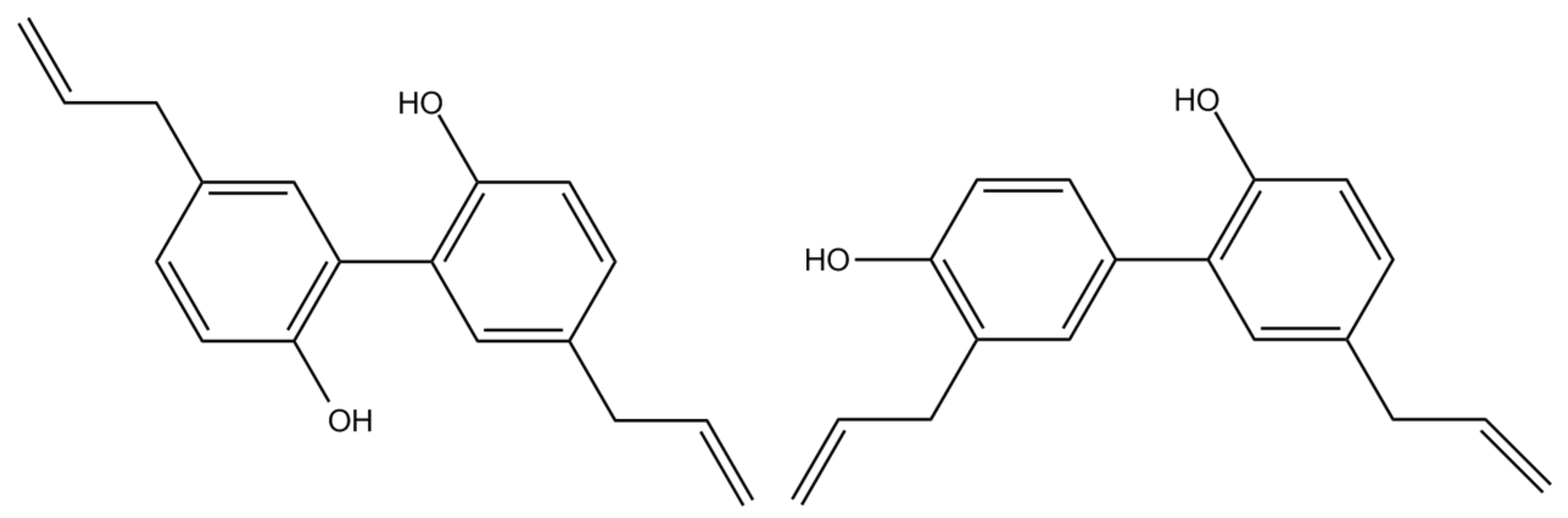
Magnolol and Honokiol Inhibited the Function and Expression of BCRP with Mechanism Exploration
Abstract
:1. Introduction
2. Results
2.1. Cell Viability Assay
2.2. Effects of MN and HK on the Function of BCRP
2.3. Intracellular Accumulations of MN and HK in MDCKII-WT and MDCKII-BCRP Cells
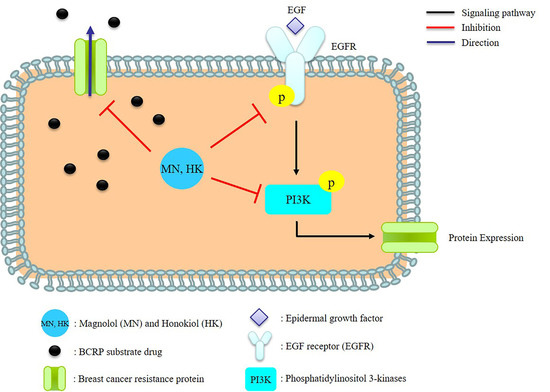
2.4. Effects of MN and HK on the Expressions of BCRP, EGFR, and PI3K
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Lines and Culture Conditions
4.3. Cell Viability Assay
4.4. Effects of MN and HK on the Function of BCRP
4.5. The Intracellular Accumulations of MN and HK in MDCKII-WT and MDCKII-BCRP Cells
4.6. Effects of MN and HK on the Expression of BCRP
4.7. Effects of MN and HK on BCRP Expression and EGFR/PI3K Signaling Pathway
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
References
- Gillet, J.P.; Gottesman, M.M. Mechanisms of multidrug resistance in cancer. Methods Mol. Biol. 2010, 596, 47–76. [Google Scholar] [PubMed]
- Wu, C.P.; Calcagno, A.M.; Ambudkar, S.V. Reversal of ABC drug transporter-mediated multidrug resistance in cancer cells: Evaluation of current strategies. Curr. Mol. Pharmacol. 2008, 1, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Tinoush, B.; Shirdel, I.; Wink, M. Phytochemicals: Potential Lead Molecules for MDR Reversal. Front. Pharmacol. 2020, 11, 832. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.M.F.; Cardoso, D.S.P.; Ferreira, M.-J.U. Overcoming Multidrug Resistance: Flavonoid and Terpenoid Nitrogen-Containing Derivatives as ABC Transporter Modulators. Molecules 2020, 25, 3364. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Jaitak, V. Natural products as multidrug resistance modulators in cancer. Eur. J. Med. Chem. 2019, 176, 268–291. [Google Scholar] [CrossRef] [PubMed]
- Silvia, D.; Laura, B.; Maria, N.R.; Elisabetta, T. Recent advances in the search of BCRP- and dual P-gp/BCRP-based multidrug resistance modulators. Cancer Drug Resist. 2019, 2, 710–743. [Google Scholar]
- Mao, Q.; Unadkat, J.D. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport—An update. AAPS J. 2015, 17, 65–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakanishi, T.; Ross, D.D. Breast cancer resistance protein (BCRP/ABCG2): Its role in multidrug resistance and regulation of its gene expression. Chin. J. Cancer 2012, 31, 73–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaoglu, M.; Güllüce, M.; Karradayi, M. Plant-Derived Natural Products as Multidrug Resistance Modulators in Cancer Therapy. Anatol. J. Biol. 2020, 1, 1–51. [Google Scholar]
- Sarrica, A.; Kirika, N.; Romeo, M.; Salmona, M.; Diomede, L. Safety and Toxicology of Magnolol and Honokiol. Planta Med. 2018, 84, 1151–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Wang, J.; Xu, W.; Sun, Y.; You, J.; Lu, H.; Song, Y.; Wei, J.; Li, L. Magnolol inhibits Streptococcus suis-induced inflammation and ROS formation via TLR2/MAPK/NF-kappaB signaling in RAW264.7 cells. Pol. J. Vet. Sci. 2018, 21, 111–118. [Google Scholar] [PubMed]
- Chen, H.; Fu, W.; Chen, H.; You, S.; Liu, X.; Yang, Y.; Wei, Y.; Huang, J.; Rui, W. Magnolol attenuates the inflammation and enhances phagocytosis through the activation of MAPK, NF-kappaB signal pathways in vitro and in vivo. Mol. Immunol. 2019, 105, 96–106. [Google Scholar] [CrossRef]
- Xie, Z.; Zhao, J.; Wang, H.; Jiang, Y.; Yang, Q.; Fu, Y.; Zeng, H.; Holscher, C.; Xu, J.; Zhang, Z. Magnolol alleviates Alzheimer’s disease-like pathology in transgenic C. elegans by promoting microglia phagocytosis and the degradation of beta-amyloid through activation of PPAR-gamma. Biomed. Pharmacother. 2020, 124, 109886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.H.; Ren, H.Y.; Shen, J.X.; Zhang, X.Y.; Ye, H.M.; Shen, D.Y. Magnolol suppresses the proliferation and invasion of cholangiocarcinoma cells via inhibiting the NF-kappaB signaling pathway. Biomed. Pharmacother. 2017, 94, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Ma, H.; Zhang, T.; Liu, H.; Yu, L.; Li, G.; Li, H.; Hu, M. Magnolol Inhibits the Growth of Non-Small Cell Lung Cancer via Inhibiting Microtubule Polymerization. Cell Physiol. Biochem. 2017, 42, 1789–1801. [Google Scholar] [CrossRef]
- Chang, H.; Chang, C.Y.; Lee, H.J.; Chou, C.Y.; Chou, T.C. Magnolol ameliorates pneumonectomy and monocrotaline-induced pulmonary arterial hypertension in rats through inhibition of angiotensin II and endothelin-1 expression. Phytomedicine 2018, 51, 205–213. [Google Scholar] [CrossRef]
- Chen, M.C.; Lee, C.F.; Huang, W.H.; Chou, T.C. Magnolol suppresses hypoxia-induced angiogenesis via inhibition of HIF-1alpha/VEGF signaling pathway in human bladder cancer cells. Biochem. Pharmacol. 2013, 85, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Baschieri, A.; Pulvirenti, L.; Muccilli, V.; Amorati, R.; Tringali, C. Chain-breaking antioxidant activity of hydroxylated and methoxylated magnolol derivatives: The role of H-bonds. Org. Biomol. Chem. 2017, 15, 6177–6184. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Ding, H.; Liu, Y.; Yang, Q.; Xu, N.; Yang, Y.; Ai, X. Magnolol protects channel catfish from Aeromonas hydrophila infection via inhibiting the expression of aerolysin. Vet. Microbiol. 2017, 211, 119–123. [Google Scholar] [CrossRef]
- Wang, H.; Liao, Z.; Sun, X.; Shi, Q.; Huo, G.; Xie, Y.; Tang, X.; Zhi, X.; Tang, Z. Intravenous administration of Honokiol provides neuroprotection and improves functional recovery after traumatic brain injury through cell cycle inhibition. Neuropharmacology 2014, 86, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.P.; Lee, W.L.; Tang, Y.Q.; Yap, W.H. Honokiol: A Review of Its Anticancer Potential and Mechanisms. Cancers 2020, 12, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fried, L.E.; Arbiser, J.L. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxid. Redox Signal. 2009, 11, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.L.; Man, K.M.; Huang, P.H.; Chen, W.C.; Chen, D.C.; Cheng, Y.W.; Liu, P.L.; Chou, M.C.; Chen, Y.H. Honokiol and magnolol as multifunctional antioxidative molecules for dermatologic disorders. Molecules 2010, 15, 6452–6465. [Google Scholar] [CrossRef] [PubMed]
- Han, H.K.; Anh, L.T.V. Modulation of P-Glycoprotein Expression by Honokiol, Magnolol and 4-O-Methylhonokiol, the Bioactive Components of Magnolia officinalis. Anticancer Res. 2012, 32, 4445–4452. [Google Scholar]
- Nabekura, T.; Hiroi, T.; Kawasaki, T.; Uwai, Y. Effects of natural nuclear factor-kappa B inhibitors on anticancer drug efflux transporter human P-glycoprotein. Biomed. Pharmacother. 2015, 70, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Levitzki, A.; Klein, S. My journey from tyrosine phosphorylation inhibitors to targeted immune therapy as strategies to combat cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 11579–11586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pick, A.; Wiese, M. Tyrosine kinase inhibitors influence ABCG2 expression in EGFR-positive MDCK BCRP cells via the PI3K/Akt signaling pathway. ChemMedChem 2012, 7, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Chen, Y.-J.; Hung, M.-C. Implication of nuclear EGFR in the development of resistance to anticancer therapies. BioMedicine 2011, 1, 2–10. [Google Scholar] [CrossRef]
- Mottaghi, S.; Abbaszadeh, H. Natural Lignans Honokiol and Magnolol as Potential Anticarcinogenic and Anticancer Agents. A Comprehensive Mechanistic Review. Nutr. Cancer 2021, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Szczepanski, M.J.; Lee, Y.J. Magnolol induces apoptosis via inhibiting the EGFR/PI3K/Akt signaling pathway in human prostate cancer cells. J. Cell. Biochem. 2009, 106, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Li, R.Z.; Jiang, Z.B.; Wei, C.L.; Luo, L.X.; Yao, X.J.; Li, G.P.; Leung, E.L. Honokiol Inhibits Proliferation, Invasion and Induces Apoptosis Through Targeting Lyn Kinase in Human Lung Adenocarcinoma Cells. Front. Pharmacol. 2018, 9, 558. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, L.; Liu, R.; Zhang, D.; Lan, X.; Huang, C.; Xin, W.; Wang, C.; Zhang, D.; Du, G. A novel naturally occurring salicylic acid analogue acts as an anti-inflammatory agent by inhibiting nuclear factor-kappaB activity in RAW264.7 macrophages. Mol. Pharm. 2012, 9, 671–677. [Google Scholar] [CrossRef]
- Labbozzetta, M.; Notarbartolo, M.; Poma, P. Can NF-kappaB Be Considered a Valid Drug Target in Neoplastic Diseases? Our Point of View. Int. J. Mol. Sci. 2020, 21, 3070. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020, 11, 797. [Google Scholar] [CrossRef]
- Nabekura, T.; Kawasaki, T.; Furuta, M.; Kaneko, T.; Uwai, Y. Effects of Natural Polyphenols on the Expression of Drug Efflux Transporter P-Glycoprotein in Human Intestinal Cells. ACS Omega 2018, 3, 1621–1626. [Google Scholar] [CrossRef]
- Dong, C.; Wu, J.; Chen, Y.; Nie, J.; Chen, C. Activation of PI3K/AKT/mTOR Pathway Causes Drug Resistance in Breast Cancer. Front. Pharmacol. 2021, 12, 628690. [Google Scholar] [CrossRef] [PubMed]
- Akinleye, A.; Avvaru, P.; Furqan, M.; Song, Y.; Liu, D. Phosphatidylinositol 3-kinase (PI3K) inhibitors as cancer therapeutics. J. Hematol. Oncol. 2013, 6, 88. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, I.; Nishikawa, S.; Yamamoto, A.; Kono, Y.; Fujita, T. The Impact of Breast Cancer Resistance Protein (BCRP/ABCG2) on Drug Transport Across Caco-2 Cell Monolayers. Drug Metab. Dispos. 2020, 48, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.J.; Huang, W.C.; Wei, Y.L.; Hsu, S.C.; Yuan, P.; Lin, H.Y.; Wistuba, I.I.; Lee, J.J.; Yen, C.J.; Su, W.C.; et al. Elevated BCRP/ABCG2 expression confers acquired resistance to gefitinib in wild-type EGFR-expressing cells. PLoS ONE 2011, 6, e21428. [Google Scholar] [CrossRef]
- Twentyman, P.R.; Luscombe, M. A Study of Some Variables in a Tetrazolium Dye (Mtt) Based Assay for Cell-Growth and Chemosensitivity. Br. J. Cancer 1987, 56, 279–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.P.; Hsieh, Y.C.; Shia, C.S.; Hsu, P.W.; Chen, J.Y.; Hou, Y.C.; Hsieh, Y.W. Increased systemic exposure of methotrexate by a polyphenol-rich herb via modulation on efflux transporters multidrug resistance-associated protein 2 and breast cancer resistance protein. J. Pharm. Sci. 2016, 105, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Li, P.Y.; Liang, Y.C.; Sheu, M.J.; Huang, S.S.; Chao, C.Y.; Kuo, Y.H.; Huang, G.J. Alpinumisoflavone attenuates lipopolysaccharide-induced acute lung injury by regulating the effects of anti-oxidation and anti-inflammation both in vitro and in vivo. RSC Adv. 2018, 8, 31515–31528. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.-P.; Li, P.-Y.; Chen, S.-Y.; Lin, S.-P.; Hou, Y.-C. Magnolol and Honokiol Inhibited the Function and Expression of BCRP with Mechanism Exploration. Molecules 2021, 26, 7390. https://doi.org/10.3390/molecules26237390
Yu C-P, Li P-Y, Chen S-Y, Lin S-P, Hou Y-C. Magnolol and Honokiol Inhibited the Function and Expression of BCRP with Mechanism Exploration. Molecules. 2021; 26(23):7390. https://doi.org/10.3390/molecules26237390
Chicago/Turabian StyleYu, Chung-Ping, Pei-Ying Li, Szu-Yu Chen, Shiuan-Pey Lin, and Yu-Chi Hou. 2021. "Magnolol and Honokiol Inhibited the Function and Expression of BCRP with Mechanism Exploration" Molecules 26, no. 23: 7390. https://doi.org/10.3390/molecules26237390
APA StyleYu, C.-P., Li, P.-Y., Chen, S.-Y., Lin, S.-P., & Hou, Y.-C. (2021). Magnolol and Honokiol Inhibited the Function and Expression of BCRP with Mechanism Exploration. Molecules, 26(23), 7390. https://doi.org/10.3390/molecules26237390