Study on Purification and Characterization of Polyphenol Oxidase from Acetes chinensis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
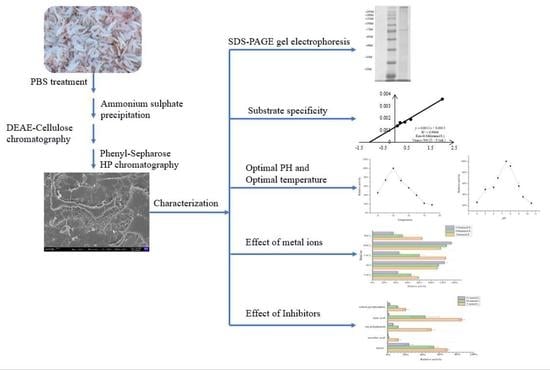
2.2. Purification of Acetes chinensis PPO
2.3. Determination of Acetes chinensis PPO Activity
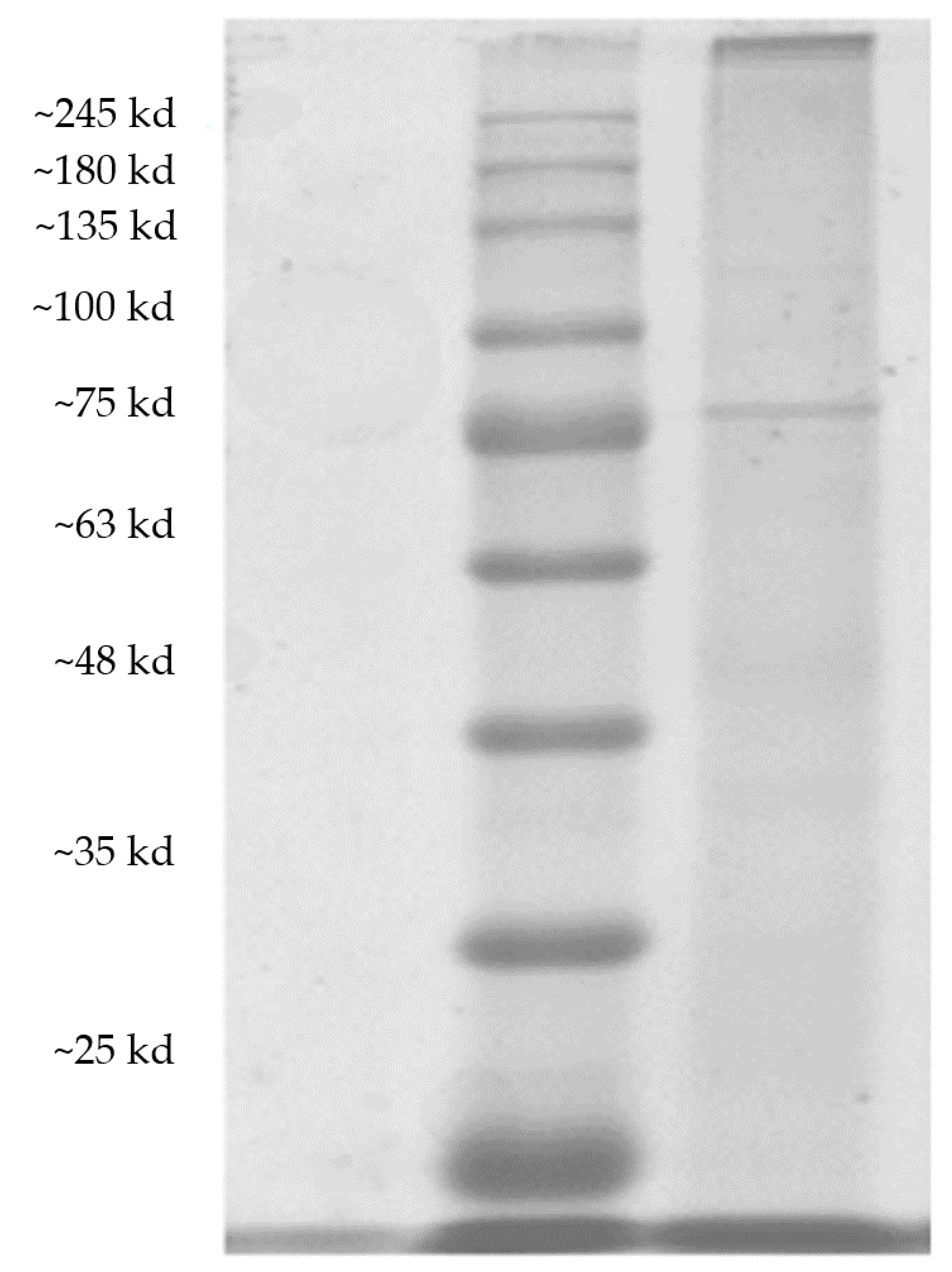
2.4. SDS-PAGE Gel Electrophoresis Determination
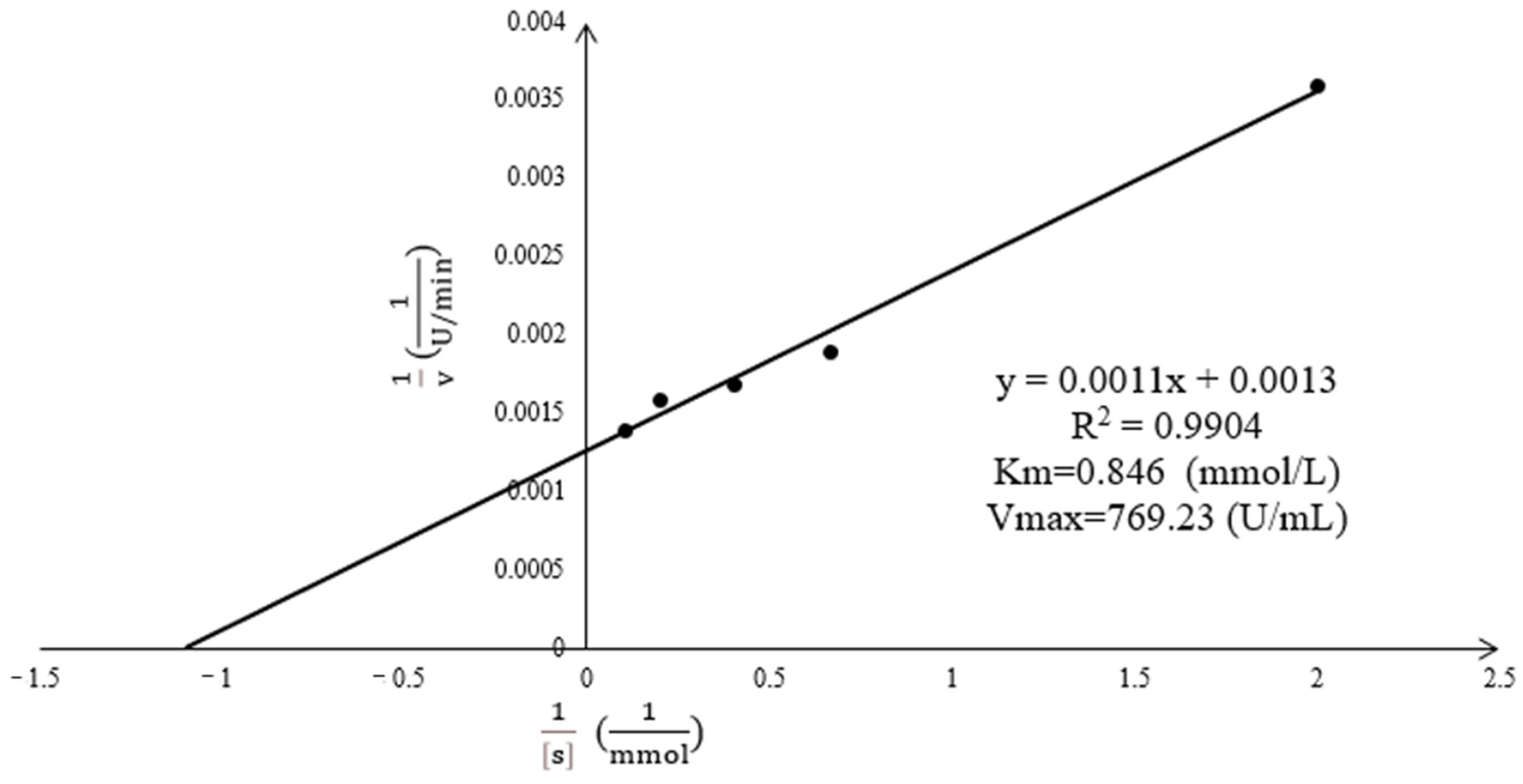
2.5. Enzyme Kinetic Curve of Acetes chinensis PPO
2.6. Optimal pH of Acetes chinensis PPO Activity
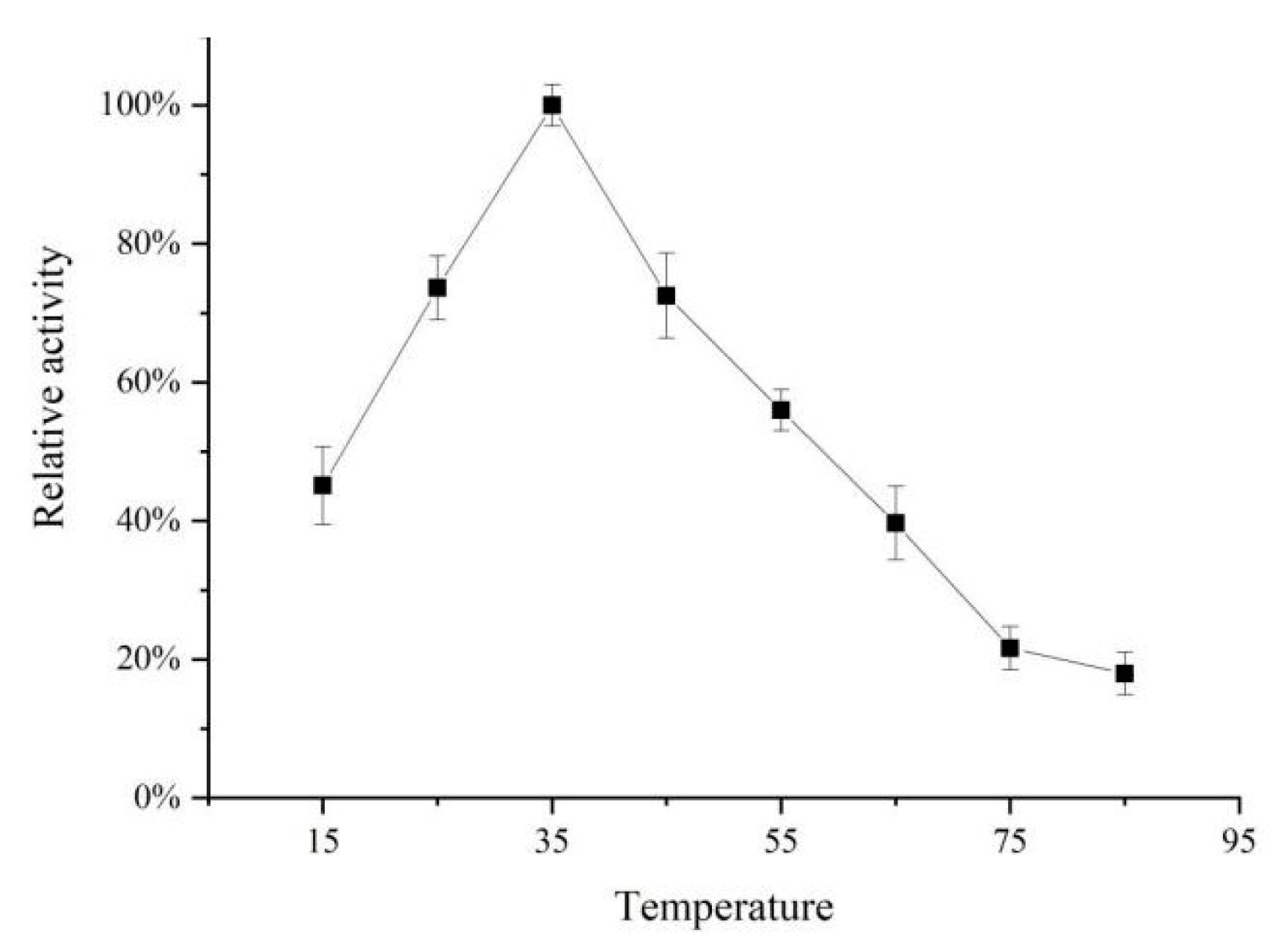
2.7. Optimal Temperature of Acetes chinensis PPO Activity
2.8. Effect of Metal Ions on Acetes chinensis PPO Activity
2.9. Effect of Inhibitor on Acetes chinensis PPO Activity
2.10. Statistical Analysis
3. Results and Discussion
3.1. Purification of Polyphenol Oxidase from Acetes chinensis
3.2. Enzyme Kinetic Curve of the Acetes chinensis PPO Activity
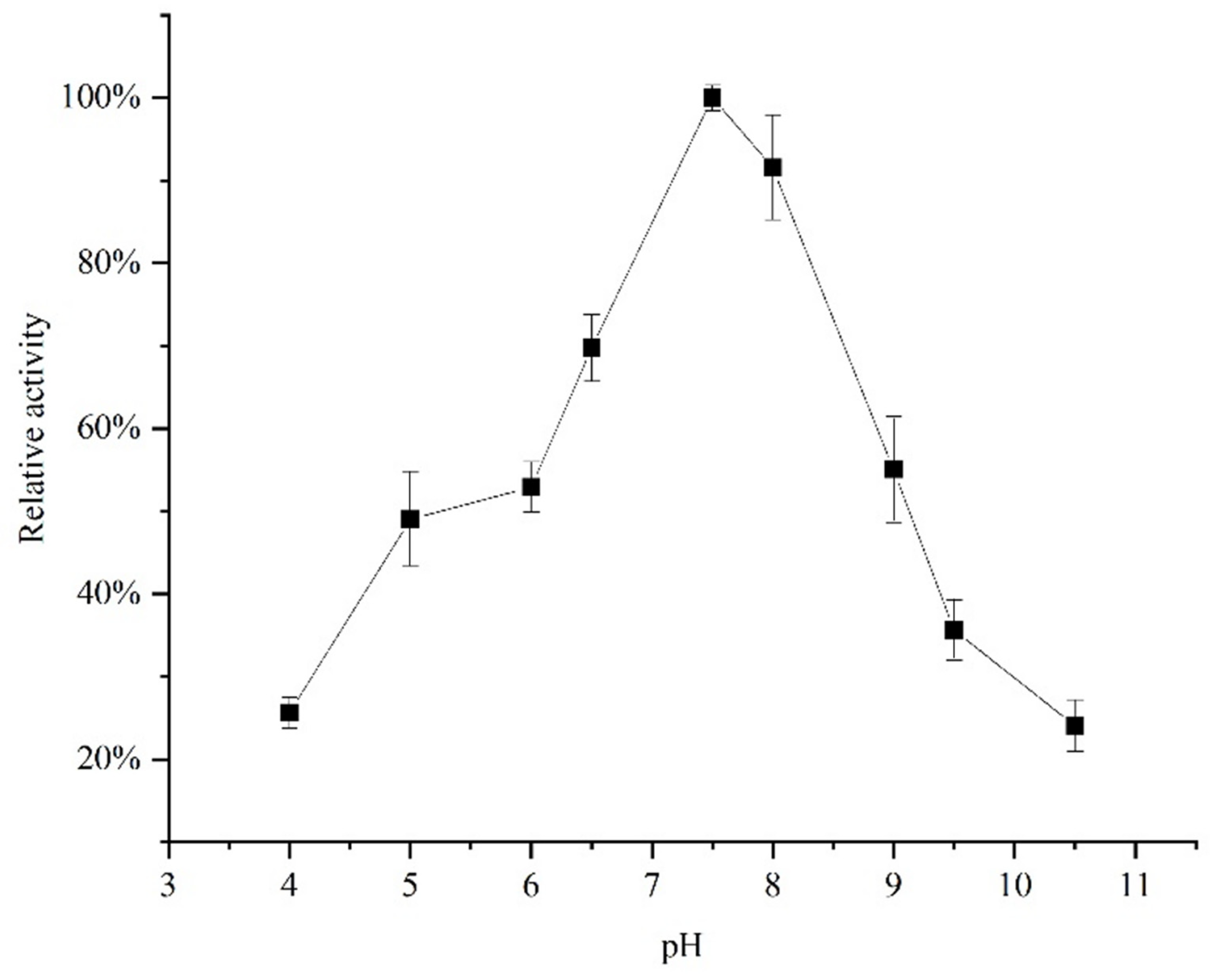
3.3. Effect of Temperature and pH on the Acetes chinensis PPO Activity
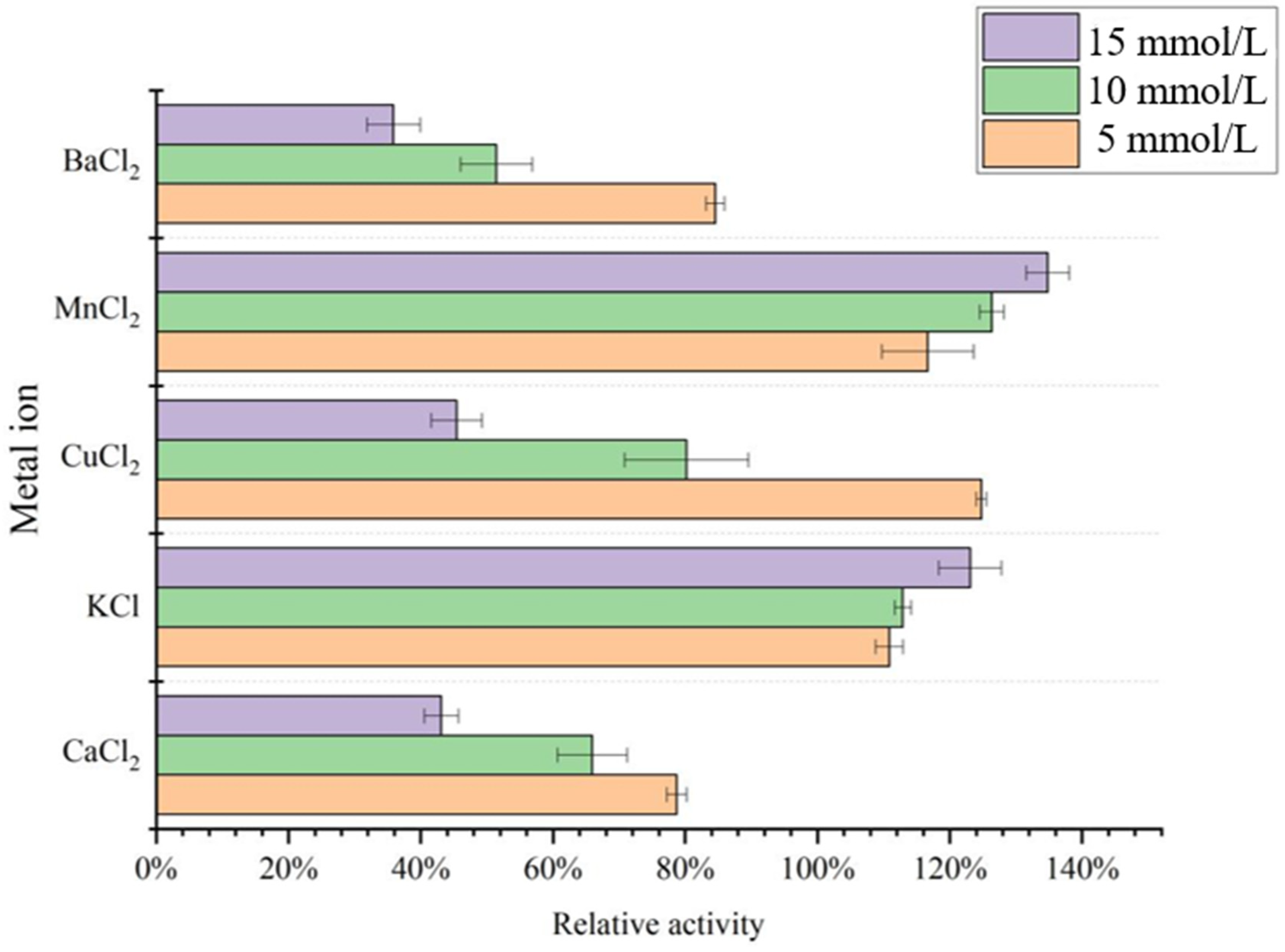
3.4. Effect of Metal Ions on the Acetes chinensis PPO Activity
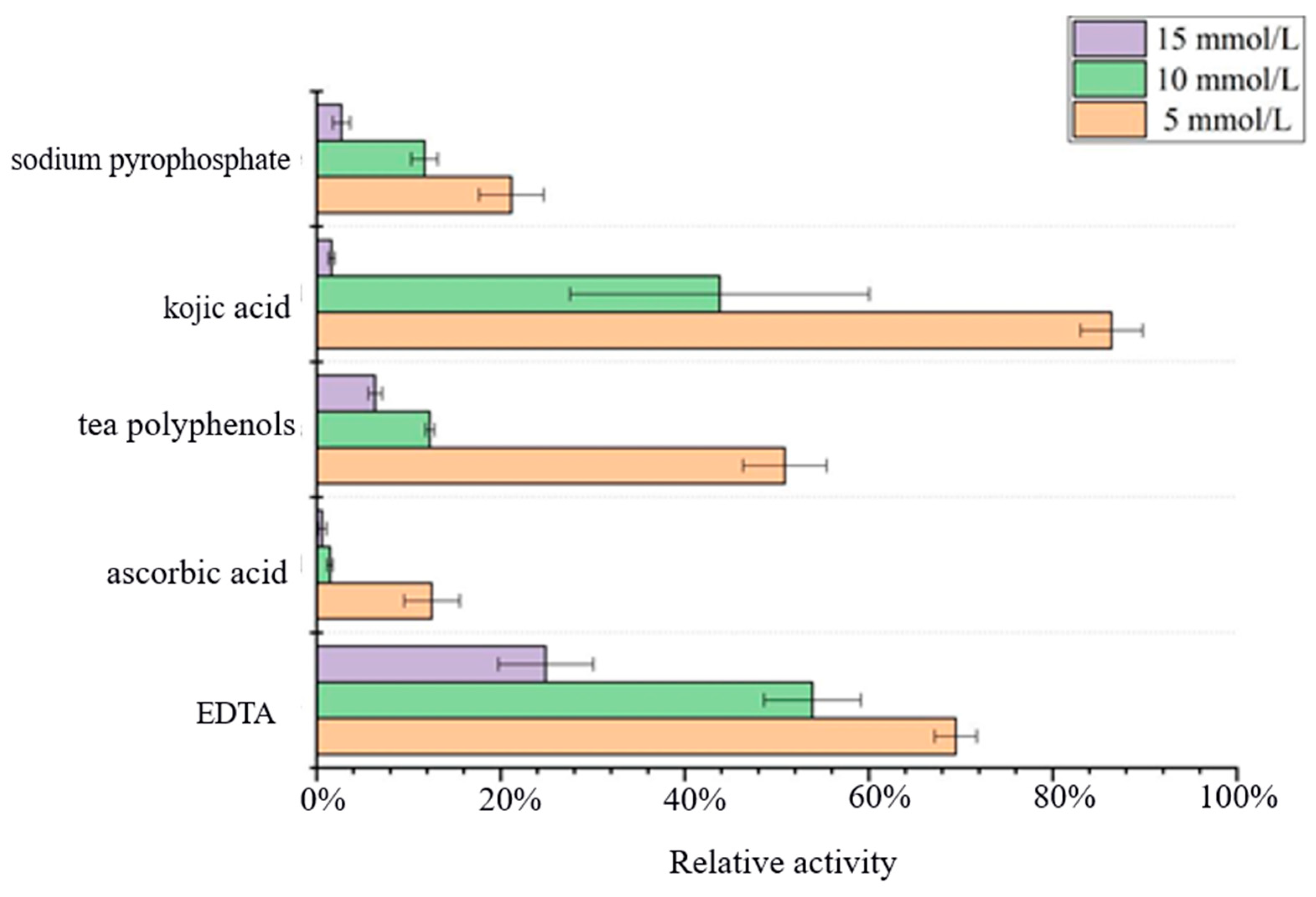
3.5. Effect of Inhibitors on Acetes chinensis PPO Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kim, S.; Kim, J.; Choi, H.G.; Park, J.K.; Min, G.S. Complete mitochondrial genome of the northern mauxia shrimp Acetes chinensis (Decapoda, Dendrobranchiata, Sergestoidae). Mitochondrial DNA 2012, 23, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yang, X.; Wang, Y.; Sun, L. Cascading Mechanism Triggering the Activation of Polyphenol Oxidase Zymogen in Shrimp Litopenaeus vannamei After Postmortem and the Correlation with Melanosis Development. Food Bioprocess Technol. 2020, 13, 1131–1145. [Google Scholar] [CrossRef]
- Yadav, D.K.; Prasad, A.; Kruk, J.; Pospisil, P. Evidence for the involvement of loosely bound plastosemiquinones in superoxide anion radical production in photosystem II. PLoS ONE 2014, 9, e115466. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jongberg, S.; Andersen, M.L.; Davies, M.J.; Lund, M.N. Quinone-induced protein modifications: Kinetic preference for reaction of 1,2-benzoquinones with thiol groups in proteins. Free Radic. Biol. Med. 2016, 97, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Onwude, D.I.; Hashim, N.; Abdan, K.; Janius, R.; Chen, G. The effectiveness of combined infrared and hot-air drying strategies for sweet potato. J. Food Eng. 2019, 241, 75–87. [Google Scholar] [CrossRef]
- de Jesus Rivas, N.; Whitaker, J.R. Purification and Some Properties of Two Polyphenol Oxidases from Bartlett Pears. Plant Physiol. 1973, 52, 501–507. [Google Scholar]
- Salehi, F.; Kashaninejad, M. Modeling of moisture loss kinetics and color changes in the surface of lemon slice during the combined infrared-vacuum drying. Inf. Process. Agric. 2018, 5, 516–523. [Google Scholar] [CrossRef]
- Zamorano, J.-P.; Martínez-Álvarez, O.; Montero, P.; Gómez-Guillén, M.d.C. Characterisation and tissue distribution of polyphenol oxidase of deepwater pink shrimp (Parapenaeus longirostris). Food Chem. 2009, 112, 104–111. [Google Scholar] [CrossRef]
- Sritunyalucksana, K.; Cerenius, L.; Söderhäll, K. Molecular cloning and characterization of prophenoloxidase in the black tiger shrimp, Penaeus monodon. Dev. Comp. Immunol. 1999, 23, 179–186. [Google Scholar] [CrossRef]
- Benjakul, S.; Visessanguan, W.; Tanaka, N. Properties of phenoloxidase isolated from the cephalothorax of kuruma prawn (Penaeus japonicus). J. Food Biochem. 2005, 29, 470–485. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Benjakul, S. Effect of catechin and ferulic acid on melanosis and quality of Pacific white shrimp subjected to prior freeze–thawing during refrigerated storage. Food Control 2010, 21, 1263–1271. [Google Scholar] [CrossRef]
- ASHIDA, M.; DOHKE, K. Activation of pro-phenoloxidase by the activating enzyme of the silkworm, Bombyx mori. Insect Biochem. 1980, 10, 37–47. [Google Scholar] [CrossRef]
- Bravo, K.; Osorio, E. Characterization of polyphenol oxidase from Cape gooseberry (Physalis peruviana L.) fruit. Food Chem. 2016, 197, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Qi, X.; Zheng, X. Purification and some properties of cuttlefish ink polyphenol oxidase. Dev. Food Sci. 2004, 42, 223–232. [Google Scholar]
- Han, S.Y.; Wang, M.Q.; Wang, B.J.; Liu, M.; Jiang, K.Y.; Wang, L. A comparative study on oxidative stress response in the hepatopancreas and midgut of the white shrimp Litopenaeus vannamei under gradual changes to low or high pH environment. Fish Shellfish Immunol. 2018, 76, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Saby John, K.; Bhat, S.G.; Prasada Rao, U.J.S. Isolation and partial characterization of phenol oxidases from Mangifera indica L. sap (latex). J. Mol. Catal. B Enzym. 2011, 68, 30–36. [Google Scholar] [CrossRef]
- Liu, N.N.; Liu, W.; Wang, D.J.; Zhou, Y.B.; Lin, X.J.; Wang, X.; Li, S.B. Purification and partial characterization of polyphenol oxidase from the flower buds of Lonicera japonica Thunb. Food Chem. 2013, 138, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Sartori, E.R.; Vicentini, F.C.; Fatibello-Filho, O. Indirect determination of sulfite using a polyphenol oxidase biosensor based on a glassy carbon electrode modified with multi-walled carbon nanotubes and gold nanoparticles within a poly(allylamine hydrochloride) film. Talanta 2011, 87, 235–242. [Google Scholar] [CrossRef] [Green Version]
- López-Caballero, M.E.; Martínez-Álvarez, O.; Gómez-Guillén, M.C.; Montero, P. Several melanosis-inhibiting formulas to enhance the quality of deepwater pink shrimp (Parapenaeus longirostris). Innov. Food Sci. Emerg. Technol. 2019, 51, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Rocha, A.M.C.N.; Morais, A.M.M.B. Characterization of polyphenoloxidase (PPO) extracted from ‘Jonagored’ apple. Food Control 2001, 12, 85–90. [Google Scholar] [CrossRef]
| Purification Stages | Volume (mL) | Total Activity (U) | Total Protein (mg) | Specific Activity (U/mg) | Yield (%) | Purification Fold |
|---|---|---|---|---|---|---|
| crude extract:0.05 M phosphate buffer | 1000 a ± 0 | 7532 a ± 756 | 355 a ± 65.11 | 21.2 d ± 4.5 | 100 a ± 0 | 1 c ± 0 |
| 20–80% (NH4)2SO4 precipitation | 248 b ± 16 | 4128 b ± 364 | 75 b ± 3.21 | 54.4 c ± 7.4 | 54.8 b ± 0.58 | 2.56 c ± 1.54 |
| anion exchange:DEAE Sephacel column | 20 c ± 7 | 1814 c ± 214 | 12 c ± 0.24 | 150.4 b ± 21.7 | 24.1 c ± 0.35 | 7.09 b ± 4.62 |
| hydrophobic interaction:phynyl-Sepharose HP column | 1 d ± 0.5 | 1351 c ± 133 | 2.1 d ± 0.04 | 643.4 a ± 65.2 | 17.9 c ± 0.12 | 30.35 a ± 14.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhou, G.; Fei, L.; Chen, L.; Sun, L.; Lyu, F.; Ding, Y. Study on Purification and Characterization of Polyphenol Oxidase from Acetes chinensis. Molecules 2021, 26, 7545. https://doi.org/10.3390/molecules26247545
Zhang J, Zhou G, Fei L, Chen L, Sun L, Lyu F, Ding Y. Study on Purification and Characterization of Polyphenol Oxidase from Acetes chinensis. Molecules. 2021; 26(24):7545. https://doi.org/10.3390/molecules26247545
Chicago/Turabian StyleZhang, Jianyou, Guangcheng Zhou, Lifeng Fei, Lifan Chen, Lei Sun, Fei Lyu, and Yuting Ding. 2021. "Study on Purification and Characterization of Polyphenol Oxidase from Acetes chinensis" Molecules 26, no. 24: 7545. https://doi.org/10.3390/molecules26247545
APA StyleZhang, J., Zhou, G., Fei, L., Chen, L., Sun, L., Lyu, F., & Ding, Y. (2021). Study on Purification and Characterization of Polyphenol Oxidase from Acetes chinensis. Molecules, 26(24), 7545. https://doi.org/10.3390/molecules26247545