Mapping the Chemistry of Hair Strands by Mass Spectrometry Imaging—A Review
Abstract
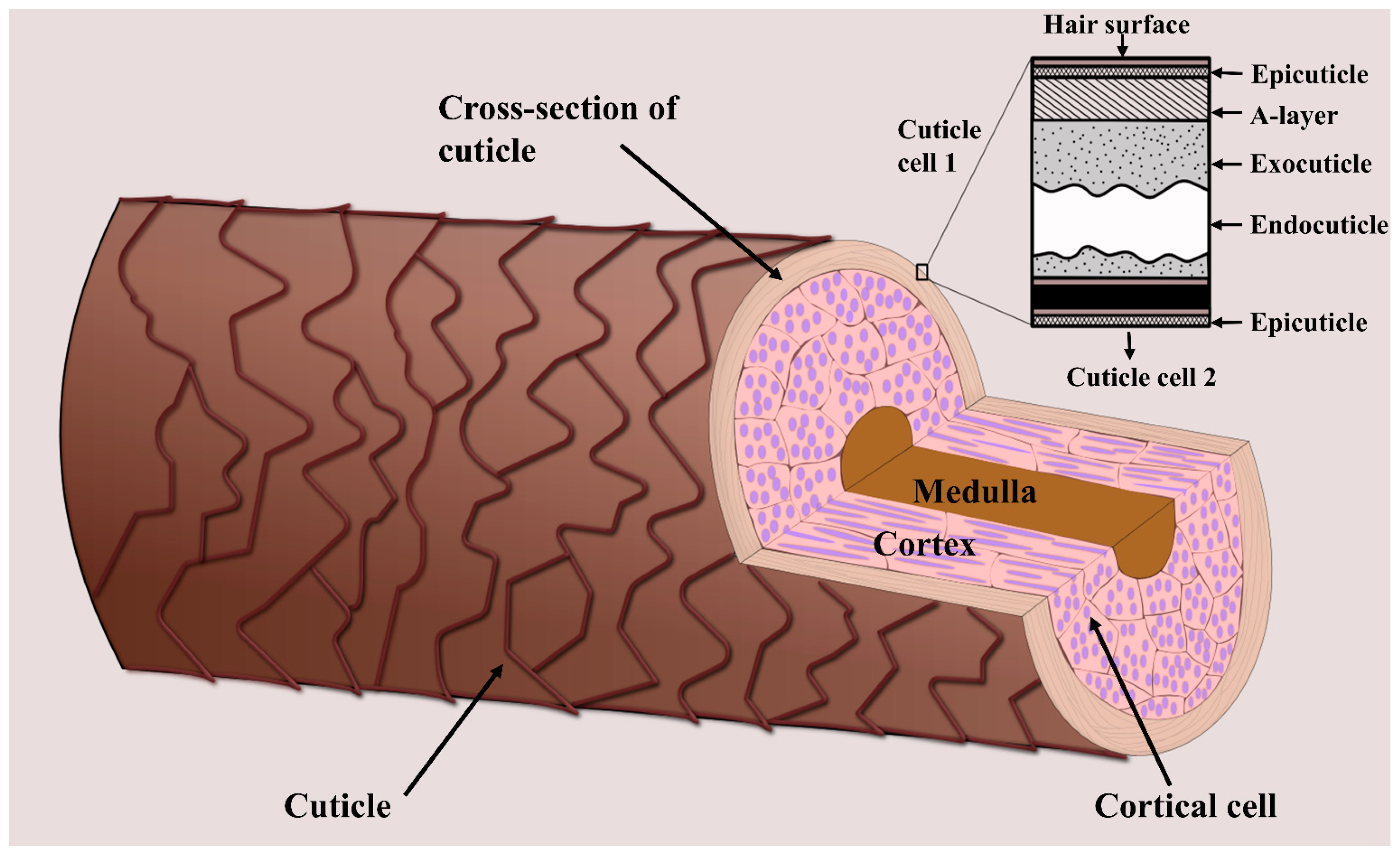
1. Introduction
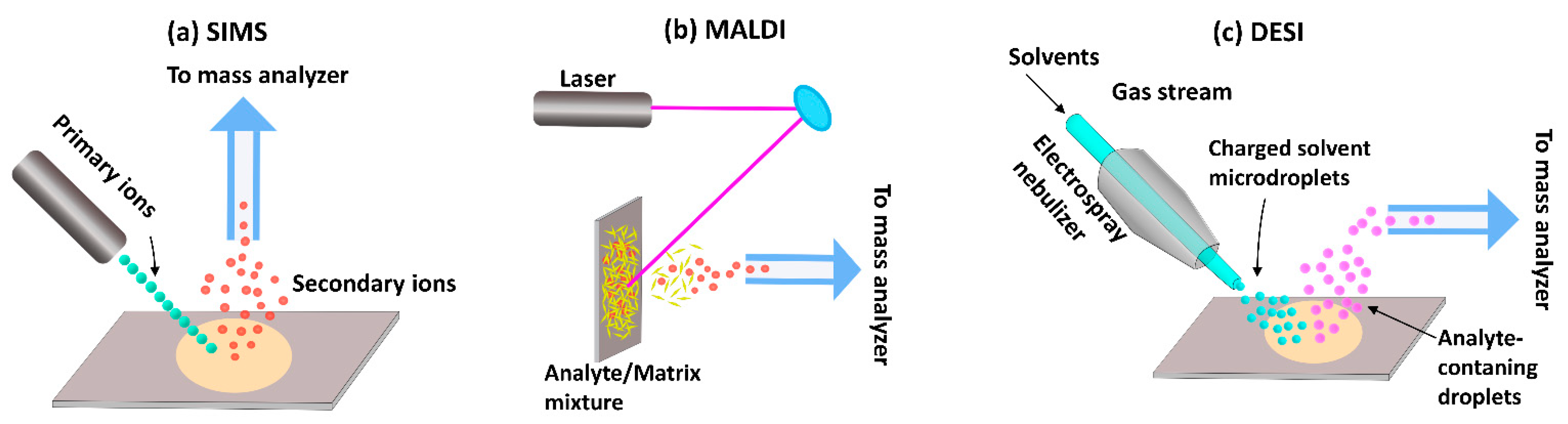
2. Techniques over Time—From Bulk Analysis to High Resolution Mapping
| TOF Reflectron | Magnetic Sector | Orbitrap | FTICR | |
|---|---|---|---|---|
| Primary ion | Pulsed | Continuous | Pulsed | Pulsed |
| Upper mass limit | 10,000 | 20,000 | 50,000 | 30,000 |
| Mass resolution | 15,000 | <100,000 | >100,000 | 1,000,000 |
| Mass accuracy | <5 ppm | <3 ppm | <5 ppm | <1 ppm |
| MS/MS | MS | MS2 | No | MSn |
| Advantages | Good mass accuracy Fast scan speed | High mass accuracy and resolution | High mass accuracy and resolution | High mass accuracy and resolution |
| Drawbacks | Medium mass resolution | Expensive | Expensive | Low scan speed Expensive |
3. Analysis of Hair by Mass Spectrometry Imaging
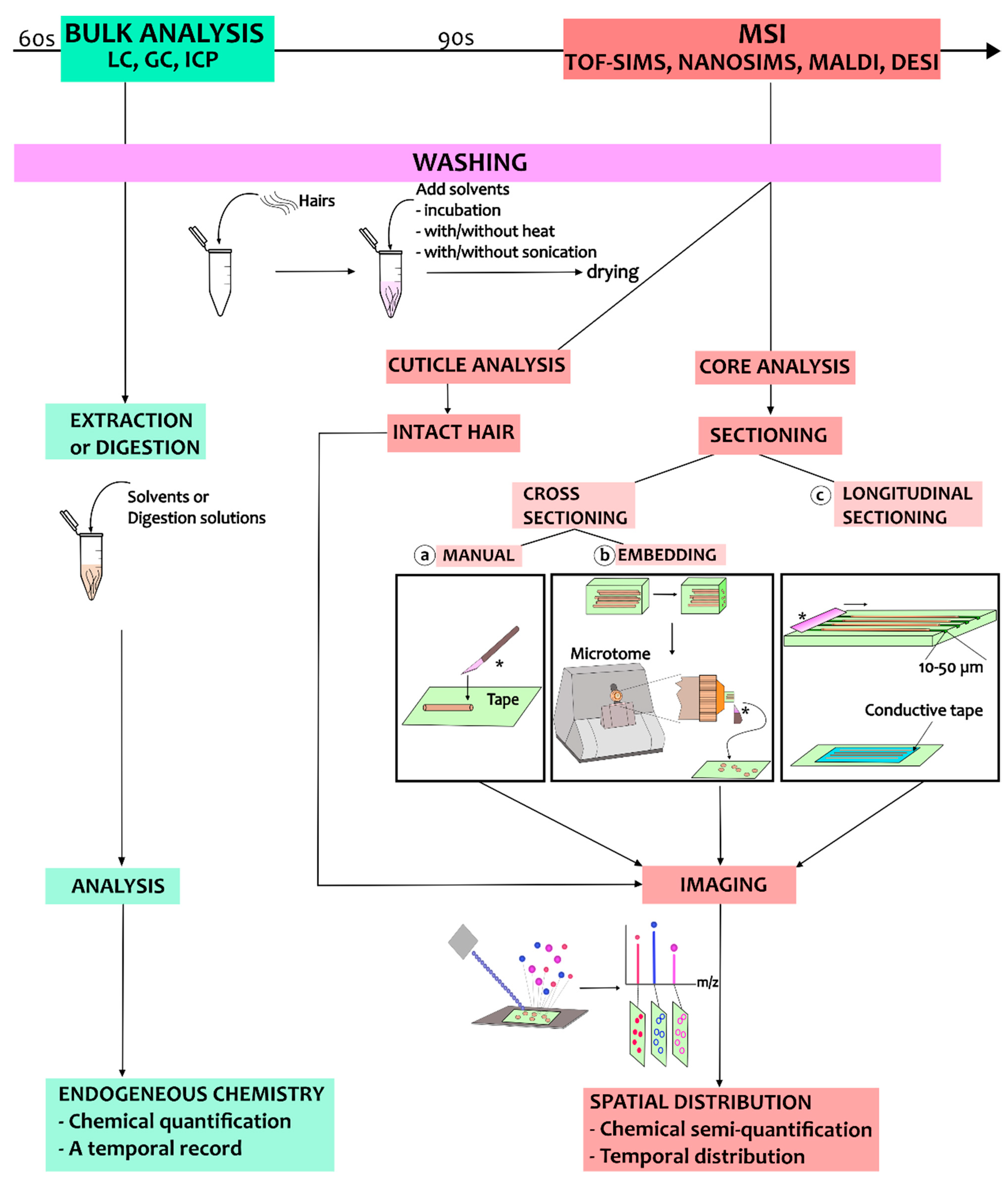
4. Sample Preparation Procedures: Considerations, Pitfalls, and Use Cases
4.1. Hair Core Analysis
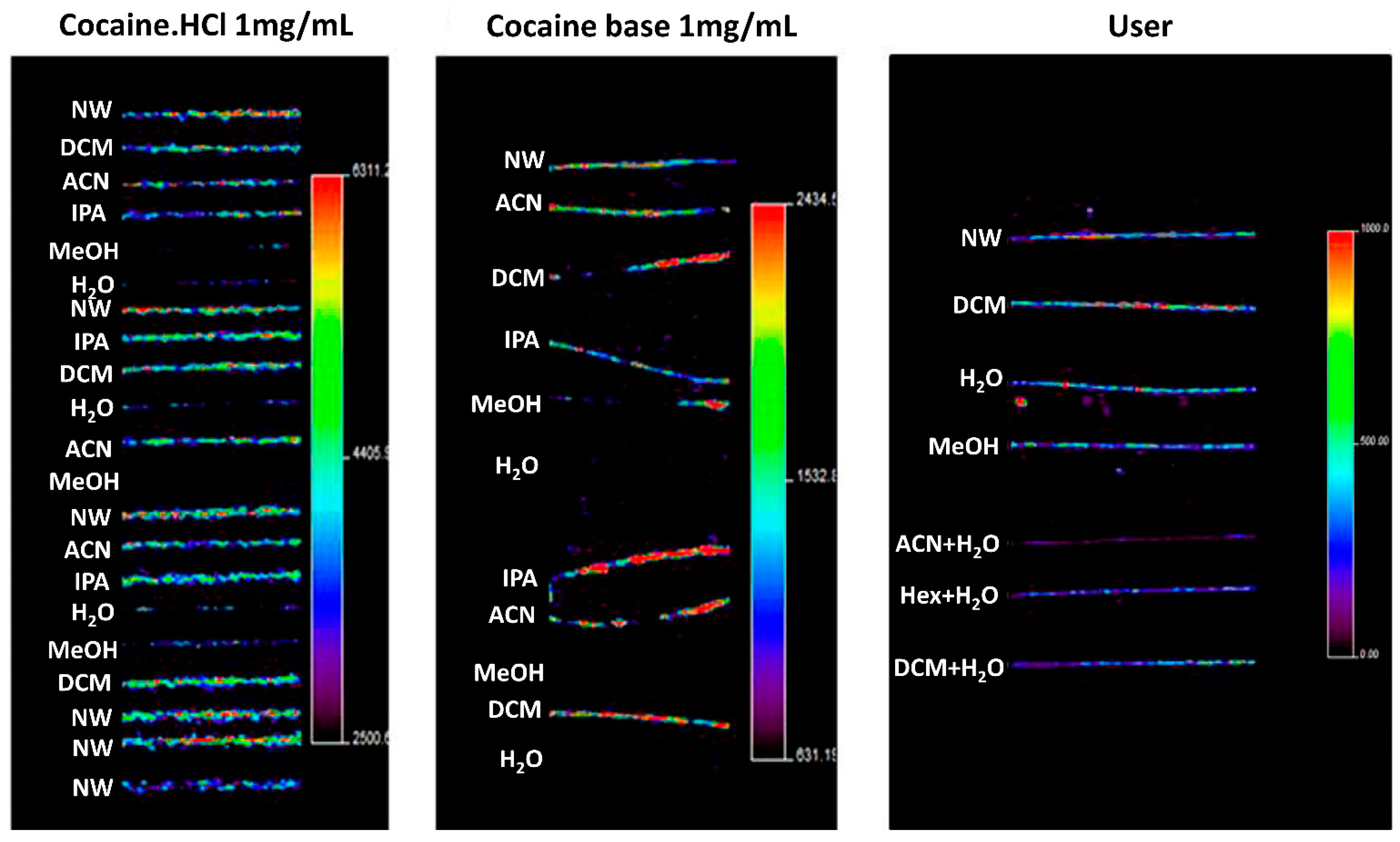
4.1.1. Washing Procedures
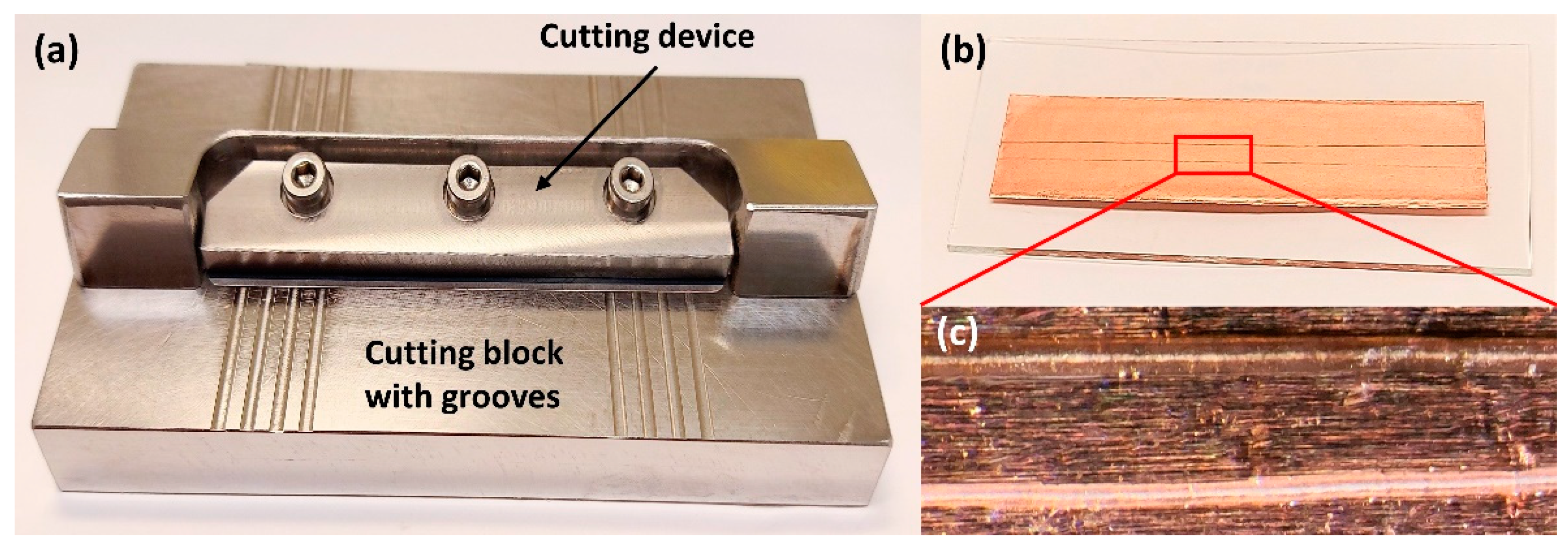
4.1.2. Cross-Sectioning of Hair Strands
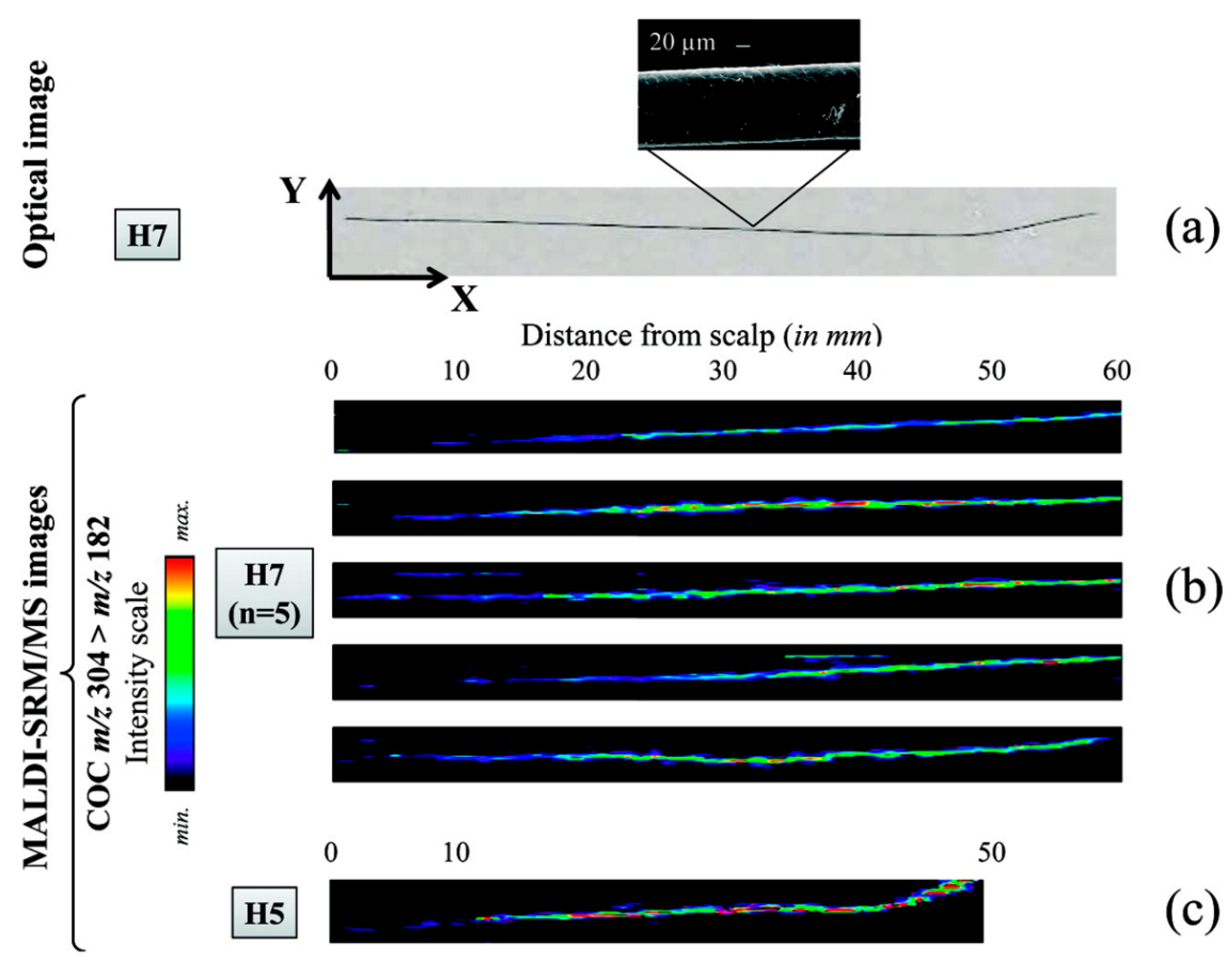
4.1.3. Longitudinal Sectioning of Hair Strands
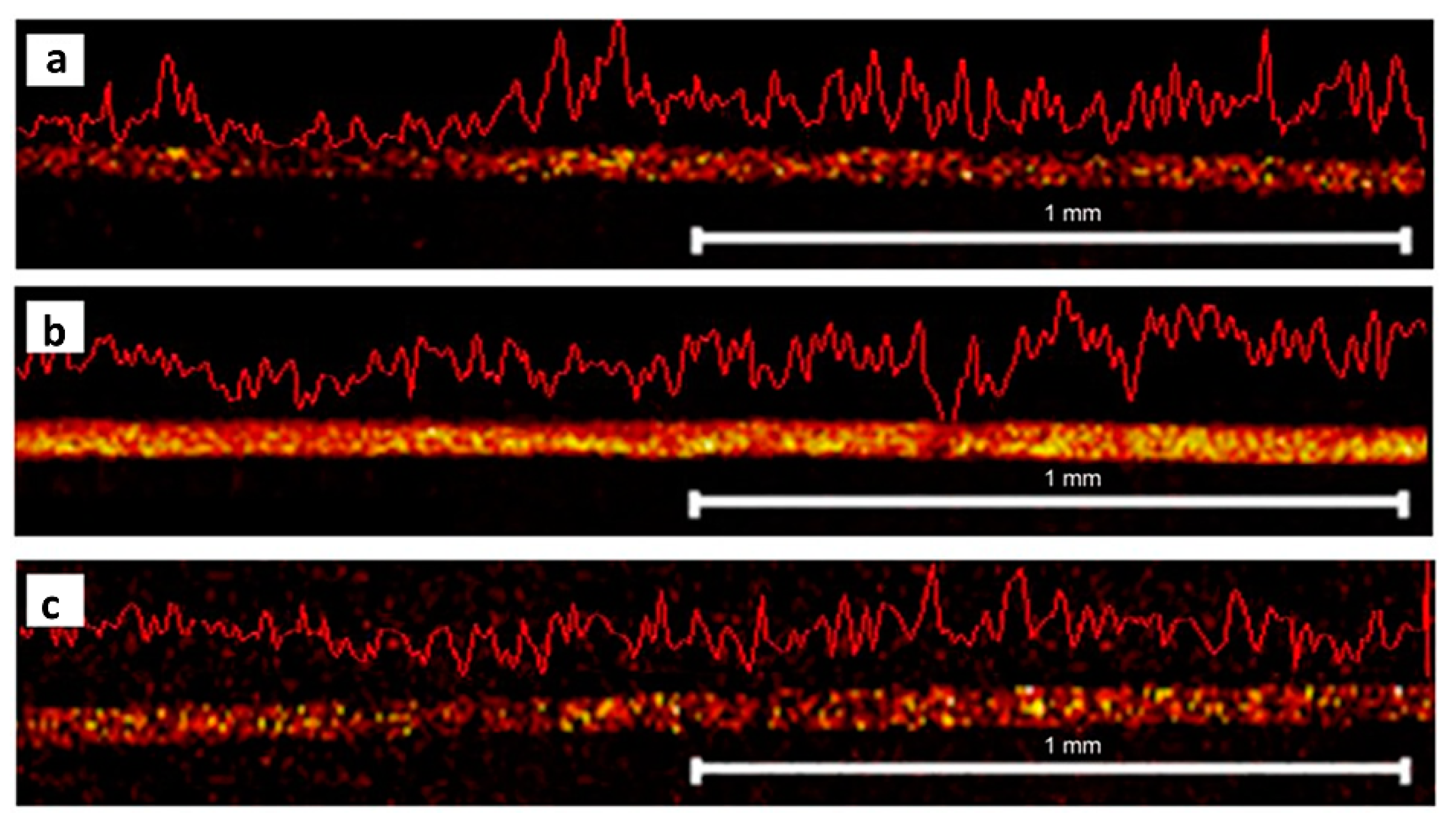
4.2. Hair Cuticle Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nuttall, K.L. Review: Interpreting hair mercury levels in individual patients. Ann. Clin. Lab. Sci. 2006, 36, 248–261. [Google Scholar] [PubMed]
- Tipple, B.J.; Valenzuela, L.O.; Ehleringer, J.R. Strontium isotope ratios of human hair record intra-city variations in tap water source. Sci. Rep. 2018, 8, 3334. [Google Scholar] [CrossRef]
- Ammer, S.T.M.; Kootker, L.M.; Bartelink, E.J.; Anderson, B.E.; Cunha, E.; Davies, G.R. Comparison of strontium isotope ratios in Mexican human hair and tap water as provenance indicators. Forensic Sci. Int. 2020, 314, 110422. [Google Scholar] [CrossRef]
- Saitoh, M.; Uzuka, M.; Sakamoto, M. Human hair cycle. J. Investig. Dermatol. 1970, 54, 65. [Google Scholar] [CrossRef] [PubMed]
- Pragst, F.; Balikova, M.A. State of the art in hair analysis for detection of drug and alcohol abuse. Clin. Chim. Acta 2006, 370, 17–49. [Google Scholar] [CrossRef]
- Vandevenne, M.; Vandenbussche, H.; Verstraete, A. Detection time of drugs of abuse in urine. Acta Clin. Belg. 2000, 55, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, A.G. Detection times of drugs of abuse in blood, urine, and oral fluid. Ther. Drug. Monit. 2004, 26, 200–205. [Google Scholar] [CrossRef]
- Shima, N.; Sasaki, K.; Kamata, T.; Matsuta, S.; Wada, M.; Kakehashi, H.; Nakano, S.; Kamata, H.; Nishioka, H.; Sato, T.; et al. Incorporation of zolpidem into hair and its distribution after a single administration. Drug Metab. Dispos. 2017, 45, 286–293. [Google Scholar] [CrossRef]
- Uhl, M. Determination of drugs in hair using GC/MS/MS. Forensic Sci. Int. 1997, 84, 281–294. [Google Scholar] [CrossRef]
- Rodushkin, I.; Axelsson, M.D. Application of double focusing sector field ICP-MS for multielemental characterization of human hair and nails. Part, I. Analytical methodology. Sci. Total Environ. 2000, 250, 83–100. [Google Scholar] [CrossRef]
- Kronstrand, R.; Nystrom, I.; Strandberg, J.; Druid, H. Screening for drugs of abuse in hair with ion spray LC-MS-MS. Forensic Sci. Int. 2004, 145, 183–190. [Google Scholar] [CrossRef]
- Salmela, S.; Vuori, E.; Kilpio, J.O. The effect of washing procedures on trace-element content of human-hair. Anal. Chim. Acta 1981, 125, 131–137. [Google Scholar] [CrossRef]
- Borella, P.; Rovesti, S.; Caselgrandi, E.; Bargellini, A. Quality control in hair analysis: A systematic study on washing procedures for trace element determinations. MikroChim. Acta 1996, 123, 271–280. [Google Scholar] [CrossRef]
- Pineda-Vargas, C.A.; Eisa, M.E.M. Analysis of human hair cross sections from two different population groups by Nuclear Microscopy. Nucl. Instrum. Meth. B 2010, 268, 2164–2167. [Google Scholar] [CrossRef]
- Beasley, D.; Gomez-Morilla, I.; Spyrou, N. Elemental analysis of hair using PIXE-tomography and INAA. J. Radioanal. Nucl. Chem. 2008, 276, 101–105. [Google Scholar] [CrossRef]
- Gillen, G.; Roberson, S.; Ng, C.; Stranick, M. Elemental and molecular imaging of human hair using secondary ion mass spectrometry. Scanning 1999, 21, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Erne, R.; Bernhard, L.; Kawecki, M.; Baumgartner, M.R.; Kraemer, T. Using time-of-flight secondary ion mass spectrometry (ToF-SIMS) and matrix assisted laser desorption/ionization mass spectrometry (MALDI-MS) for investigations on single hair samples to solve the contamination versus incorporation issue of hair analysis in the case of cocaine and methadone. Analyst 2020, 145, 4906–4919. [Google Scholar] [PubMed]
- Robbins, C.R. Chemical and Physical Behavior of Human Hair, 5th ed.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Rogers, G.E. Known and unknown features of hair cuticle structure: E brief review. Cosmetics 2019, 6, 32. [Google Scholar] [CrossRef]
- Parry, D.A.D.; Smith, T.A.; Rogers, M.A.; Schweizer, J. Human hair keratin-associated proteins: Sequence regularities and structural implications. J. Struct. Biol. 2006, 155, 361–369. [Google Scholar] [CrossRef]
- Rogers, M.A.; Langbein, L.; Praetzel-Wunder, S.; Winter, H.; Schweizer, J. Human hair keratin-associated proteins (KAPs). Int. Rev. Cytol. 2006, 251, 209–263. [Google Scholar]
- Sinclair, R.D. Healthy hair: What is it? J. Investig. Derm. Symp. Proc. 2007, 12, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Gavazzoni Dias, M.F. Hair cosmetics: An overview. Int. J. Trichol. 2015, 7, 2–15. [Google Scholar] [CrossRef]
- Kintz, P.; Salomone, A.; Vincenti, M. Hair Analysis in Clinical and Forensic Toxicology, 1st ed.; Academic Press; Elsevier: London, UK, 2015. [Google Scholar]
- Valentine, J.L.; Kang, H.K.; Spivey, G. Arsenic levels in human blood, urine, and hair in response to exposure via drinking water. Environ. Res. 1979, 20, 24–32. [Google Scholar] [CrossRef]
- Harrison, W.W.; Yurachek, J.P.; Benson, C.A. Determination of trace elements in human hair by atomic absorption spectroscopy. Clin. Chim. Acta 1969, 23, 83–91. [Google Scholar] [CrossRef]
- Backer, E.T. Chloric acid digestion in determination of trace metals (Fe, Zn and Cu) in brain and hair by atomic absorption spectrophotometry. Clin. Chim. Acta 1969, 24, 233–238. [Google Scholar] [CrossRef]
- Rodushkin, I.; Axelsson, M.D. Application of double focusing sector field ICP-MS for multielemental characterization of human hair and nails. Part II. A study of the inhabitants of northern Sweden. Sci. Total Environ. 2000, 262, 21–36. [Google Scholar] [CrossRef]
- Ulrich, A.; Moor, C.; Vonmont, H.; Jordi, H.R.; Lory, M. ICP-MS trace-element analysis as a forensic tool. Anal. Bioanal. Chem. 2004, 378, 1059–1068. [Google Scholar] [CrossRef]
- Moor, C.; Lymberopoulou, T.; Dietrich, V.J. Determination of heavy metals in soils, sediments and geological materials by ICP-AES and ICP-MS. MikroChim. Acta 2001, 136, 123–128. [Google Scholar] [CrossRef]
- Baumgartner, A.M.; Jones, P.F.; Baumgartner, W.A.; Black, C.T. Radioimmunoassay of hair for determining opiate-abuse histories. J. Nucl. Med. 1979, 20, 748–752. [Google Scholar]
- Cheze, M.; Duffort, G.; Deveaux, M.; Pepin, G. Hair analysis by liquid chromatography-tandem mass spectrometry in toxicological investigation of drug-facilitated crimes: Report of 128 cases over the period June 2003-May 2004 in metropolitan Paris. Forensic Sci. Int. 2005, 153, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Hegstad, S.; Khiabani, H.Z.; Kristoffersen, L.; Kunoe, N.; Lobmaier, P.P.; Christophersen, A.S. Drug screening of hair by liquid chromatography-tandem mass spectrometry. J. Anal. Toxicol. 2008, 32, 364–372. [Google Scholar] [CrossRef]
- Klausz, G.; Keller, E.; Rona, K. Hair analysis of abused drugs with gas-chromatography mass spectrometry. Acta Pharm. Hung. 2009, 79, 47–56. [Google Scholar] [PubMed]
- Wainhaus, S.B.; Tzanani, N.; Dagan, S.; Miller, M.L.; Amirav, A. Fast analysis of drugs in a single hair. J. Am. Soc. Mass Spectrom. 1998, 9, 1311–1320. [Google Scholar] [CrossRef]
- Kintz, P.; Mangin, P. Simultaneous determination of opiates, cocaine and major metabolites of cocaine in human hair by gas chromotography/mass spectrometry (GC/MS). Forensic Sci. Int. 1995, 73, 93–100. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cheong, J.C.; Lee, J.I.; In, M.K. Improved gas chromatography-negative ion chemical ionization tandem mass spectrometric method for determination of 11-nor-Δ9-tetrahydrocannabinol-9-carboxylic acid in hair using mechanical pulverization and bead-assisted liquid-liquid extraction. Forensic Sci. Int. 2011, 206, e99–e102. [Google Scholar] [CrossRef]
- Stranorossi, S.; Bermejobarrera, A.; Chiarotti, M. Segmental hair analysis for cocaine and heroin abuse determination. Forensic Sci. Int. 1995, 70, 211–216. [Google Scholar] [CrossRef]
- Zimmerley, M.; Lin, C.Y.; Oertel, D.C.; Marsh, J.M.; Ward, J.L.; Potma, E.O. Quantitative detection of chemical compounds in human hair with coherent anti-Stokes Raman scattering microscopy. J. Biomed. Opt. 2009, 14, 044019. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lorentz, K.O.; de Nolf, W.; Cotte, M.; Ioannou, G.; Foruzanfar, F.; Zaruri, M.R.; Sajjadi, S.M.S. Synchrotron radiation micro X-Ray Fluorescence (SR-μXRF) elemental mapping of ancient hair: Metals and health at 3rd millennium BCE Shahr-i Sokhta, Iran. J. Archaeol. Sci. 2020, 120, 105193. [Google Scholar] [CrossRef]
- Smart, K.E.; Kilburn, M.; Schroeder, M.; Martin, B.G.H.; Hawes, C.; Marsh, J.M.; Grovenor, C.R.M. Copper and calcium uptake in colored hair. J. Cosmet. Sci. 2009, 60, 337–345. [Google Scholar] [CrossRef]
- Waki, M.L.; Onoue, K.; Takahashi, T.; Goto, K.; Saito, Y.; Inami, K.; Makita, I.; Angata, Y.; Suzuki, T.; Yamashita, M.; et al. Investigation by imaging mass spectrometry of biomarker candidates for aging in the hair cortex. PLoS ONE 2011, 6, e26721. [Google Scholar] [CrossRef]
- Solazzo, C. Follow-up on the characterization of peptidic markers in hair and fur for the identification of common North American species. Rapid Commun. Mass Spectrom. 2017, 31, 1375–1384. [Google Scholar] [CrossRef]
- Audinot, J.N.; Schneider, S.; Yegles, M.; Hallegot, P.; Wennig, R.; Migeon, H.N. Imaging of arsenic traces in human hair by nano-SIMS 50. Appl. Surf. Sci. 2004, 231, 490–496. [Google Scholar] [CrossRef]
- Holzlechner, G.; Kubicek, M.; Hutter, H.; Fleig, J. A novel ToF-SIMS operation mode for improved accuracy and lateral resolution of oxygen isotope measurements on oxides. J. Anal. Atom. Spectrom. 2013, 28, 1080–1089. [Google Scholar] [CrossRef]
- Weber, R.J.M.; Southam, A.D.; Sommer, U.; Viant, M.R. Characterization of isotopic abundance measurements in high resolution ft-icr and orbitrap mass spectra for improved confidence of metabolite identification. Anal. Chem. 2011, 83, 3737–3743. [Google Scholar] [CrossRef] [PubMed]
- Erne, R.; Bernard, L.; Steuer, A.E.; Baumgartner, M.R.; Kraemer, T. Hair analysis: Contamination versus incorporation from the circulatory system—investigations on single hair samples using time-of-flight secondary ion mass spectrometry and matrix-assisted laser desorption/ionization mass spectrometry. Anal. Chem. 2019, 91, 4132–4139. [Google Scholar] [CrossRef]
- Miki, A.; Katagi, M.; Kamata, T.; Zaitsu, K.; Tatsuno, M.; Nakanishi, T.; Tsuchihashi, H.; Takubo, T.; Suzuki, K. MALDI-TOF and MALDI-FTICR imaging mass spectrometry of methamphetamine incorporated into hair. J. Mass Spectrom. 2011, 46, 411–416. [Google Scholar] [CrossRef]
- Shen, M.; Xiang, P.; Shi, Y.; Pu, H.; Yan, H.; Shen, B. Mass imaging of ketamine in a single scalp hair by MALDI-FTMS. Anal. Bioanal. Chem. 2014, 406, 4611–4616. [Google Scholar] [CrossRef] [PubMed]
- Kamata, T.; Shima, N.; Miki, A.; Matsuo, E.; Yamamoto, T.; Tsuchihashi, H.; Sato, T.; Shimma, S.; Katagi, M. High spatial-resolution matrix-assisted Laser desorption/ionization-ion trap-time-of-flight tandem mass spectrometry imaging for depicting longitudinal and transverse distribution of drugs incorporated into hair. Anal. Chem. 2020, 92, 5821–5829. [Google Scholar] [CrossRef]
- Stoeckli, M.; Staab, D.; Schweitzer, A. Compound and metabolite distribution measured by MALDI mass spectrometric imaging in whole-body tissue sections. Int. J. Mass Spectrom. 2007, 260, 195–202. [Google Scholar] [CrossRef]
- De Hoffmann, E.; Stroobant, V. Mass Spectrometry: Principles and Applications; John Wiley, and Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Ghaste, M.; Mistrik, R.; Shulaev, V. Applications of Fourier Transform Ion Cyclotron Resonance (FT-ICR) and orbitrap based high resolution mass spectrometry in metabolomics and lipidomics. Int. J. Mol. Sci. 2016, 17, 816. [Google Scholar] [CrossRef]
- Castaing, R.; Slodzian, G. Optique corpusculaire-premiers essais de microanalyse par emission ionique secondaire. J. Microsc. 1962, 1, 395–399. [Google Scholar]
- Vickerman, J.C. Molecular imaging and depth profiling by mass spectrometry-SIMS, MALDI or DESI? Analyst 2011, 136, 2199–2217. [Google Scholar] [CrossRef] [PubMed]
- Benninghoven, A.; Sichtermann, W.K. Detection, identification and structural investigation of biologically important compounds by secondary ion mass-spectrometry. Anal. Chem. 1978, 50, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Touboul, D.; Kollmer, F.; Niehuis, E.; Brunelle, A.; Laprevote, O. Improvement of biological time-of-flight-secondary ion mass spectrometry imaging with a bismuth cluster ion source. J. Am. Soc. Mass Spectr. 2005, 16, 1608–1618. [Google Scholar] [CrossRef]
- Fletcher, J.S.; Lockyer, N.P.; Vaidyanathan, S.; Vickerman, J.C. TOF-SIMS 3D biomolecular imaging of Xenopus laevis oocytes using buckminsterfullerene (C-60) primary ions. Anal. Chem. 2007, 79, 2199–2206. [Google Scholar] [CrossRef]
- Tian, H.; Sparvero, L.J.; Blenkinsopp, P.; Amoscato, A.A.; Watkins, S.C.; Bayir, H.; Kagan, V.E.; Winograd, N. Secondary-ion mass spectrometry images cardiolipins and phosphatidylethanolamines at the subcellular level. Angew. Chem. Int. Ed. 2019, 58, 3156–3161. [Google Scholar] [CrossRef]
- Nilsson, K.D.; Granden, J.; Farewell, A.; Fletcher, J.S. Interrogation of chemical changes on, and through, the bacterial envelope of Escherichia coli FabF mutant using time-of-flight secondary ion mass spectrometry. Surf. Interface Anal. 2021, 53, 1006–1012. [Google Scholar] [CrossRef]
- Rubakhin, S.S.; Sweedler, J.V. A mass spectrometry primer for mass spectrometry imaging. Methods Mol. Biol. 2010, 656, 21–49. [Google Scholar]
- Beasley, E.; Francese, S.; Bassindale, T. Detection and mapping of cannabinoids in single hair samples through rapid derivatization and matrix-assisted laser desorption ionization mass spectrometry. Anal. Chem. 2016, 88, 10328–10334. [Google Scholar] [CrossRef]
- Bai, H.R.; Wang, S.J.; Liu, J.J.; Gao, D.; Jiang, Y.Y.; Liu, H.X.; Cai, Z.W. Localization of ginsenosides in Panax ginseng with different age by matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry imaging. J. Chromatogr. B 2016, 1026, 263–271. [Google Scholar] [CrossRef]
- Kaya, I.; Brinet, D.; Michno, W.; Baskurt, M.; Zetterbergt, H.; Blenow, K.; Hanrieder, J. Novel trimodal MALDI imaging mass spectrometry (ims3) at 10 mu m reveals spatial lipid and peptide correlates implicated in a beta plaque pathology in alzheimer’s disease. ACS Chem. NeuroSci. 2017, 8, 2778–2790. [Google Scholar] [CrossRef] [PubMed]
- Stoeckli, M.; Chaurand, P.; Hallahan, D.E.; Caprioli, R.M. Imaging mass spectrometry: A new technology for the analysis of protein expression in mammalian tissues. Nat. Med. 2001, 7, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Hopfgartner, G.; Varesio, E.; Stoeckli, M. Matrix-assisted laser desorption/ionization mass spectrometric imaging of complete rat sections using a triple quadrupole linear ion trap. Rapid Commun. Mass Spectrom. 2009, 23, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Parrot, D.; Papazian, S.; Foil, D.; Tasdemir, D. Imaging the unimaginable: Desorption electrospray ionization-imaging mass spectrometry (desi-ims) in natural product research. Planta Med. 2018, 84, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Richel, A.; Vanderghem, C.; Simon, M.; Wathelet, B.; Paquot, M. Evaluation of matrix-assisted laser desorption/ionization mass spectrometry for second-generation lignin analysis. Anal. Chem. Insights 2012, 7, 79–89. [Google Scholar] [CrossRef]
- Wojtowicz, A.; Wietecha-Posluszny, R. DESI-MS analysis of human fluids and tissues for forensic applications. Appl. Phys. A 2019, 125, 1–9. [Google Scholar] [CrossRef]
- Lin, L.E.; Chen, C.L.; Huang, Y.C.; Chung, H.H.; Lin, C.W.; Chen, K.C.; Peng, Y.J.; Ding, S.T.; Wang, M.Y.; Shen, T.L.; et al. Precision biomarker discovery powered by microscopy image fusion-assisted high spatial resolution ambient ionization mass spectrometry imaging. Anal. Chim. Acta 2020, 1100, 75–87. [Google Scholar] [CrossRef]
- Soudah, T.; Zoabi, A.; Margulis, K. Desorption electrospray ionization mass spectrometry imaging in discovery and development of novel therapies. Mass Spectrom. Rev. 2021. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, A.; Kollmer, F.; Sohn, S.; Hook, F.; Sjovall, P. Spatial-resolution limits in mass spectrometry imaging of supported lipid bilayers and individual lipid vesicles. Anal. Chem. 2010, 82, 2426–2433. [Google Scholar] [CrossRef]
- Zavalin, A.; Yang, J.H.; Haase, A.; Holle, A.; Caprioli, R. Implementation of a gaussian beam laser and aspheric optics for high spatial resolution MALDI imaging MS. J. Am. Soc. Mass Spectr. 2014, 25, 1079–1082. [Google Scholar] [CrossRef]
- Bouschen, W.; Schulz, O.; Eikel, D.; Spengler, B. Matrix vapor deposition/recrystallization and dedicated spray preparation for high-resolution scanning microprobe matrix-assisted laser desorption/ionization imaging mass spectrometry (SMALDI-MS) of tissue and single cells. Rapid Commun. Mass Spectrom. 2010, 24, 355–364. [Google Scholar] [CrossRef]
- Kaya, I.; Jennische, E.; Lange, S.; Malmberg, P. Multimodal chemical imaging of a single brain tissue section using ToF-SIMS, MALDI-ToF and immuno/histochemical staining. Analyst 2021, 146, 1169–1177. [Google Scholar] [CrossRef]
- Flinders, B.; Cuypers, E.; Porta, T.; Varesio, E.; Hopfgartner, G.; Heeren, R.M.A. Mass spectrometry imaging of drugs of abuse in hair. Methods Mol. Biol. 2017, 1618, 137–147. [Google Scholar]
- Lanekoff, I.; Laskin, J. Quantitative mass spectrometry imaging of molecules in biological systems. Adv. Chromatogr. 2018, 54, 43–72. [Google Scholar]
- Seah, M.P.; Shard, A.G. The matrix effect in secondary ion mass spectrometry. Appl Surf. Sci. 2018, 439, 605–611. [Google Scholar] [CrossRef]
- Angerer, T.B.; Pour, M.D.; Malmberg, P.; Fletcher, J.S. Improved molecular imaging in rodent brain with time-of-flight-secondary ion mass spectrometry using gas cluster ion beams and reactive vapor exposure. Anal. Chem. 2015, 87, 4305–4313. [Google Scholar] [CrossRef] [PubMed]
- Pour, M.D.; Malmberg, P.; Ewing, A. An investigation on the mechanism of sublimed DHB matrix on molecular ion yields in SIMS imaging of brain tissue. Anal. Bioanal. Chem. 2016, 408, 3071–3081. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y. Matrix-assisted laser desorption/ionization mass spectrometric imaging for the rapid segmental analysis of methamphetamine in a single hair using umbelliferone as a matrix. Anal. Chim. Acta 2017, 975, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Stenn, K.S.; Paus, R. Controls of hair follicle cycling. Physiol. Rev. 2001, 81, 449–494. [Google Scholar] [CrossRef]
- Nakanishi, T.; Nirasawa, T.; Takubo, T. Quantitative mass barcode-like image of nicotine in single longitudinally sliced hair sections from long-term smokers by matrix-assisted laser desorption time-of-flight mass spectrometry imaging. J. Anal. Toxicol. 2014, 38, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Kempson, I.M.; Skinner, W.M. A comparison of washing methods for hair mineral analysis: Internal versus external effects. Biol. Trace Elem. Res. 2012, 150, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.A.; Kronstrand, R.; Kintz, P.; Society of Hair, T. Society of hair testing guidelines for drug testing in hair. Forensic Sci. Int. 2012, 218, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Society of Hair, T. Recommendations for hair testing in forensic cases. Forensic Sci. Int. 2004, 145, 83–84. [Google Scholar]
- Cuypers, E.; Flinders, B.; Boone, C.M.; Bosman, I.J.; Lusthof, K.J.; Van Asten, A.C.; Tytgat, J.; Heeren, R.M. Consequences of decontamination procedures in forensic hair analysis using metal-assisted secondary ion mass spectrometry analysis. Anal. Chem. 2016, 88, 3091–3097. [Google Scholar] [CrossRef] [PubMed]
- Kintz, P. Analytical and Practical Aspects of Drug Testing in Hair, 1st ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Poetzsch, M.; Baumgartner, M.R.; Steuer, A.E.; Kraemer, T. Segmental hair analysis for differentiation of tilidine intake from external contamination using LC-ESI-MS/MS and MALDI-MS/MS imaging. Drug Test. Anal. 2015, 7, 143–149. [Google Scholar] [CrossRef]
- Wikramanayake, T.C.; Mauro, L.M.; Tabas, I.A.; Chen, A.L.; Llanes, I.C.; Jimenez, J.J. Cross-section trichometry: A clinical tool for assessing the progression and treatment response of alopecia. Int. J. Trichol. 2012, 4, 259–264. [Google Scholar] [PubMed]
- Ruetsch, S.B.; Kamath, Y.K.; Rele, A.S.; Mohile, R.B. Secondary ion mass spectrometric investigation of penetration of coconut and mineral oils into human hair fibers: Relevance to hair damage. J. Cosmet Sci. 2001, 52, 169–184. [Google Scholar]
- Kojima, T.; Yamada, H.; Isobe, M.; Yamamoto, T.; Takeuchi, M.; Aoki, D.; Matsushita, Y.; Fukushima, K. Compositional changes of human hair melanin resulting from bleach treatment investigated by nanoscale secondary ion mass spectrometry. Skin Res. Technol. 2014, 20, 416–421. [Google Scholar] [CrossRef]
- Hindmarsh, J.T. Caveats in hair analysis in chronic arsenic poisoning. Clin. Biochem. 2002, 35, 1–11. [Google Scholar] [CrossRef]
- Hallegot, P.; Corcuff, P. High-spatial-resolution maps of sulphur from human hair sections: An EELS study. J. Microsc. 1993, 172, 131–136. [Google Scholar] [CrossRef]
- Cooper, R.B.; Kirk, P.L. An improved technique for sectioning hairs. J. Crim. Law Criminol. Police Sci. 1953, 44, 124–127. [Google Scholar] [CrossRef]
- O’Malley, J.T.; Merchant, S.N.; Burgess, B.J.; Jones, D.D.; Adams, J.C. Effects of fixative and embedding medium on morphology and immunostaining of the cochlea. Audiol. Neurotol. 2009, 14, 78–87. [Google Scholar]
- Hess, W.M.; Seegmiller, R.E. Computerized image-analysis of resin-embedded hair. Trans. Am. Microsc. Soc. 1988, 107, 421–425. [Google Scholar] [CrossRef]
- Bertrand, L.; Vichi, A.; Doucet, J.; Walter, P.; Blanchard, P. The fate of archaeological keratin fibres in a temperate burial context: Microtaphonomy study of hairs from Marie de Bretagne (15th c., Orléans, France). J. Archaeol. Sci. 2014, 42, 487–499. [Google Scholar] [CrossRef]
- Gill, E.L.; Yost, R.A.; Vedam-Mai, V.; Garrett, T.J. Precast gelatin-based molds for tissue embedding compatible with mass spectrometry imaging. Anal. Chem. 2017, 89, 576–580. [Google Scholar] [CrossRef]
- Chen, R.; Hui, L.; Sturm, R.M.; Li, L. Three dimensional mapping of neuropeptides and lipids in crustacean brain by mass spectral imaging. J. Am. Soc. Mass Spectrom. 2009, 20, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.A.; Reyzer, M.L.; Caprioli, R.M. Direct tissue analysis using matrix-assisted laser desorption/ionization mass spectrometry: Practical aspects of sample preparation. J. Mass Spectrom. 2003, 38, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Wortmann, F.J.; Wortmann, G.; Sripho, T. Why is hair curly?—Deductions from the structure and the biomechanics of the mature hair shaft. Exp. Dermatol. 2020, 29, 366–372. [Google Scholar] [CrossRef]
- Mohammad, N.I. Simple modified freezing technique for identification of human scalp and pubic hairs. Egypt. J. Forensic Sci. 2012, 2, 69–72. [Google Scholar] [CrossRef]
- Hutchinson, P.E.; Thompson, J.R. The cross-sectional size and shape of human terminal scalp hair. Br. J. Dermatol. 1997, 136, 159–165. [Google Scholar]
- Kempson, I.M.; Skinner, W.M. ToF-SIMS analysis of elemental distributions in human hair. Sci. Total Environ. 2005, 338, 213–227. [Google Scholar] [CrossRef]
- Kempson, I.M.; Skinner, W.M.; Kirkbride, P.K. A method for the longitudinal sectioning of single hair samples. J. Forensic Sci. 2002, 47, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Kamata, T.; Shima, N.; Sasaki, K.; Matsuta, S.; Takei, S.; Katagi, M.; Miki, A.; Zaitsu, K.; Nakanishi, T.; Sato, T.; et al. Time-course mass spectrometry imaging for depicting drug incorporation into hair. Anal. Chem. 2015, 87, 5476–5481. [Google Scholar] [CrossRef] [PubMed]
- Flinders, B.; Cuypers, E.; Zeijlemaker, H.; Tytgat, J.; Heeren, R.M. Preparation of longitudinal sections of hair samples for the analysis of cocaine by MALDI-MS/MS and TOF-SIMS imaging. Drug Test. Anal. 2015, 7, 859–865. [Google Scholar] [CrossRef]
- Kojima, T.; Yamada, H.; Saito, Y.; Nawa, T.; Isobe, M.; Yamamoto, T.; Aoki, D.; Matsushita, Y.; Fukushima, K. Investigation of dyeing behavior of oxidative dye in fine structures of the human hair cuticle by nanoscale secondary ion mass spectrometry. Skin Res. Technol. 2015, 21, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Porta, T.; Grivet, C.; Kraemer, T.; Varesio, E.; Hopfgartner, G. Single hair cocaine consumption monitoring by mass spectrometric imaging. Anal. Chem. 2011, 83, 4266–4272. [Google Scholar] [CrossRef] [PubMed]
- Poetzsch, M.; Steuer, A.E.; Roemmelt, A.T.; Baumgartner, M.R.; Kraemer, T. Single hair analysis of small molecules using MALDI-triple quadrupole MS imaging and LC-MS/MS: Investigations on opportunities and pitfalls. Anal. Chem. 2014, 86, 11758–11765. [Google Scholar] [CrossRef]
- Gilliland, W.M., Jr.; Prince, H.M.A.; Poliseno, A.; Kashuba, A.D.M.; Rosen, E.P. Infrared matrix-assisted laser desorption electrospray ionization mass spectrometry imaging of human hair to characterize longitudinal profiles of the antiretroviral maraviroc for adherence monitoring. Anal. Chem. 2019, 91, 10816–10822. [Google Scholar] [CrossRef] [PubMed]
- Gilliland, W.M.; White, N.R.; Yam, B.H.; Mwangi, J.N.; Prince, H.M.A.; Weideman, A.M.; Kashuba, A.D.M.; Rosen, E.P. Influence of hair treatments on detection of antiretrovirals by mass spectrometry imaging. Analyst 2020, 145, 4540–4550. [Google Scholar] [CrossRef]
- Harkey, M.R. Anatomy and physiology of hair. Forensic Sci. Int. 1993, 63, 9–18. [Google Scholar] [CrossRef]
- Cone, E.J.; Yousefnejad, D.; Darwin, W.D.; Maguire, T. Testing human hair for drugs of abuse. II. Identification of unique cocaine metabolites in hair of drug abusers and evaluation of decontamination procedures. J. Anal. Toxicol. 1991, 15, 250–255. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baumgartner, W.A.; Hill, V.A. Sample preparation techniques. Forensic Sci. Int. 1993, 63, 121–135. [Google Scholar] [CrossRef]
- Stout, P.R.; Ropero-Miller, J.D.; Baylor, M.R.; Mitchell, J.M. External contamination of hair with cocaine: Evaluation of external cocaine contamination and development of performance-testing materials. J. Anal. Toxicol. 2006, 30, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.; Carolan, V.A.; Gardiner, P.H.E. Removal of exogenously bound elements from human hair by various washing procedures and determination by inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2002, 455, 23–34. [Google Scholar] [CrossRef]
- Mantinieks, D.; Wright, P.; Di Rago, M.; Gerostamoulos, D. A systematic investigation of forensic hair decontamination procedures and their limitations. Drug Test. Anal. 2019, 11, 1542–1555. [Google Scholar] [CrossRef] [PubMed]
- Bossers, L.C.; Paul, R.; Berry, A.J.; Kingston, R.; Middendorp, C.; Guwy, A.J. An evaluation of washing and extraction techniques in the analysis of ethyl glucuronide and fatty acid ethyl esters from hair samples. J. Chromatogr. B 2014, 953–954. [Google Scholar] [CrossRef]
- Swift, J.A.; Holmes, A.W. Degradation of human hair by papain: Part III: Some electron microscope observations. Text. Res. J. 1965, 35, 1014–1019. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philipsen, M.H.; Haxen, E.R.; Manaprasertsak, A.; Malmberg, P.; Hammarlund, E.U. Mapping the Chemistry of Hair Strands by Mass Spectrometry Imaging—A Review. Molecules 2021, 26, 7522. https://doi.org/10.3390/molecules26247522
Philipsen MH, Haxen ER, Manaprasertsak A, Malmberg P, Hammarlund EU. Mapping the Chemistry of Hair Strands by Mass Spectrometry Imaging—A Review. Molecules. 2021; 26(24):7522. https://doi.org/10.3390/molecules26247522
Chicago/Turabian StylePhilipsen, Mai H., Emma R. Haxen, Auraya Manaprasertsak, Per Malmberg, and Emma U. Hammarlund. 2021. "Mapping the Chemistry of Hair Strands by Mass Spectrometry Imaging—A Review" Molecules 26, no. 24: 7522. https://doi.org/10.3390/molecules26247522
APA StylePhilipsen, M. H., Haxen, E. R., Manaprasertsak, A., Malmberg, P., & Hammarlund, E. U. (2021). Mapping the Chemistry of Hair Strands by Mass Spectrometry Imaging—A Review. Molecules, 26(24), 7522. https://doi.org/10.3390/molecules26247522






