes. New J. Chem. 2004, 28, 874–879. [Google Scholar] [CrossRef]
- Ke, F.; Peng, C.; Zhang, T.; Zhang, M.; Zhou, C.; Cai, H.; Zhu, J.; Wan, X. Fumarate-based metal-organic frameworks as a new platform for highly selective removal of fluoride from brick tea. Sci. Rep. 2018, 8, 939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Kim, J.; Ahn, W.S. Efficient carbon dioxide capture over a nitrogen-rich carbon having a hierarchical micro-mesopore structure. Fuel 2012, 95, 360–364. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W. Adsorption by Powders and Porous Solids; Press, A., Ed.; Academic Press: Cambridge, MA, USA, 1999. [Google Scholar]
- Zahn, G.; Schulze, H.A.; Lippke, J.; König, S.; Sazama, U.; Fröba, M.; Behrens, P. A water-born Zr-based porous coordination polymer: Modulated synthesis of Zr-fumarate MOF. Microporous Mesoporous Mater. 2015, 203, 186–194. [Google Scholar] [CrossRef]
- Vitillo, J.G.; Savonnet, M.; Ricchiardi, G.; Bordiga, S. Tailoring metal-organic frameworks for CO2 capture: The amino effect. ChemSusChem 2011, 4, 1281–1290. [Google Scholar] [CrossRef]
- Becker, T.M.; Heinen, J.; Dubbeldam, D.; Lin, L.C.; Vlugt, T.J.H. Polarizable Force Fields for CO2 and CH4 Adsorption in M-MOF-74. J. Phys. Chem. C 2017, 121, 4659–4673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuruoka, T.; Furukawa, S.; Takashima, Y.; Yoshida, K.; Isoda, S.; Kitagawa, S. Nanoporous Nanorods Fabricated by Coordination Modulation and Oriented Attachment Growth. Angew. Chem. 2009, 121, 4833–4837. [Google Scholar] [CrossRef]
- Diring, S.; Furukawa, S.; Takashima, Y.; Tsuruoka, T.; Kitagawa, S. Controlled Multiscale Synthesis of Porous Coordination Polymer in Nano/Micro Regimes. Chem. Mater. 2010, 22, 4531–4538. [Google Scholar] [CrossRef]
- Ramsahye, N.A.; Trung, T.K.; Bourrelly, S.; Yang, Q.; Devic, T.; Maurin, G.; Horcajada, P.; Llewellyn, P.L.; Yot, P.; Serre, C.; et al. Influence of the organic ligand functionalization on the breathing of the porous iron terephthalate metal organic framework type material upon hydrocarbon adsorption. J. Phys. Chem. C 2011, 115, 18683–18695. [Google Scholar] [CrossRef]
- Trens, P.; Belarbi, H.; Shepherd, C.; Gonzalez, P.; Ramsahye, N.A.; Lee, U.-H.; Seo, Y.-K.; Chang, J.-S. Adsorption and separation of xylene isomers vapors onto the chromium terephthalate-based porous material MIL-101(Cr): An experimental and computational study. Microporous Mesoporous Mater. 2014, 183, 17–22. [Google Scholar] [CrossRef]
- Dassault Systèmes. BIOVIA Materials Studio. Available online: https://www.3ds.com/products-services/biovia/products/molecular-modeling-simulation/biovia-materials-studio/ (accessed on 14 November 2021).
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 76, 3865. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.G.; Yun, K.H. Carbon Dioxide’s Liquid-Vapor Coexistence Curve and Critical Properties As Predicted by a Simple Molecular Model. J. Phys. Chem. 1995, 99, 12021–12024. [Google Scholar] [CrossRef]
- Chen, B.; Potoff, J.J.; Siepmann, J.I. Monte Carlo Calculations for Alcohols and Their Mixtures with Alkanes. Transferable Potentials for Phase Equilibria. 5. United-Atom Description of Primary, Secondary, and Tertiary Alcohols. J. Phys. Chem. B 2001, 105, 3093–3104. [Google Scholar] [CrossRef]
- Mamontova, E.; Trens, P.; Salles, F.; Fraisse, B.; Gimello, O.; Guari, Y.; Larionova, J.; Long, J. Enantioselective separation under humid conditions by chiral Hofmann clathrates: New opportunities for vintage materials. Inorg. Chem. Front. 2019, 6, 3245–3254. [Google Scholar] [CrossRef]
- Benzaria, S.; Mamontova, E.; Guari, Y.; Larionova, J.; Long, J.; Trens, P.; Salles, F.; Zajac, J. Heat Release Kinetics upon Water Vapor Sorption Using Cation-Exchanged Zeolites and Prussian Blue Analogues as Adsorbents: Application to Short-Term Low-Temperature Thermochemical Storage of Energy. Energies 2021, 14, 3505. [Google Scholar] [CrossRef]

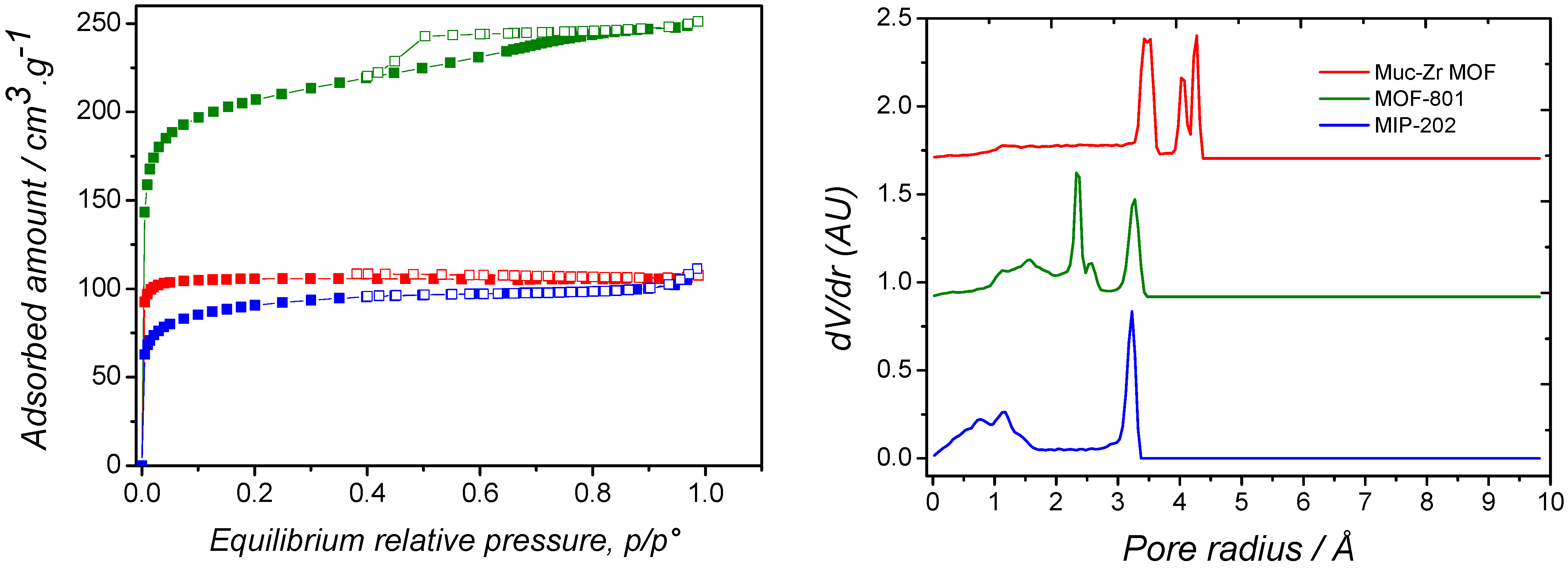
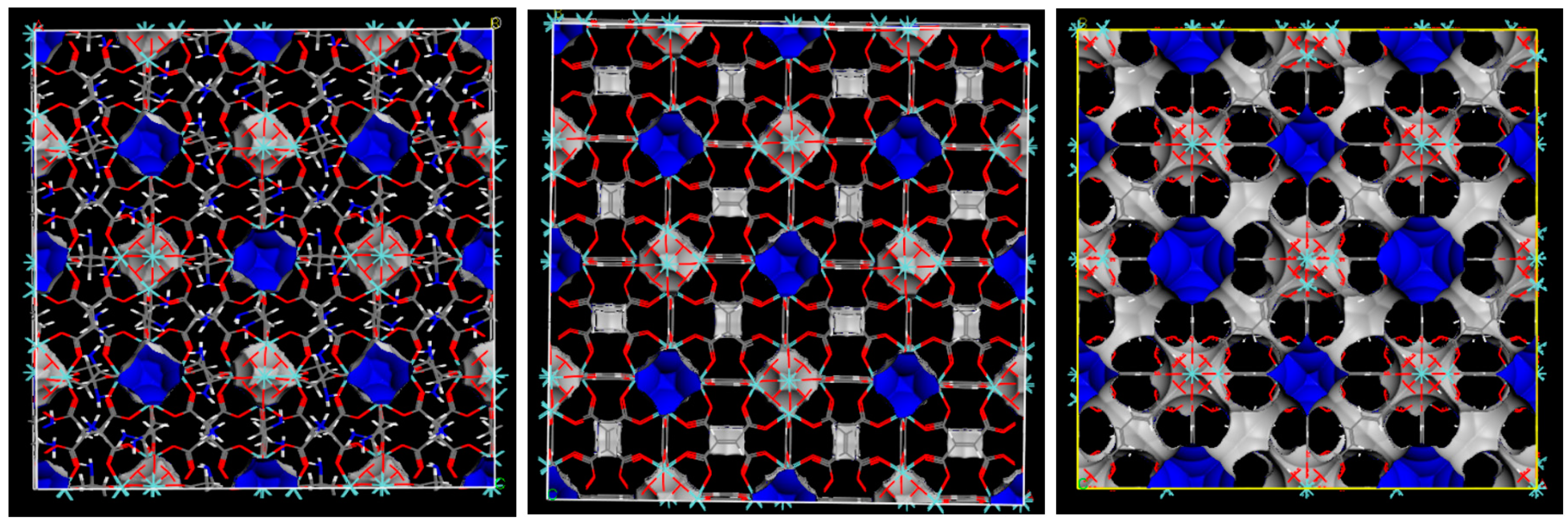
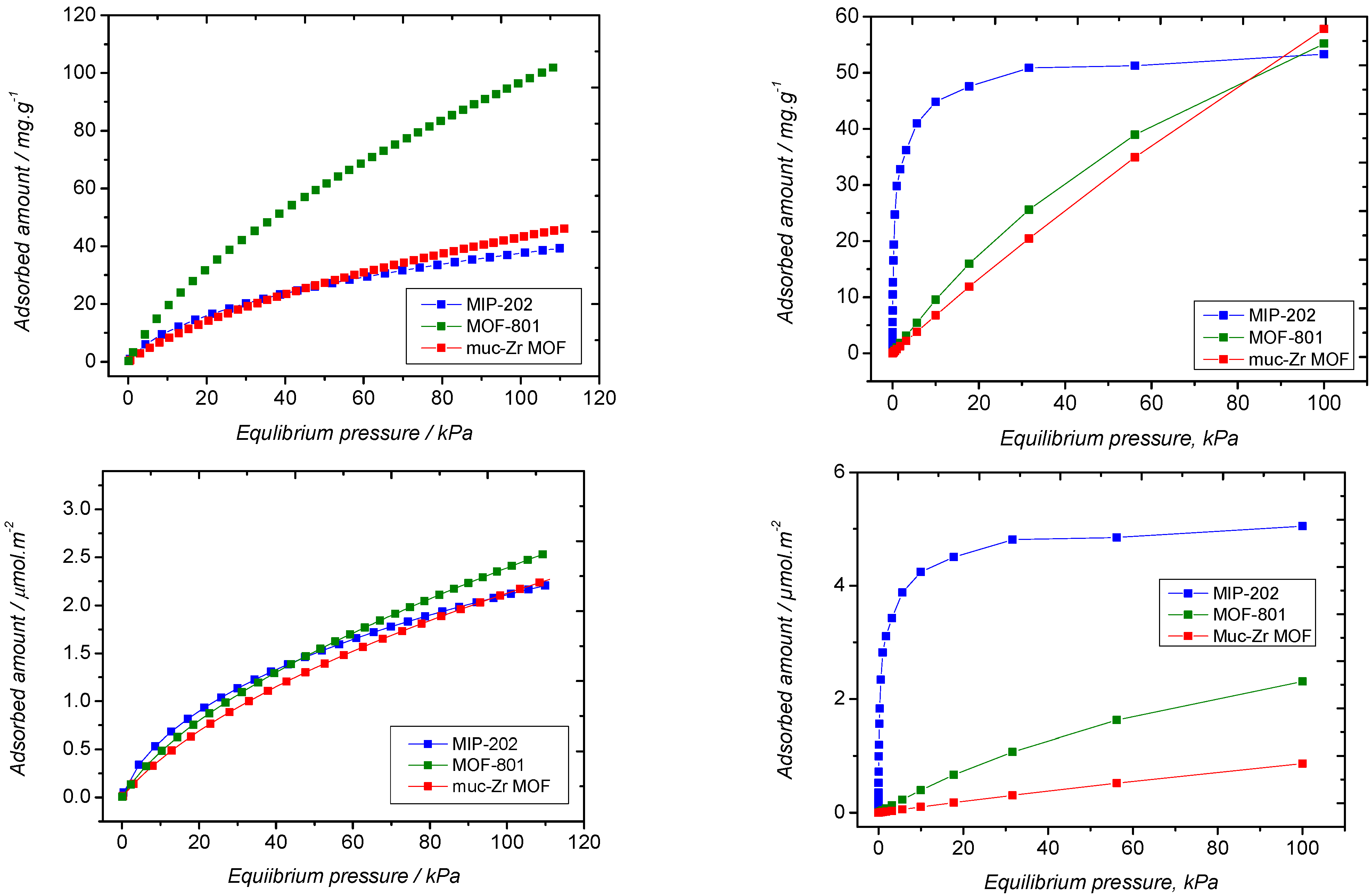
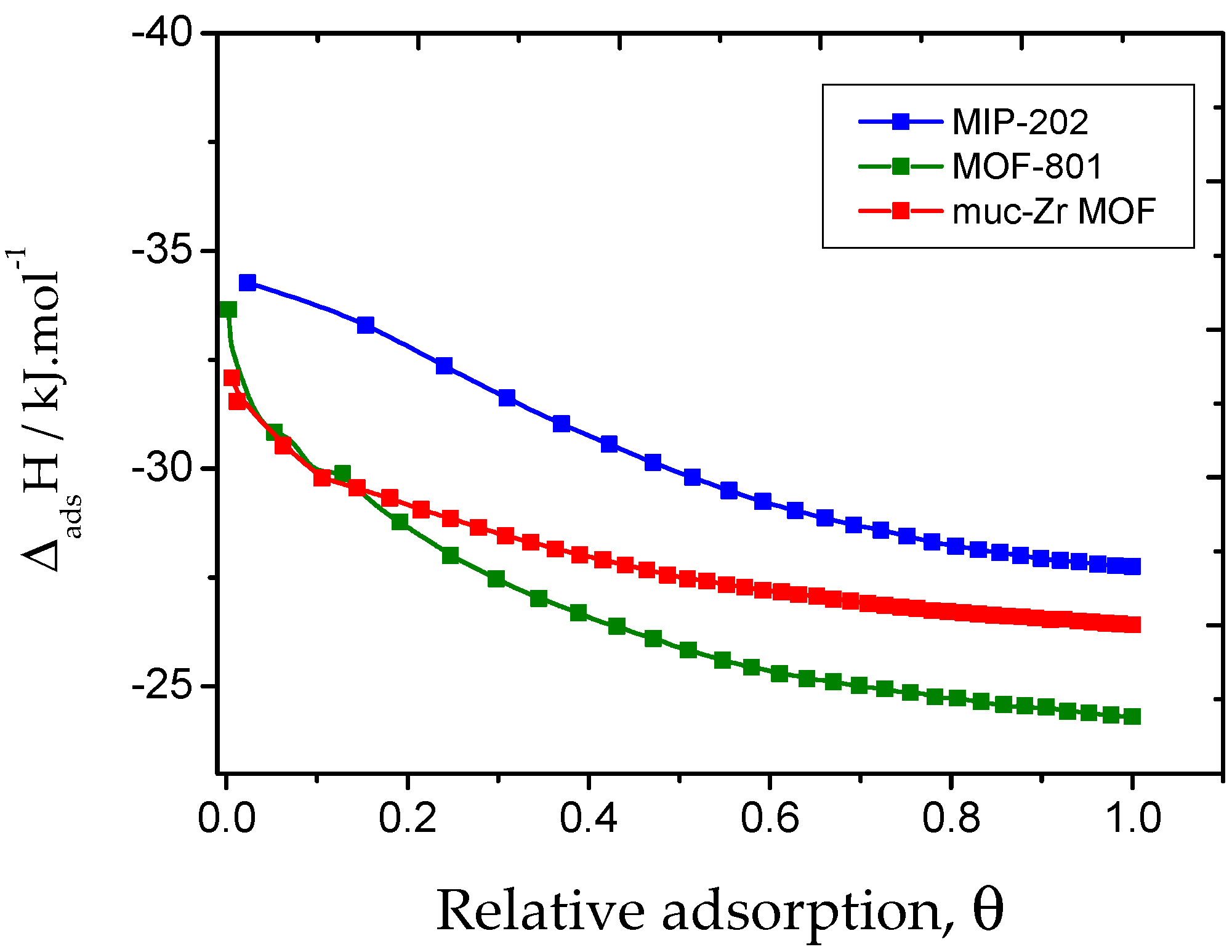
| Specific Surface Area (Langmuir)/m2·g−1 | Pore Volume/cm3·g−1 | External Surface Area/m2·g−1 | Average Mesopores Diameter/Å | |
|---|---|---|---|---|
| Muc-Zr MOF | 462 | 0.157 | 14 | - |
| MOF-801 | 920 | 0.230 | 205 | 68 |
| MIP-202 | 405 | 0.09 | 106 | - |
| Specific Surface Area/m2·g−1 | Pore Volume/cm3·g−1 | |
|---|---|---|
| MIP-202 | 240 | 0.27 |
| MOF-801 | 543 | 0.32 |
| Muc-Zr MOF | 1518 | 0.50 |
| Experimental Data | Simulated Data | ||
|---|---|---|---|
| ΔHads/−kJ·mol−1 Low Coverage | ΔHads/−kJ·mol−1 p = 105 Pa | ΔHads/−kJ·mol−1 Low Coverage | |
| Muc-Zr MOF | 32 | 26 | 19 |
| MOF-801 | 33 | 24 | 23 |
| MIP-202 | 34 | 28 | 50 |
| Enthalpy of condensation of CO2 at 288 K | 16.7 | ||
| Experimental Data | Simulated Data | ||
|---|---|---|---|
| ΔHads/−kJ·mol−1 Extrapolation at Low Coverage | ΔHads/−kJ·mol−1 p = 105 Pa | ΔHads/−kJ·mol−1 Low Coverage | |
| Muc-Zr MOF | ~100 | ~35 | 54 |
| MOF-801 | ~90 | ~40 | 39 |
| MIP-202 | ~145 | ~40 | 90 |
| Enthalpy of condensation of ethanol at 298 K | 42.3 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saidi, M.; Ho, P.H.; Yadav, P.; Salles, F.; Charnay, C.; Girard, L.; Boukli-Hacene, L.; Trens, P. Zirconium-Based Metal Organic Frameworks for the Capture of Carbon Dioxide and Ethanol Vapour. A Comparative Study. Molecules 2021, 26, 7620. https://doi.org/10.3390/molecules26247620
Saidi M, Ho PH, Yadav P, Salles F, Charnay C, Girard L, Boukli-Hacene L, Trens P. Zirconium-Based Metal Organic Frameworks for the Capture of Carbon Dioxide and Ethanol Vapour. A Comparative Study. Molecules. 2021; 26(24):7620. https://doi.org/10.3390/molecules26247620
Chicago/Turabian StyleSaidi, Meryem, Phuoc Hoang Ho, Pankaj Yadav, Fabrice Salles, Clarence Charnay, Luc Girard, Leila Boukli-Hacene, and Philippe Trens. 2021. "Zirconium-Based Metal Organic Frameworks for the Capture of Carbon Dioxide and Ethanol Vapour. A Comparative Study" Molecules 26, no. 24: 7620. https://doi.org/10.3390/molecules26247620
APA StyleSaidi, M., Ho, P. H., Yadav, P., Salles, F., Charnay, C., Girard, L., Boukli-Hacene, L., & Trens, P. (2021). Zirconium-Based Metal Organic Frameworks for the Capture of Carbon Dioxide and Ethanol Vapour. A Comparative Study. Molecules, 26(24), 7620. https://doi.org/10.3390/molecules26247620