In Vitro Antioxidant, Antitumor and Photocatalytic Activities of Silver Nanoparticles Synthesized Using Equisetum Species: A Green Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Green Synthesis of AgNPs
2.3. Physico-Chemical Characterization of the Obtained AgNPs
2.4. Photocatalytic Activity
2.5. Biological Evaluation Methods
2.5.1. Antioxidant Activity Assays
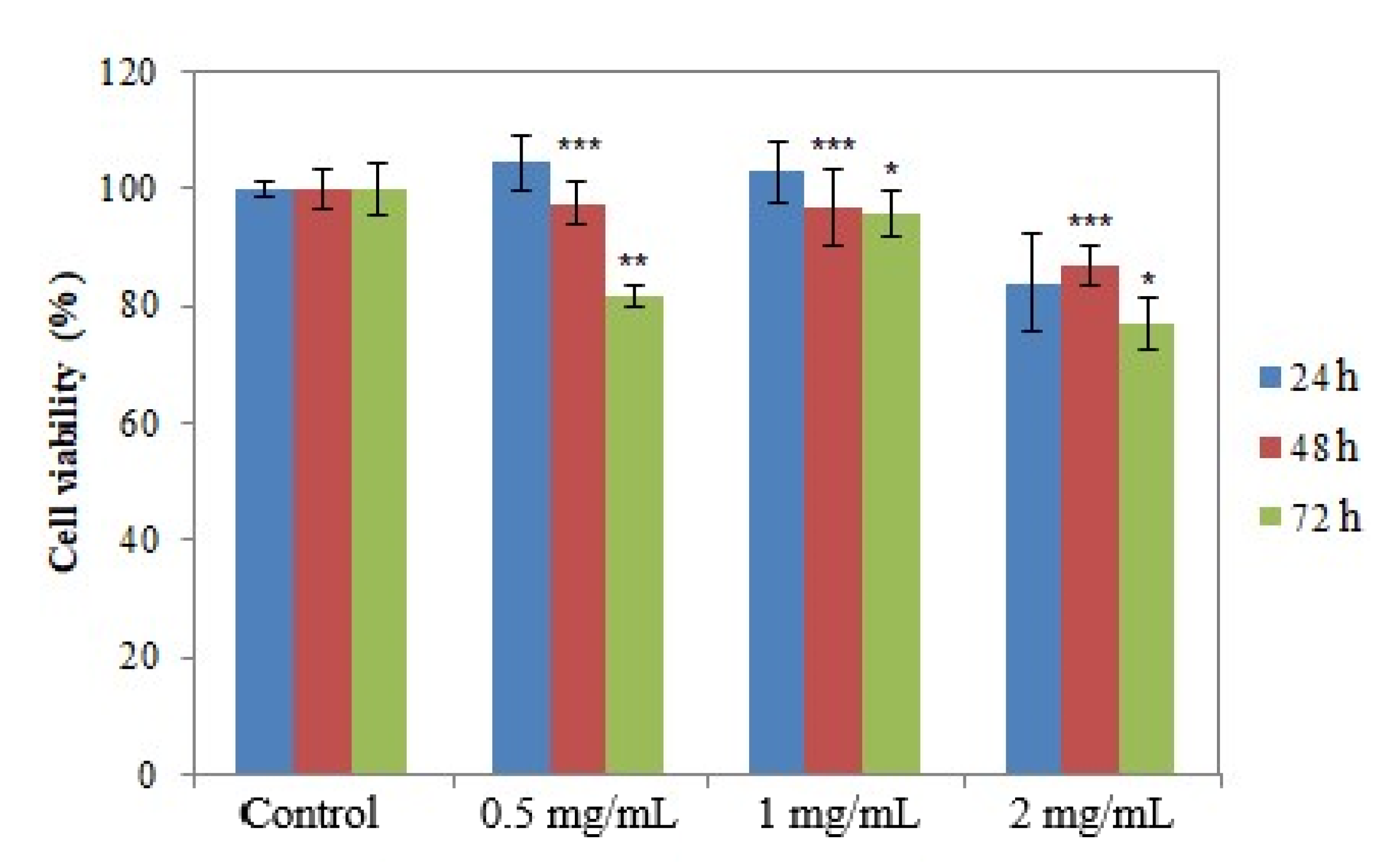
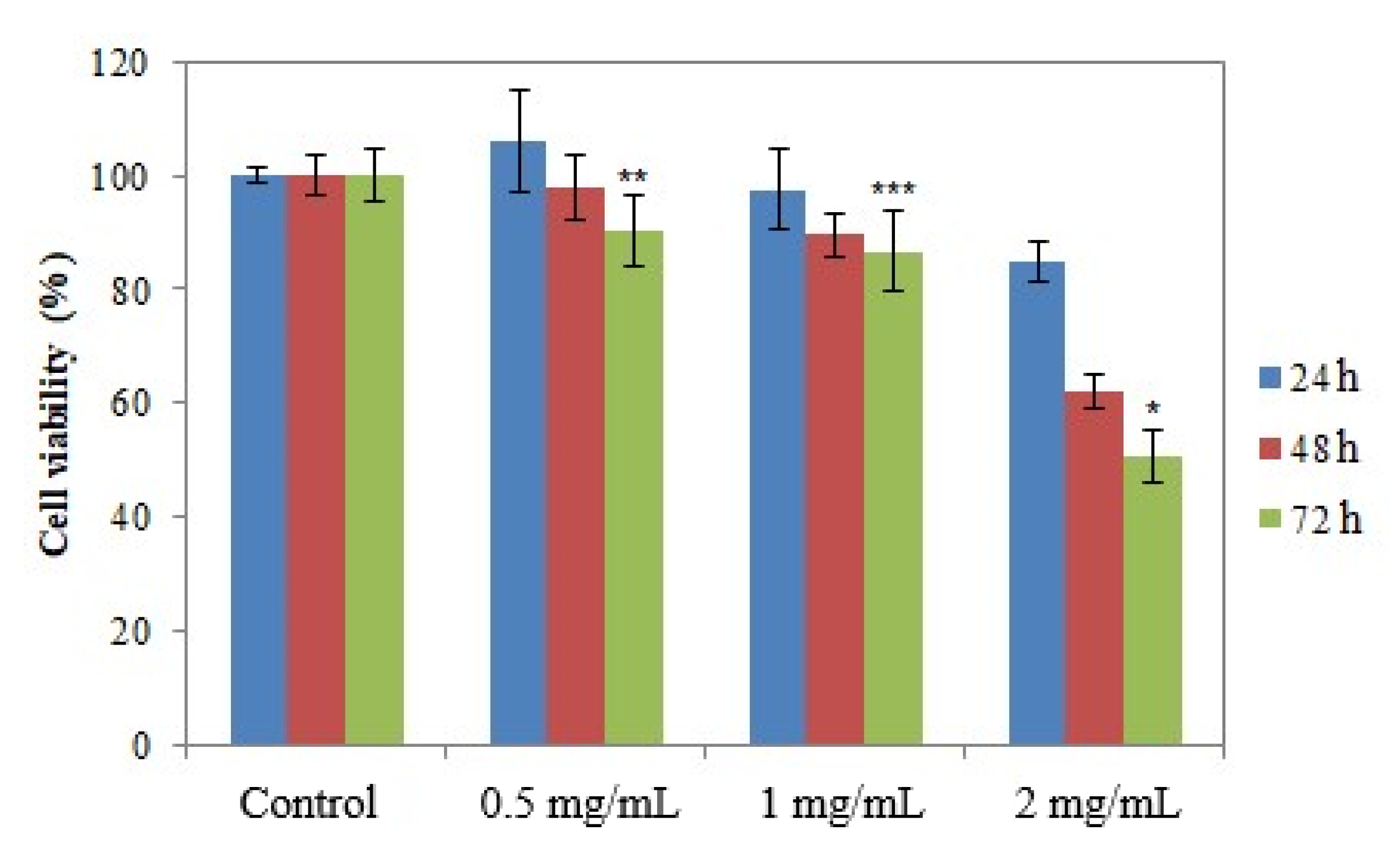
2.5.2. In Vitro Cytotoxic Efficacy of AgNPs
3. Results and Discussion
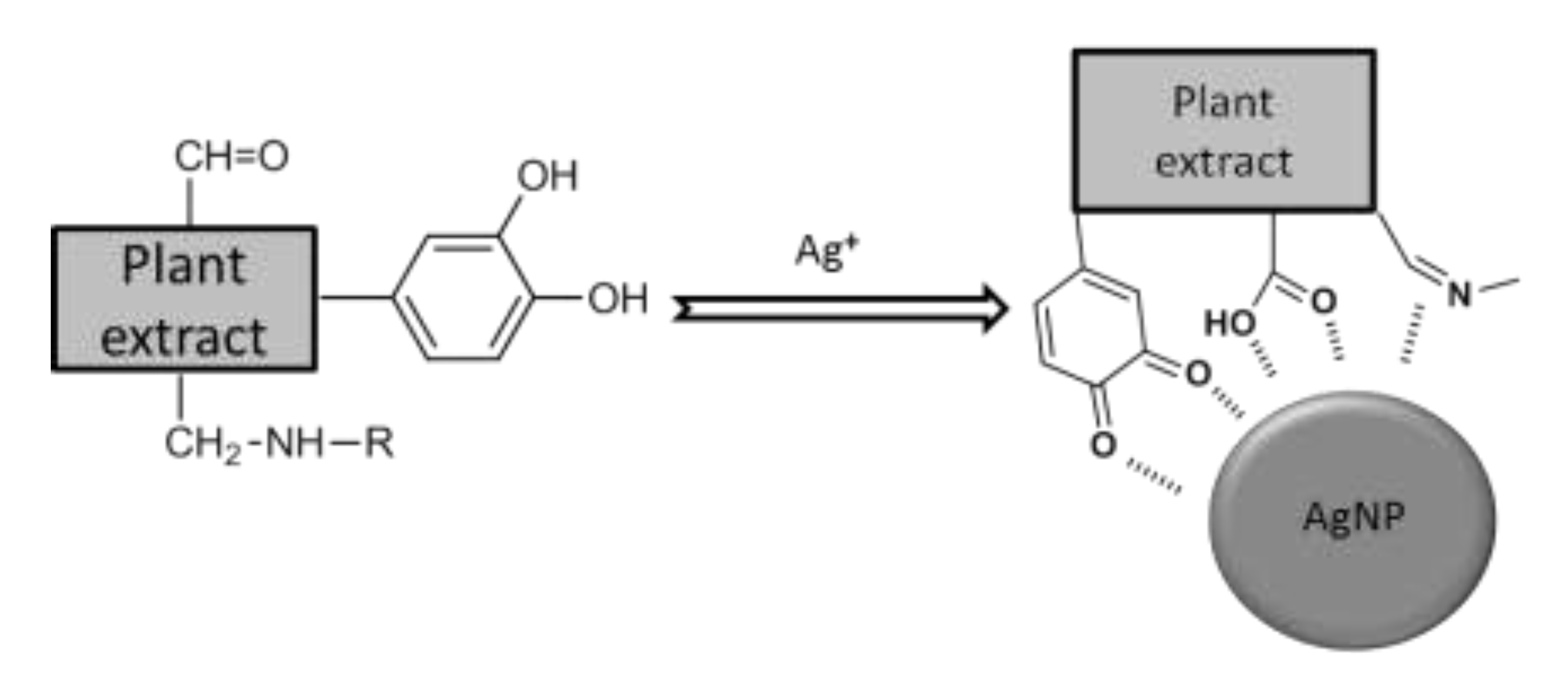
3.1. AgNPs Synthesis
3.2. Physico-Chemical Characterization of the Obtained AgNPs
3.2.1. TEM Analysis, DLS Characterization and Zeta Potential Determination
3.2.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.2.3. Energy Dispersive Spectroscopic Analysis of AgNPs
3.2.4. Phytochemical Evaluation of Phenolic Compounds from Equisetum Extracts Used for AgNPs Synthesis
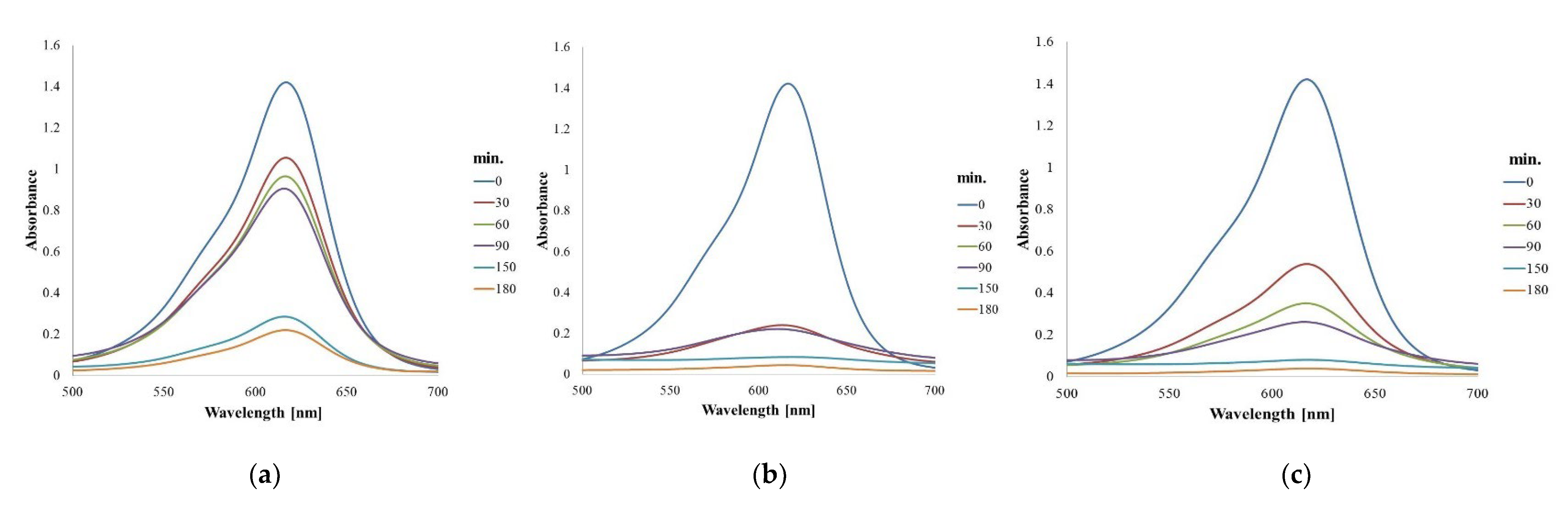
3.3. Photocatalytic Activity of the Obtained AgNPs
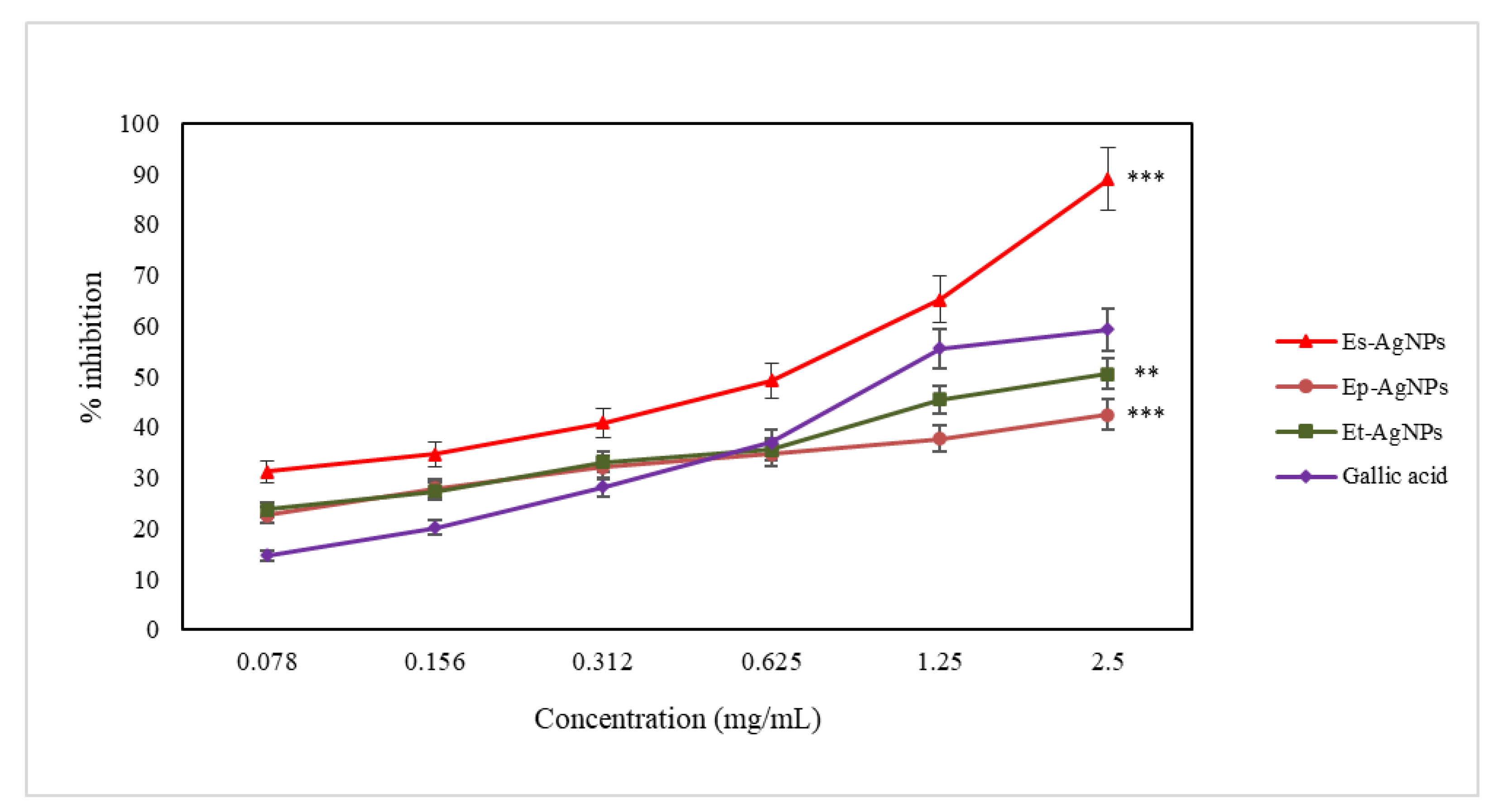
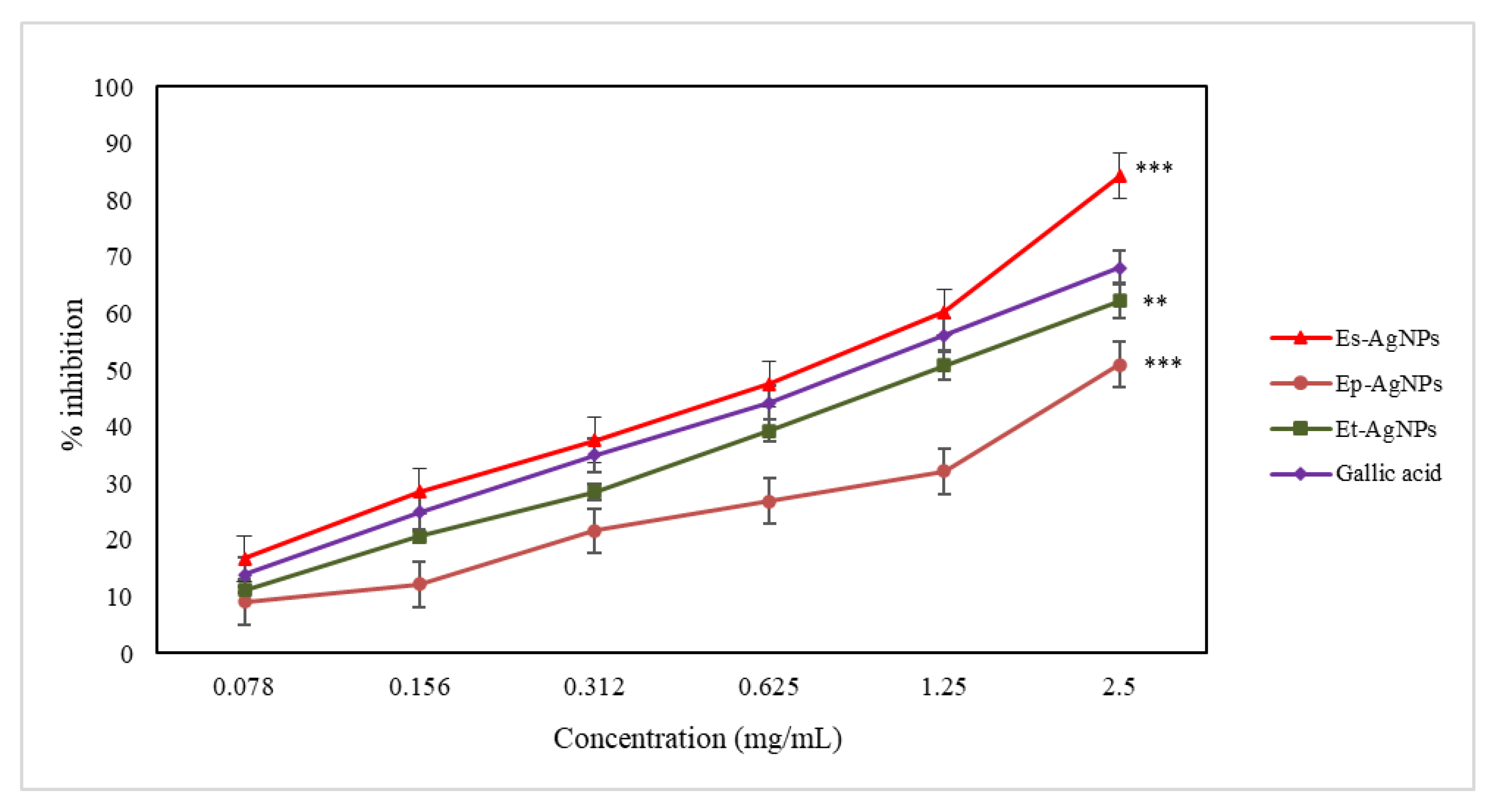
3.4. In Vitro Evaluation of the Antioxidant Activity of the Obtained AgNPs
3.4.1. Determination of the Ferrous Ion Chelating Capacity
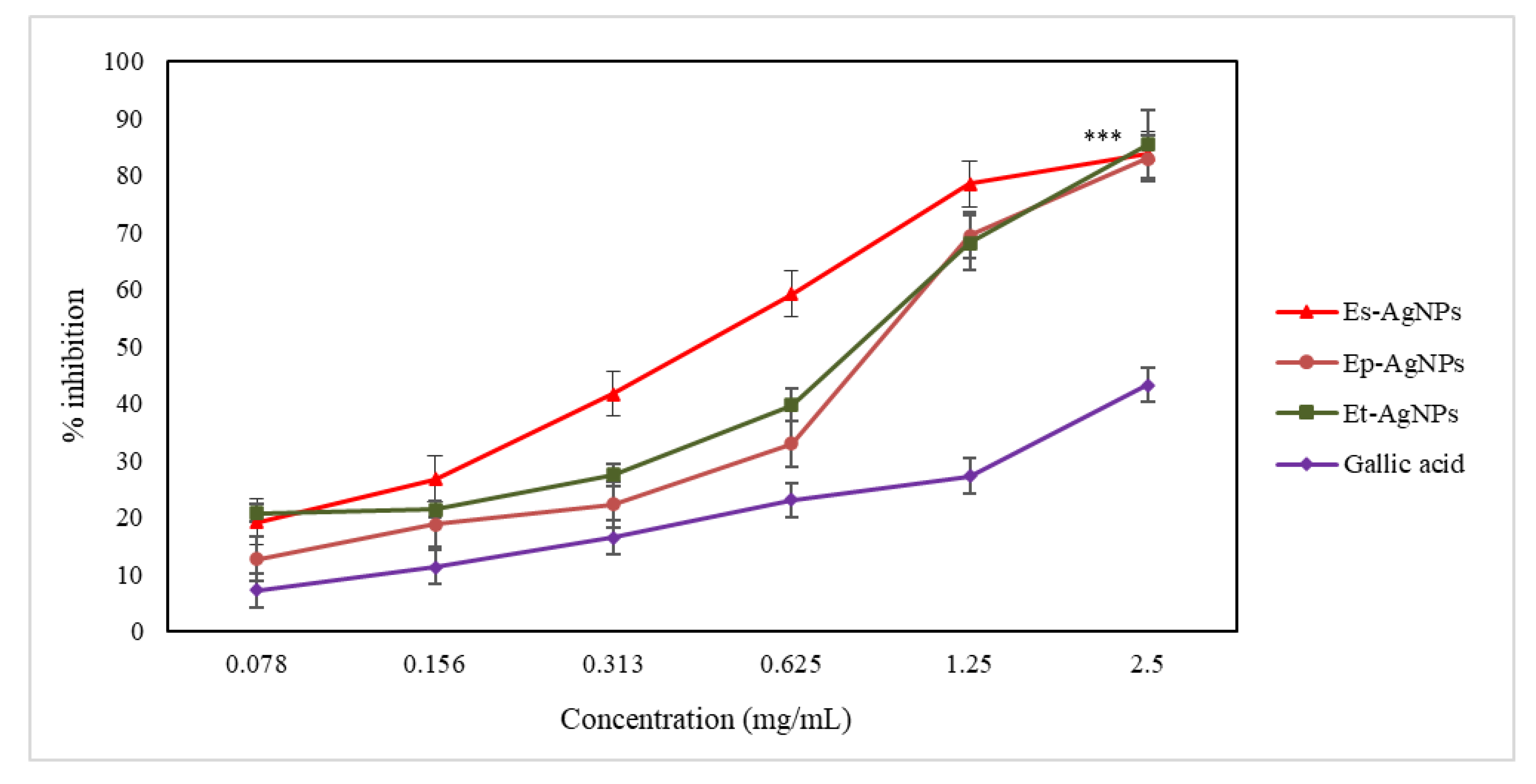
3.4.2. Determination of LOX Inhibition
3.4.3. Determination of the Hydroxyl Radical Scavenging Capacity
3.4.4. Determination of the Superoxide Anion Radical Scavenging Capacity
3.5. In Vitro Evaluation of the Antitumor Action of the Obtained AgNPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Nikalje, A.P. Nanotechnology and its applications in medicine. Med. Chem. 2015, 5, 081–089. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.-M.; Park, I.; Seung-Hyun, K.; Thiruvengadam, M.; Rajakumar, G. Plant-mediated synthesis of silver nanoparticles: Their characteristic properties and therapeutic applications. Nanoscale Res. Lett. 2016, 11, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradi, F.; Sedaghat, S.; Moradi, O.; Arab Salmanabadi, S. Review on green nano-biosynthesis of silver nanoparticles and their biological activities: With an emphasis on medicinal plants. Inorg. Nano-Metal Chem. 2021, 51, 133–142. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.; Raja, N.I.; Iqbal, M.; Aslam, S. Applications of plant flavonoids in the green synthesis of colloidal silver nanoparticles and impacts on human health. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 1381–1392. [Google Scholar] [CrossRef]
- Mashwani, Z.-R.; Khan, T.; Khan, M.A.; Nadhman, A. Synthesis in plants and plant extracts of silver nanoparticles with potent antimicrobial properties: Current status and future prospects. Appl. Microbiol. Biotechnol. 2015, 99, 9923–9934. [Google Scholar] [CrossRef]
- Anjum, S.; Abbasi, B.H.; Shinwari, Z.K. Plant-mediated green synthesis of silver nanoparticles for biomedical applications: Challenges and opportunities. Pakistan J. Bot. 2016, 48, 1731–1760. [Google Scholar]
- Mashwani, Z.R.; Khan, M.A.; Khan, T.; Nadhman, A. Applications of plant terpenoids in the synthesis of colloidal silver nanoparticles. Adv. Colloid Interface Sci. 2016, 234, 132–141. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Tian, J.; Wong, K.K.Y.; Ho, C.-M.; Lok, C.-N.; Yu, W.-Y.; Che, C.-M.; Chiu, J.-F.; Tam, P.K.H. Topical delivery of silver nanoparticles promotes wound healing. ChemMedChem 2007, 2, 129–136. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Banumathi, E.; Pandian, S.R.K.; Deepak, V.; Muniyandi, J.; Eom, S.H.; Gurunathan, S. Silver nanoparticles inhibit VEGF induced cell proliferation and migration in bovine retinal endothelial cells. Colloids Surf. B Biointerfaces 2009, 73, 51–57. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S. Bactericidal and cytotoxic properties of silver nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef] [Green Version]
- Milovanović, V.; Radulović, N.; Todorović, Z.; Stanković, M.; Stojanović, G. Antioxidant, antimicrobial and genotoxicity screening of hydro-alcoholic extracts of five Serbian Equisetum species. Plant Foods Hum. Nutr. 2007, 62, 113–119. [Google Scholar] [CrossRef]
- Do Monte, F.H.M.; Dos Santos, J.G.; Russi, M.; Bispo Lanziotti, V.M.N.; Moreira Leal, L.K.A.; De Andrade Cunha, G.M. Antinociceptive and anti-inflammatory properties of the hydroalcoholic extract of stems from Equisetum arvense L. in mice. Pharmacol. Res. 2004, 49, 239–243. [Google Scholar] [CrossRef]
- Sandhu, N.S.; Kaur, S.; Chopra, D. Equisetum arvense: Pharmacology and phytochemistry—A review. Asian J. Pharm. Clin. Res. 2010, 3, 146–150. [Google Scholar]
- Miljković, M.; Lazić, V.; Davidović, S.; Milivojević, A.; Papan, J.; Fernandes, M.M.; Lanceros-Mendez, S.; Ahrenkiel, S.P.; Nedeljković, J.M. Selective antimicrobial performance of biosynthesized silver nanoparticles by horsetail extract against E. coli. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2598–2607. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. The pharmacology of Equisetum arvense—A review. IOSR J. Pharm. 2017, 7, 31–42. [Google Scholar] [CrossRef]
- Sedaghat, S.; Omidi, S. Batch process biosynthesis of silver nanoparticles using Equisetum arvense leaf extract. Bioinspired Biomim. Nanobiomaterials 2019, 8, 190–197. [Google Scholar] [CrossRef]
- Boeing, T.; Tafarelo Moreno, K.G.; Gasparotto, A., Jr.; Mota da Silva, L.; de Souza, P. Phytochemistry and pharmacology of the genus Equisetum (Equisetaceae): A narrative review of the species with therapeutic potential for kidney diseases. Evidence-Based Complement. Altern. Med. 2021, 2021, 1–17. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Podgórski, R.; Oniszczuk, T.; Zukiewicz-Sobczak, W.; Nowak, R.; Waksmundzka-Hajnos, M. Extraction methods for the determination of phenolic compounds from Equisetum arvense L. herb. Ind. Crops Prod. 2014, 61, 377–381. [Google Scholar] [CrossRef]
- Pallag, A.; Filip, G.A.; Olteanu, D.; Clichici, S.; Baldea, I.; Jurca, T.; Micle, O.; Vicaş, L.; Marian, E.; Soriţău, O.; et al. Equisetum arvense L. extract induces antibacterial activity and modulates oxidative stress, inflammation, and apoptosis in endothelial vascular cells exposed to hyperosmotic stress. Oxid. Med. Cell. Longev. 2018, 2018, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Corciova, A.; Ivanescu, B. Biosynthesis, characterization and therapeutic applications of plant-mediated silver nanoparticles. J. Serbian Chem. Soc. 2018, 83, 515–538. [Google Scholar] [CrossRef] [Green Version]
- Veerasamy, R.; Xin, T.Z.; Gunasagaran, S.; Xiang, T.F.W.; Yang, E.F.C.; Jeyakumar, N.; Dhanaraj, S.A. Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J. Saudi Chem. Soc. 2011, 15, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Rajesh Kumar, T.V.; Murthy, J.S.R.; Narayana Rao, M.; Bhargava, Y. Evaluation of silver nanoparticles synthetic potential of Couroupita guianensis Aubl., flower buds extract and their synergistic antibacterial activity. 3 Biotech 2016, 6, 92. [Google Scholar] [CrossRef] [Green Version]
- Roy, K.; Sarkar, C.; Ghosh, C. Photocatalytic activity of biogenic silver nanoparticles synthesized using potato (Solanum tuberosum) infusion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 146, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Cha, C.-J.; Doerge, D.R.; Cerniglia, C.E. Biotransformation of malachite green by the fungus Cunninghamella elegans. Appl. Environ. Microbiol. 2001, 67, 4358–4360. [Google Scholar] [CrossRef] [Green Version]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
- Malterud, K.E.; Rydland, K.M. Inhibitors of 15-lipoxygenase from orange peel. J. Agric. Food Chem. 2000, 48, 5576–5580. [Google Scholar] [CrossRef]
- Jeong, J.B.; Chul Hong, S.; Jin Jeong, H. 3,4-Dihydroxybenzaldehyde purified from the barley seeds (Hordeum vulgare) inhibits oxidative DNA damage and apoptosis via its antioxidant activity. Phytomedicine 2009, 16, 85–94. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, D. Antioxidant activities of different fractions of polysaccharide purified from Gynostemma pentaphyllum Makino. Carbohydr. Polym. 2007, 68, 54–58. [Google Scholar] [CrossRef]
- Jha, D.; Thiruveedula, P.K.; Pathak, R.; Kumar, B.; Gautam, H.K.; Agnihotri, S.; Sharma, A.K.; Kumar, P. Multifunctional biosynthesized silver nanoparticles exhibiting excellent antimicrobial potential against multi-drug resistant microbes along with remarkable anticancerous properties. Mater. Sci. Eng. C 2017, 80, 659–669. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 10993-5 Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. Available online: www.ncbi.nlm.nih.gov/books/NBK144065/ (accessed on 3 May 2021).
- Singh, N.; Chatterjee, A.; Chakraborty, K.; Chatterjee, S.; Abraham, J. Cytotoxic effect on MG-63 cell line and antimicrobial and antioxidant properties of silver nanoparticles synthesized with seed extracts of Capsicum sp. Rec. Nat. Prod. 2016, 10, 47–57. [Google Scholar]
- Corciovă, A.; Mircea, C.; Burlec, A.-F.; Cioancă, O.; Tuchiluş, C.; Fifere, A.; Lungoci, A.-L.; Marangoci, N.; Hăncianu, M. Antioxidant, antimicrobial activities and photocatalytic degradation efficacy of silver nanoparticles obtained by bee propolis extract assisted biosynthesis. Farmacia 2019, 67, 482–489. [Google Scholar] [CrossRef]
- Lashin, I.; Fouda, A.; Gobouri, A.A.; Azab, E.; Mohammedsaleh, Z.M.; Makharita, R.R. Antimicrobial and in vitro cytotoxic efficacy of biogenic silver nanoparticles (Ag-NPs) fabricated by callus extract of Solanum incanum L. Biomolecules 2021, 11, 341. [Google Scholar] [CrossRef]
- Dong, Z.-Y.; Narsing Rao, M.P.; Xiao, M.; Wang, H.-F.; Hozzein, W.N.; Chen, W.; Li, W.-J. Antibacterial activity of silver nanoparticles against Staphylococcus warneri synthesized using endophytic bacteria by photo-irradiation. Front. Microbiol. 2017, 8, 1090. [Google Scholar] [CrossRef] [Green Version]
- Wypij, M.; Czarnecka, J.; Świecimska, M.; Dahm, H.; Rai, M.; Golinska, P. Synthesis, characterization and evaluation of antimicrobial and cytotoxic activities of biogenic silver nanoparticles synthesized from Streptomyces xinghaiensis OF1 strain. World J. Microbiol. Biotechnol. 2018, 34, 23. [Google Scholar] [CrossRef] [Green Version]
- Alsharif, S.M.; Salem, S.S.; Abdel-Rahman, M.A.; Fouda, A.; Eid, A.M.; El-Din Hassan, S.; Awad, M.A.; Mohamed, A.A. Multifunctional properties of spherical silver nanoparticles fabricated by different microbial taxa. Heliyon 2020, 6, e03943. [Google Scholar] [CrossRef]
- Padalia, H.; Moteriya, P.; Chanda, S. Green synthesis of silver nanoparticles from marigold flower and its synergistic antimicrobial potential. Arab. J. Chem. 2015, 8, 732–741. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.; Chaudhari, G.; Paradeshi, J.; Mahajan, R.; Chaudhari, B.L. Instant green synthesis of silver-based herbo-metallic colloidal nanosuspension in Terminalia bellirica fruit aqueous extract for catalytic and antibacterial applications. 3 Biotech 2017, 7, 36. [Google Scholar] [CrossRef]
- Altameme, H.J.; Hameed, I.H.; Abu-Serag, N.A. Analysis of bioactive phytochemical compounds of two medicinal plants horsetail Equisetum arvense and Alchemila vulgaris seeds by using gas chromatography-mass spectrometry and Fourier-transform infrared spectroscopy. Malaysian Appl. Biol. 2015, 44, 47–58. [Google Scholar]
- Topală, C.; Tătaru, L.; Ducu, C. ATR-FTIR spectra fingerprinting of medicinal herbs extracts prepared using microwave extraction. Arab. J. Med. Aromat. Plants 2017, 3, 1–9. [Google Scholar]
- Sinha, S.N. In vitro antibacterial activity of ethanolic extract of Equisetum arvense L. Int. J. Biol. Pharm. Res. 2012, 3, 19–21. [Google Scholar]
- Dos Santos, J.G.; Blanco, M.M.; Do Monte, F.H.M.; Russi, M.; Lanziotti, V.M.N.B.; Leal, L.K.A.M.; Cunha, G.M. Sedative and anticonvulsant effects of hydroalcoholic extract of Equisetum arvense. Fitoterapia 2005, 76, 508–513. [Google Scholar] [CrossRef]
- Jyoti, K.; Singh, A. Green synthesis of nanostructured silver particles and their catalytic application in dye degradation. J. Genet. Eng. Biotechnol. 2016, 14, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Haider, M.J.; Mehdi, M.S. Study of morphology and Zeta potential analyzer for the silver nanoparticles. Int. J. Sci. Eng. Res. 2014, 5, 381–387. [Google Scholar]
- Barberia-Roque, L.; Gámez-Espinosa, E.; Viera, M.; Bellotti, N. Assessment of three plant extracts to obtain silver nanoparticles as alternative additives to control biodeterioration of coatings. Int. Biodeterior. Biodegrad. 2019, 141, 52–61. [Google Scholar] [CrossRef]
- Latha, D.; Arulvasu, C.; Prabu, P.; Narayanan, V. Photocatalytic activity of biosynthesized silver nanoparticle from leaf extract of Justicia adhatoda. Mech. Mater. Sci. Eng. 2017, 9. [Google Scholar] [CrossRef]
- Batir-Marin, D.; Boev, M.; Cioanca, O.; Mircea, C.; Burlec, A.F.; Beppe, G.J.; Spac, A.; Corciova, A.; Hritcu, L.; Hancianu, M. Neuroprotective and antioxidant enhancing properties of selective Equisetum extracts. Molecules 2021, 26, 2565. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, B. Hydroxyl radical and its scavengers in health and disease. Oxid. Med. Cell. Longev. 2011, 2011, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, H.; Kim, D.H.; Cho, J.H.; Kim, Y.C. Hepatoprotective and free radical scavenging activities of phenolic petrosins and flavonoids isolated from Equisetum arvense. J. Ethnopharmacol. 2004, 95, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Costa-Rodrigues, J.; Carmo, S.C.; Silva, J.C.; Fernandes, M.H.R. Inhibition of human in vitro osteoclastogenesis by Equisetum arvense. Cell Prolif. 2012, 45, 566–576. [Google Scholar] [CrossRef]
- Das, G.; Patra, J.K.; Shin, H.-S. Biosynthesis, and potential effect of fern mediated biocompatible silver nanoparticles by cytotoxicity, antidiabetic, antioxidant and antibacterial, studies. Mater. Sci. Eng. C 2020, 114, 111011. [Google Scholar] [CrossRef]

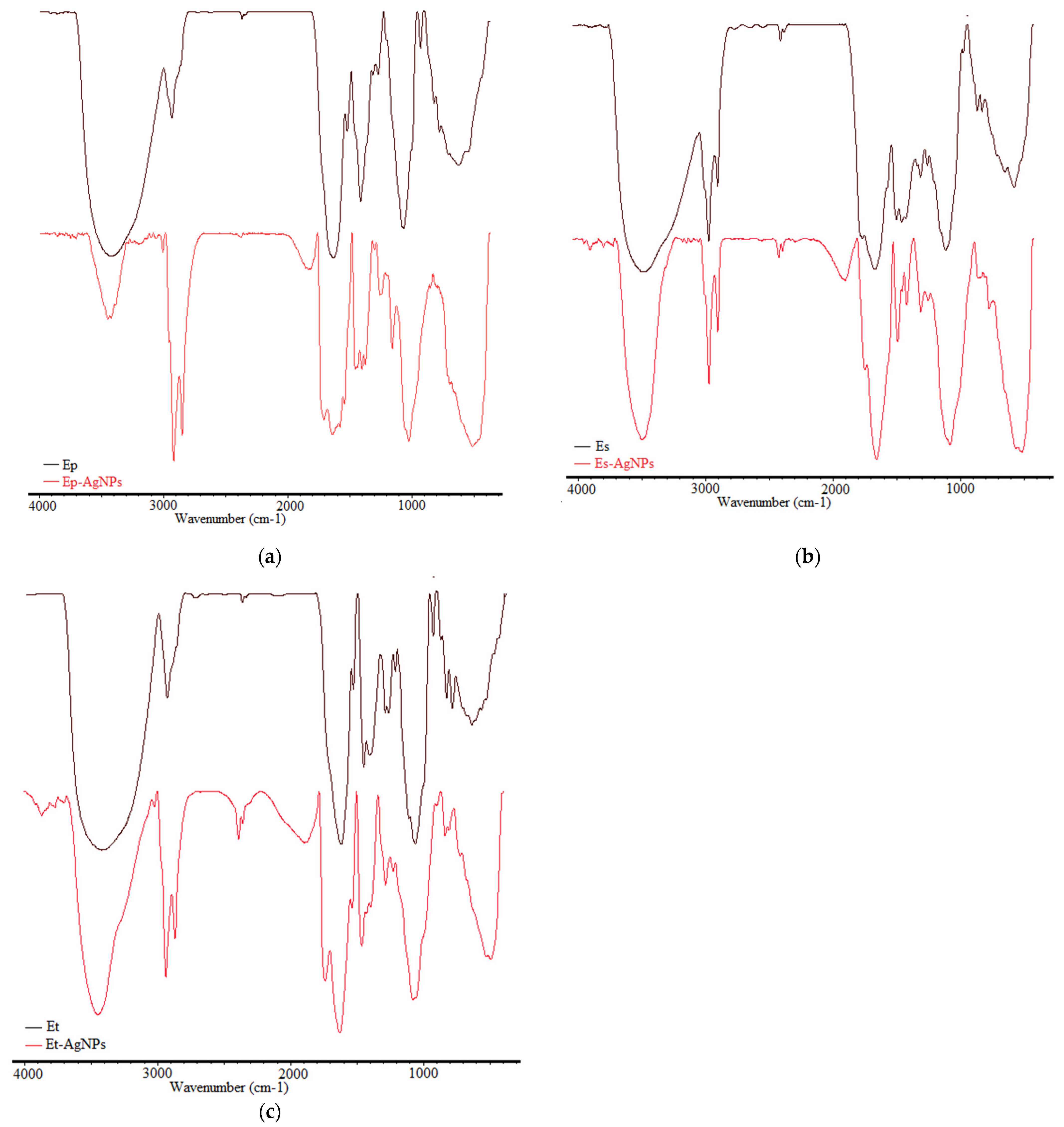



| Ep | Es | Et | Vibration Type | Ref. |
|---|---|---|---|---|
| 3433 | 3440 | 3427 | H–O stretching intermolecular hydrogen bonding from alcohols or phenols | [45] |
| 2929, 2364 | 2924, 2854 | 2927, 2362 | C–H stretching vibrations of CH3 and CH2 (alkanes) | [45,46] |
| 1629 | 1614 | 1616 | Stretching vibrations C=O, C–N (amide I), asymmetrical stretching vibrations COO- | [46] |
| 1516 | 1519 | 1523 | Deformation vibrations n-H (amide II) and aromatic bonds | [46] |
| 1408, 1307 | 1408, 1375 | 1444, 1402 | C–O (amide) stretching vibrations and C–C stretching vibrations of phenyl groups, COO- symmetric stretching vibrations and CH2 bond vibrations | [46] |
| 1265 | 1259 | 1284, 1259 | C–O stretching vibrations of alcohols, ethers, esters, carboxylic acids | [45] |
| 1060 | 1060 | 1056 | C–O and C–C stretching vibrations from carbohydrates | [46] |
| 923–621 | 921–520 | 923–630 | Bond vibrations C–H out of plane (alkenes) | [45,46] |
| Sample | Content in Phenolic Compounds (mg GAE/mL Sample) * | |
|---|---|---|
| Initial Extract | Supernatant after Separation of AgNPs | |
| Ep–AgNPs | 1.1674 ± 0.001 | 0.5584 ± 0.003 |
| Es–AgNPs | 5.2478 ± 0.001 | 3.1163 ± 0.001 |
| Et–AgNPs | 9.2046 ± 0.002 | 4.6388 ± 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batir-Marin, D.; Mircea, C.; Boev, M.; Burlec, A.F.; Corciova, A.; Fifere, A.; Iacobescu, A.; Cioanca, O.; Verestiuc, L.; Hancianu, M. In Vitro Antioxidant, Antitumor and Photocatalytic Activities of Silver Nanoparticles Synthesized Using Equisetum Species: A Green Approach. Molecules 2021, 26, 7325. https://doi.org/10.3390/molecules26237325
Batir-Marin D, Mircea C, Boev M, Burlec AF, Corciova A, Fifere A, Iacobescu A, Cioanca O, Verestiuc L, Hancianu M. In Vitro Antioxidant, Antitumor and Photocatalytic Activities of Silver Nanoparticles Synthesized Using Equisetum Species: A Green Approach. Molecules. 2021; 26(23):7325. https://doi.org/10.3390/molecules26237325
Chicago/Turabian StyleBatir-Marin, Denisa, Cornelia Mircea, Monica Boev, Ana Flavia Burlec, Andreia Corciova, Adrian Fifere, Alexandra Iacobescu, Oana Cioanca, Liliana Verestiuc, and Monica Hancianu. 2021. "In Vitro Antioxidant, Antitumor and Photocatalytic Activities of Silver Nanoparticles Synthesized Using Equisetum Species: A Green Approach" Molecules 26, no. 23: 7325. https://doi.org/10.3390/molecules26237325
APA StyleBatir-Marin, D., Mircea, C., Boev, M., Burlec, A. F., Corciova, A., Fifere, A., Iacobescu, A., Cioanca, O., Verestiuc, L., & Hancianu, M. (2021). In Vitro Antioxidant, Antitumor and Photocatalytic Activities of Silver Nanoparticles Synthesized Using Equisetum Species: A Green Approach. Molecules, 26(23), 7325. https://doi.org/10.3390/molecules26237325








