Synthesis of Energetic 7-Nitro-3,5-dihydro-4H-pyrazolo[4,3-d][1,2,3]triazin-4-one Based on a Novel Hofmann-Type Rearrangement
Abstract
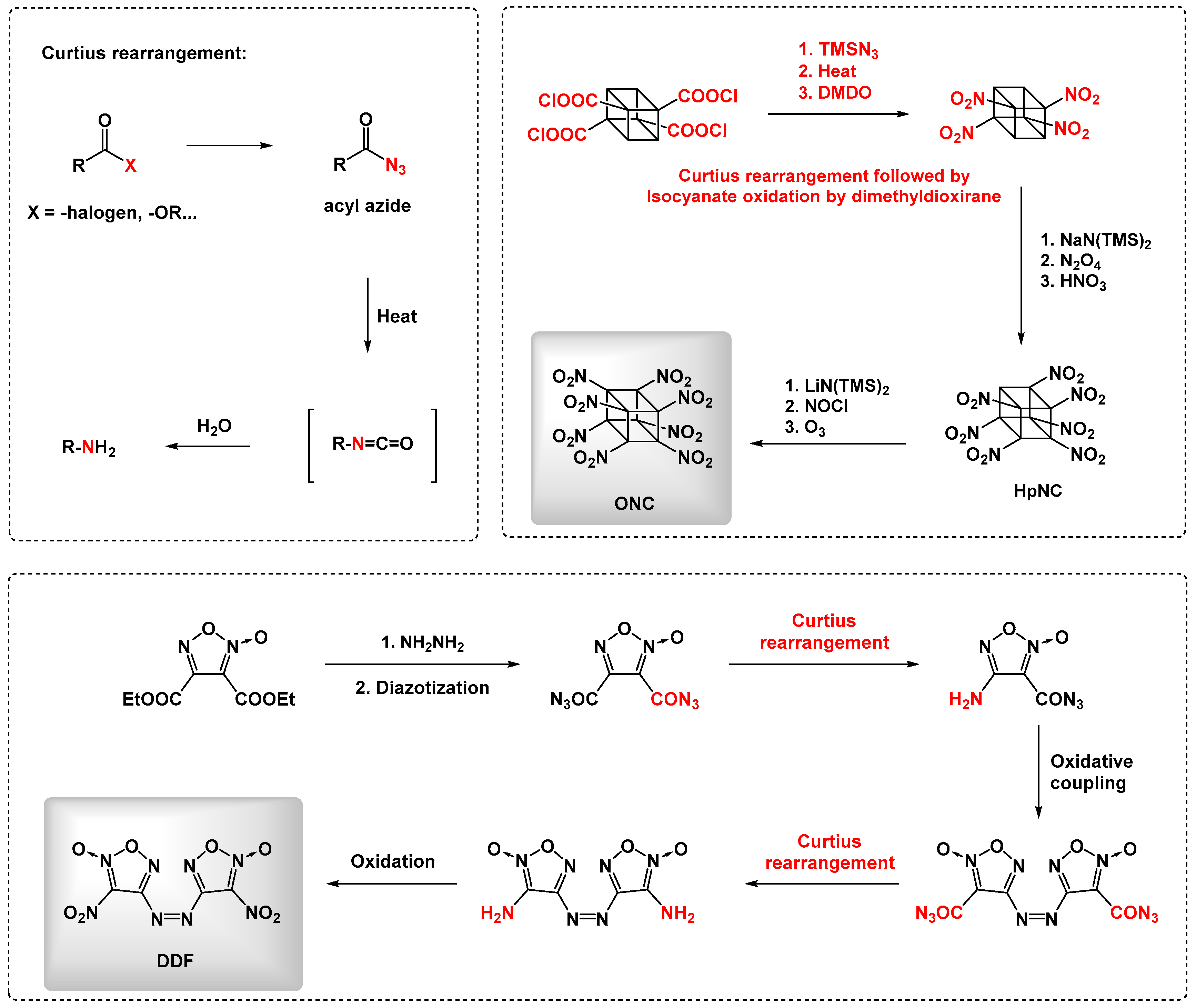
:1. Introduction
2. Results and Discussion
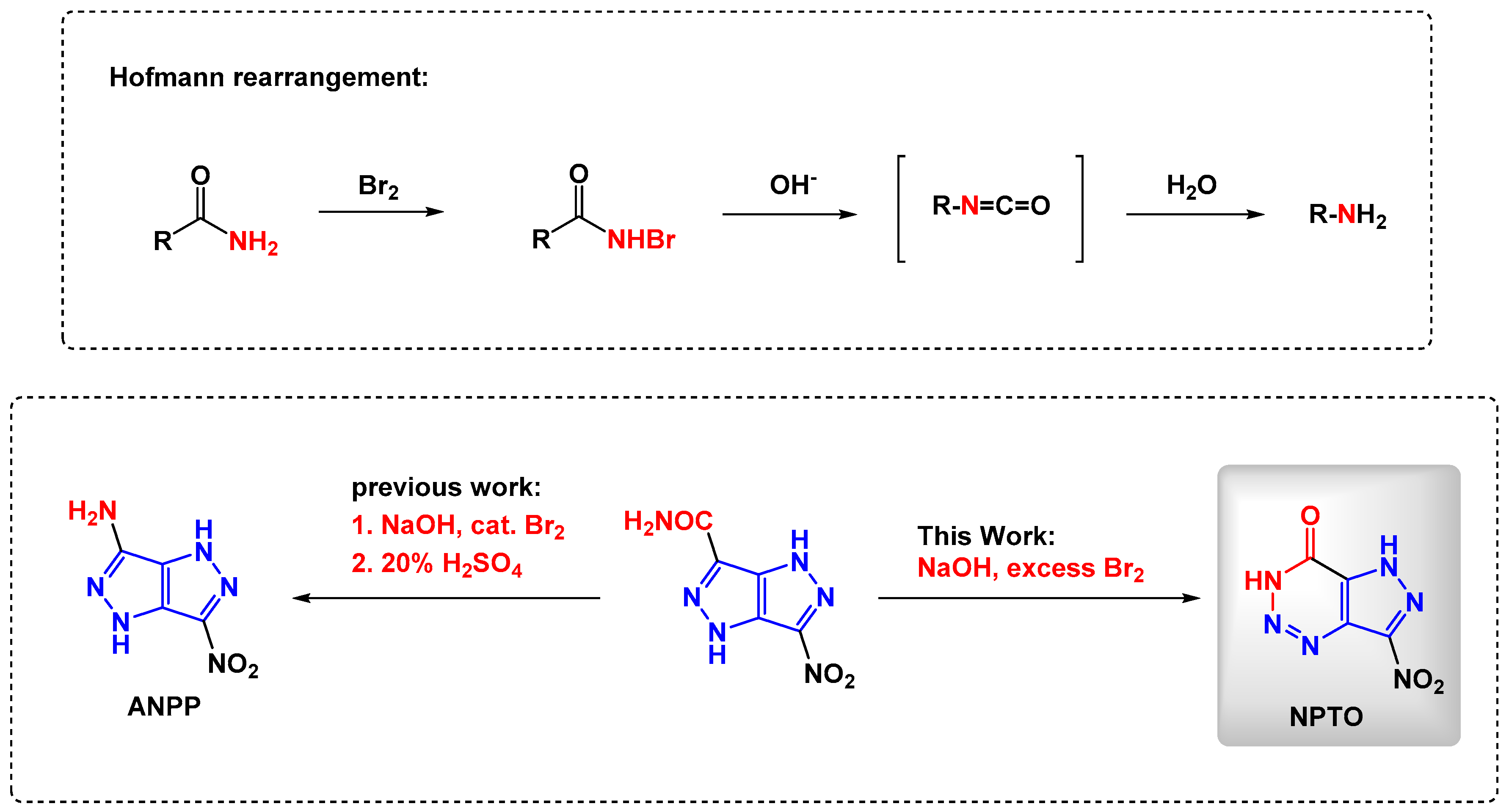
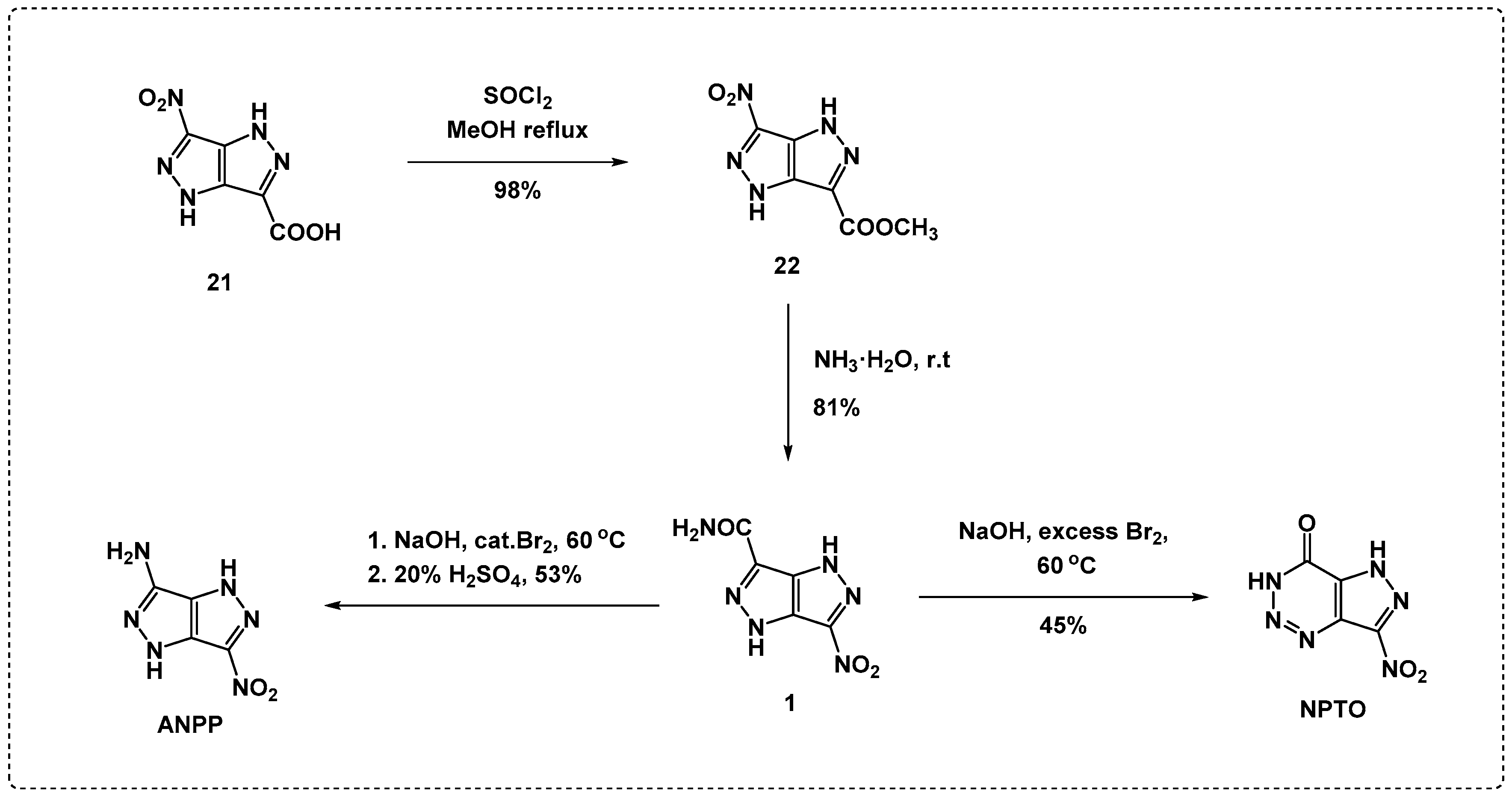
2.1. Synthesis and Characterization towards NPTO
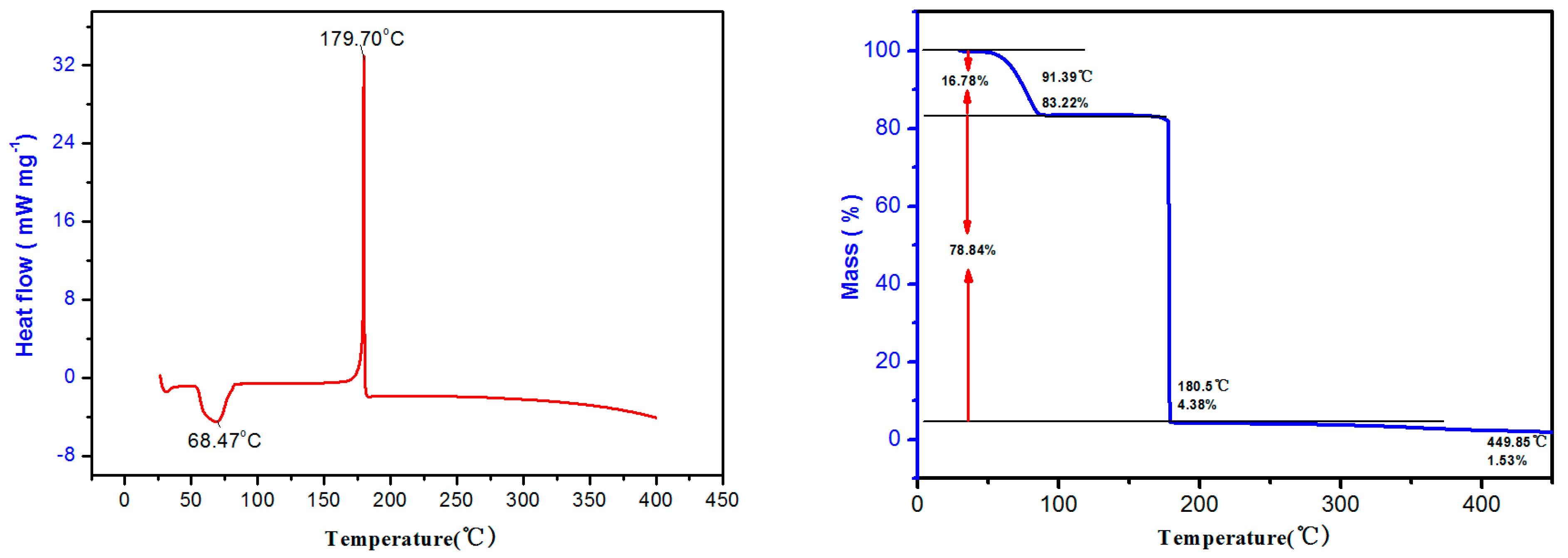
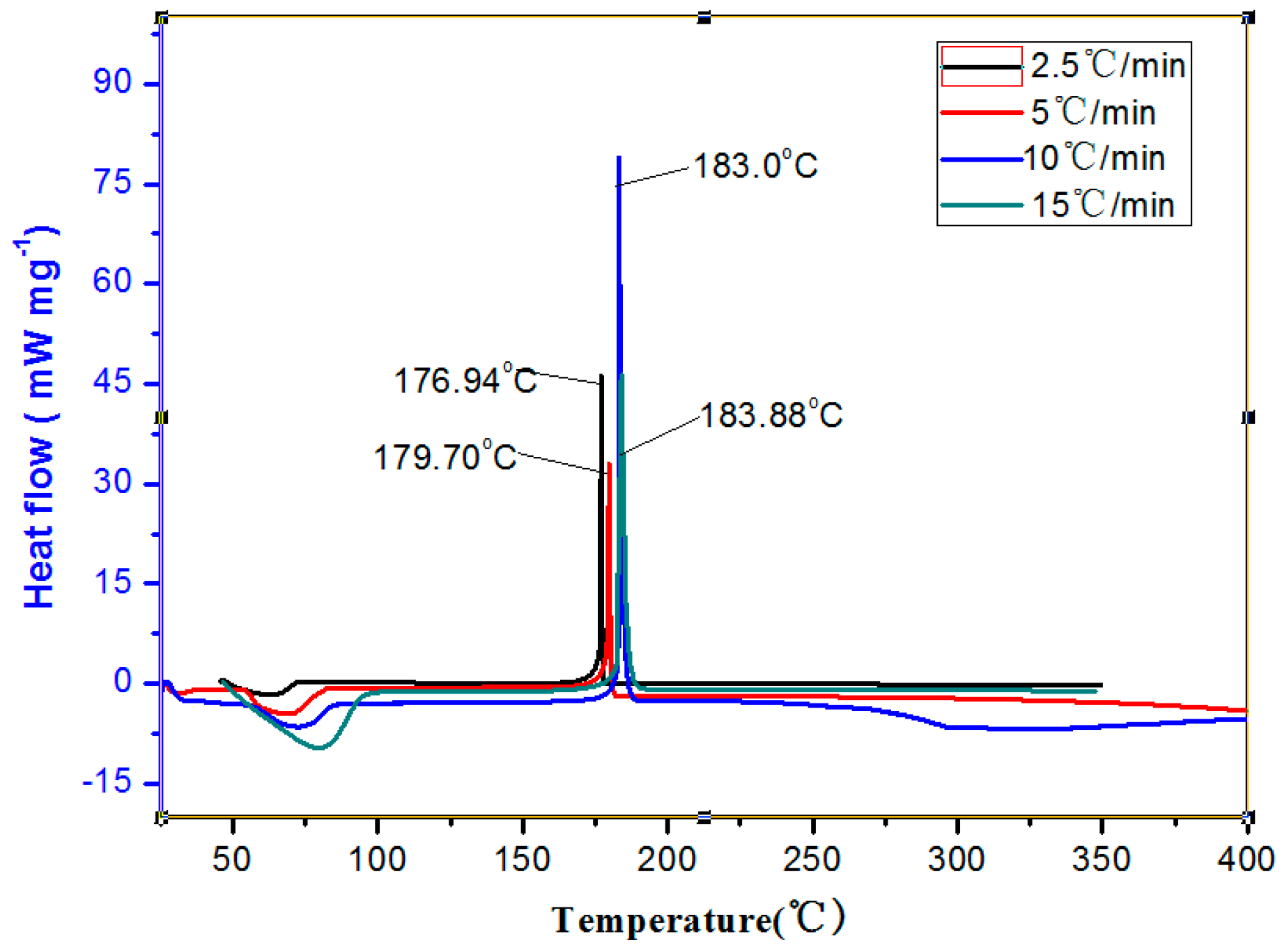
2.2. Studies on Thermal Behaviors of NPTO
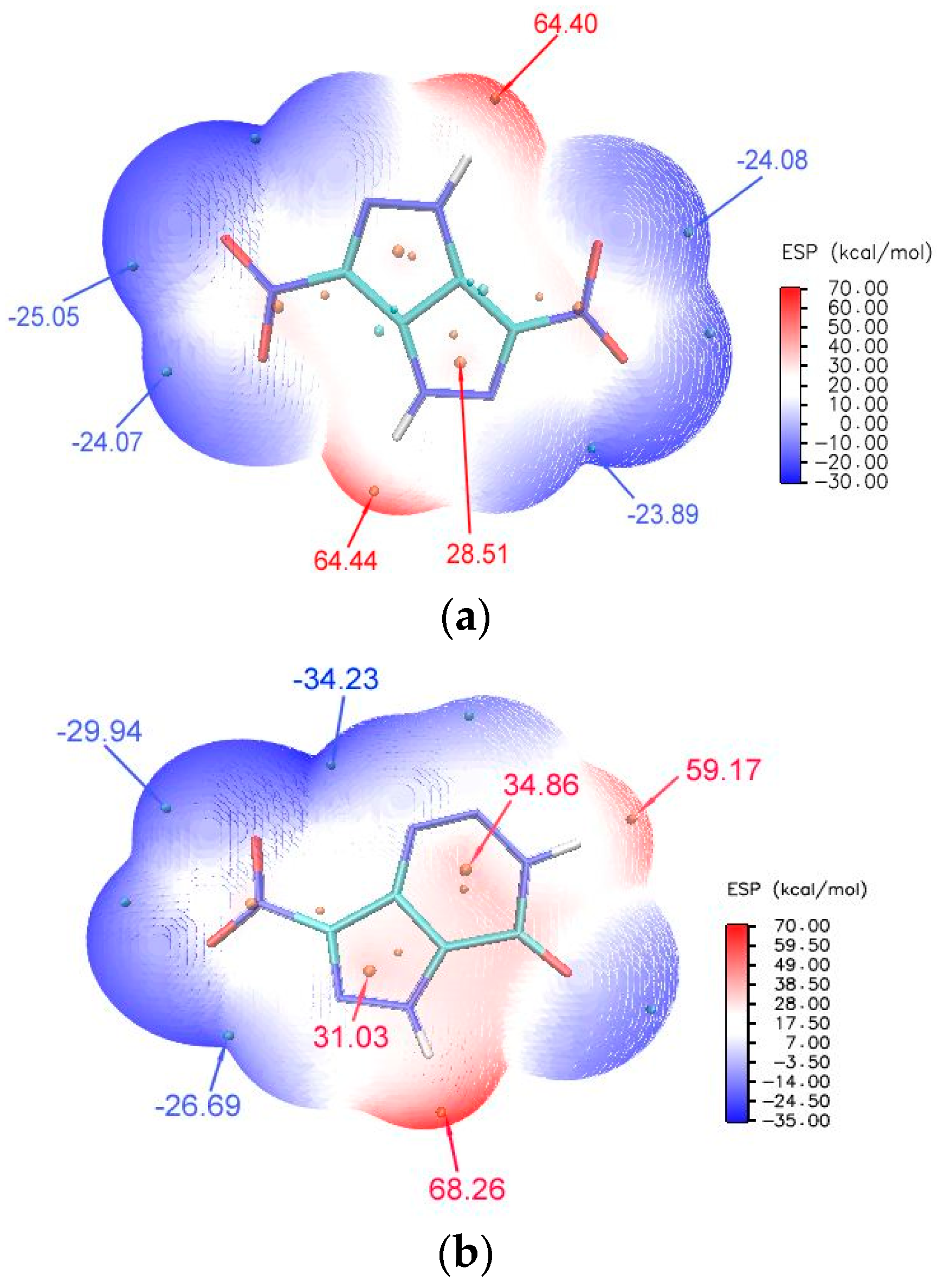
2.3. ESP Analysis Studies of NPTO
2.4. SEM Morphology of NPTO
2.5. Studies on Detonation Performances of NPTO
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Surya, K.D. Rearrangement Reactions in Applied Organic Chemistry: Reaction Mechanisms and Experimental Procedures in Medicinal Chemistry. II; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2021. [Google Scholar]
- Zeman, S.; Marcela, J. Sensitivity and performance of energetic materials. Propellants Explos. Pyrotech. 2016, 41, 426–451. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.Y. Origins of the energy and safety of energetic materials and of the energy & safety contradiction. Propellants Explos. Pyrot. 2018, 43, 855–856. [Google Scholar]
- Zhou, J.; Zhang, J.L.; Ding, L.; Bi, F.Q.; Wang, B.Z. Progress in the Construction of Cage-like Skeleton Energetic Compounds. Chin. J. Energ. Mater. 2019, 27, 708–716. [Google Scholar]
- Zhang, M.X.; Eaton, P.E.; Gilardi, R. Hepta and octanitrocubanes. Angew. Chem. Int. Ed. 2000, 39, 401–404. [Google Scholar] [CrossRef]
- Binnikov, A.N.; Kulikov, A.S.; Mahkova, N.N.; Ochinnikov, O.V.; Pivina, T.S. 4-Amino-3-azidocarbonyl furoxan as a universal synthon for the synthesis of energetic compounds of the furoxan series. In Proceedings of the 30th International Annual Conference of ICT, Karlsrruhe, Germany, 29 June–2 July 1999; pp. 58/1–58/10. [Google Scholar]
- Guo, T.; Liu, M.; Huang, X.C.; Wang, Z.J.; Qiu, S.J.; Ge, Z.X.; Meng, Z.H. Efficient preparation and comprehensive properties of thermal decomposition and detonation for 4,4′-dinitro-3,3′-azofuroxan. J. Anal. Appl. Pyrolysis 2017, 128, 451–458. [Google Scholar] [CrossRef]
- Chen, D.X.; Xiong, H.L.; Yang, H.W.; Tang, J.; Cheng, G.B. Nitropyrazole based tricyclic nitrogen-rich cation salts: A new class of promising insensitive energetic materials. FirePhysChem 2021, 1, 71–75. [Google Scholar] [CrossRef]
- Wu, B.; Yang, L.F.; Zhai, D.D.; Ma, C.M.; Pei, C.H. Facile synthesis of 4-amino-3,5-dinitropyrazolated energetic derivatives via 4-bromopyrazole and their performances. FirePhysChem 2021, 1, 76–82. [Google Scholar] [CrossRef]
- Yin, P.; Zhang, J.H.; Mitchell, L.A.; Parrish, D.A.; Shreeve, J.M. 3,6-Dinitropyrazolo[4,3-c]pyrazole -Based Multipurpose Energetic Materials through Versatile N-Functionalization Strategies. Angew. Chem. Int. Ed. 2016, 55, 12895–12897. [Google Scholar] [CrossRef]
- Li, Y.N.; Shu, Y.J.; Wang, B.Z.; Zhang, S.Y.; Zhai, L.J. Synthesis, structure and properties of neutral energetic materials based on N-functionalization of 3,6-dinitropyrazolo [4,3-c]pyrazole. RSC Adv. 2016, 6, 84760–84768. [Google Scholar] [CrossRef]
- Dalinger, I.L.; Shkineva, T.K.; Vatsadze, I.A.; Popova, G.P.; Shevelev, S.A. N-Fluoro derivatives of nitrated pyrazole-containing fused heterocycles. Mendeleev Commun. 2011, 21, 48–49. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Xia, H.L.; Yu, R.J.; Zhang, J.H.; Wang, K.C.; Zhang, Q.H. Synthesis and properties of 3,6-Dinitropyrazolo-[4,3-c] Pyrazole(DNPP) Derivatives. Propellants Explos. Pyrotech. 2020, 45, 546–553. [Google Scholar] [CrossRef]
- Li, Y.N.; Tang, T.; Lian, P.; Luo, Y.F.; Yang, W.; Wang, Y.B.; Li, H.; Zhang, Z.Z.; Wang, B.Z. Synthesis, thermal performance and quantum chemistry study on 1,4-diamino-3,5-dinitropyrazolo [4,3-c]pyrazole(LLM-119). Chin. J. Org. Chem. 2012, 32, 580–588. [Google Scholar] [CrossRef] [Green Version]
- Piercey, D.G.; Chavez, D.E.; Scott, B.L.; Imler, G.H.; Parrish, D.A. An Energetic Triazolo-1,2,4- Triazine and its N-Oxide. Angew. Chem. Int. Ed. 2016, 55, 15315–15318. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.C.; Scott, B.L.; Chavez, D.E. A high density pyrazolo-triazine explosive (PTX). J. Mater. Chem. A 2015, 3, 17963–17965. [Google Scholar] [CrossRef]
- Tang, Y.X.; He, C.L.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. Aminonitro Groups Surrounding a Fused Pyrazolo-triazine Ring: A Superior Thermally Stable and Insensitive Energetic Material. ACS Appl. Energy Mater. 2019, 2, 2263–2267. [Google Scholar] [CrossRef]
- Shevelev, S.A.; Dalimger, I.L.; Shkineva, T.K.; Ugrak, B.I.; Gulevskaya, V.I.; Kanishchev, M.I. Nitropyrazoles 1. Synthesis, transformations, and physicochemical properties of nitro derivatives of 1H,4H-pyrazolo[4,3-c]pyrazole. Russ. Chem. Bull. 1993, 42, 1063–1068. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. 2 1987, 12, S1–S19. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Wang, X.C.; Huang, H. π–stacked interactions in explosive crystals: Buffers against external mechanical stimuli. J. Am. Chem. Soc. 2008, 130, 8359–8365. [Google Scholar] [CrossRef]
- Boddu, V.M.; Viswanath, D.S.; Ghosh, T.K.; Damavarapu, R. 2,4,6-Triamino-1,3,5-trinitrobenzene (TATB) and TATB-based Formulations−A Review. J. Hazard. Mater. 2010, 181, 1–8. [Google Scholar] [CrossRef]
- Kretić, D.S.; Radovanovića, J.I.; Veljković, D.Ž. Can the sensitivity of energetic materials be tuned by using hydrogen bonds? Another look at the role of hydrogen bonding in the design of high energetic compounds. Phys. Chem. Chem. Phys. 2021, 23, 7472–7479. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.L.; Wang, B.Z.; Qiu, L.L.; Xu, R.Q.; Sheremetev, A.B. Recent Synthetic Efforts towards High Energy Density Materials: How to Design High-Performance Energetic Structures? FirePhysChem 2021, in press. [Google Scholar] [CrossRef]
- Hu, L.; Staples, R.J.; Shreeve, J.M. Hydrogen bond system generated by nitroamino rearrangement: New character for designing next generation energetic materials. Chem. Commun. 2021, 57, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Mitchell, L.A.; Parrish, D.A.; Shreeve, J.M. Energetic N-Nitramino/N-Oxyl-Functionalized Pyrazoles with Versatile π–π Stacking: Structure–Property Relationships of High-Performance Energetic Materials. Angew. Chem. Int. Ed. 2016, 55, 14409. [Google Scholar] [CrossRef]
- Xue, Q.; Bi, F.Q.; Zhang, J.L.; Wang, Z.J.; Zhai, L.J.; Huo, H.; Wang, B.Z.; Zhang, S.Y. A Family of Energetic Materials Based on 1,2,4-Oxadiazole and 1,2,5-Oxadiazole Backbones with Low Insensitivity and Good Detonation Performance. Front. Chem. 2020, 7, 942–951. [Google Scholar] [CrossRef] [Green Version]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Wu, C.L.; Cheng, H.J.; Gou, R.J.; Jia, H.Y.; Zhang, S.H. Molecular Dynamics Study on Intermolecular interaction of CL-20/MDNI Composite Explosive. Chin. J. Explos. Propellants 2017, 40, 66–72. [Google Scholar]
- Xie, Z.B.; Hu, S.Q.; Cao, X. Theoretical insight into the influence of molecular ratio on the binding energy and mechanical property of HMX/2-picoline-N-oxide cocrystal, cooperativity effect and surface electrostatic potential. Mol. Phys. 2016, 114, 2164–2176. [Google Scholar] [CrossRef]
- Yuan, S.; Gou, B.W.; Guo, S.F.; Xiao, L.; Hu, Y.B.; Chen, T.; Hao, G.Z.; Jiang, W. reparation, Characterization and Properties of A New CL-20/TKX-50 Cocrystal Explosive. Chin. J. Explos. Propellants 2020, 43, 167–172. [Google Scholar]
- Politzer, P.; Murray, J.S. The fundamental nature and role of the electrostatic potential in atoms and molecules. Theor. Chem. Acc. 2002, 108, 134–142. [Google Scholar] [CrossRef]
- Hou, C.H.; Zhang, Y.P.; Chen, Y.G.; Jia, X.L.; Zhang, S.M.; Tan, Y.X. Fabrication of Ultra-Fine TATB/HMX Cocrystal Using a Compound Solvent. Propellants Explos. Pyrotech. 2018, 43, 916–922. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, G.; Luan, J.Y.; Chen, Z.Q.; Su, P.F.; Shu, Y.J. Crystal structure, spectrum character and explosive property of a new cocrystal CL-20/DNT. J. Mol. Struct. 2016, 1110, 91–96. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Reven, T.V.; Kudin, K.N.; Burant, J.C.; et al. Gaussian~09 {R}evision {E}.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Montgomery, J.A.; Frisch, M.J.; Ochterski, J.W.; Petersson, G.A. A complete basis set model chemistry. VII. Use of the minimum population localization method. J. Chem. Phys. 2000, 112, 6532–6542. [Google Scholar] [CrossRef]
- Ochterski, J.W.; Petersson, G.A.; Montgomery, J.A. A complete basis set model chemistry. V. Extensions to six or more heavy atoms. J. Chem. Phys. 1996, 104, 2598–2619. [Google Scholar] [CrossRef]
- Westwell, M.S.; Searle, M.S.; Wales, D.J.; Williams, D.H. Empirical correlations between thermodynamic properties and intermolecular forces. J. Am. Chem. Soc. 1995, 117, 5013–5015. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Parrish, D.A.; Shreeve, J.M. Derivatives of 5-nitro-1,2,3-2H-triazole-high performance energetic materials. J. Mater. Chem. A 2013, 1, 585–593. [Google Scholar] [CrossRef]
- Suceska, M. EXPLO5, Version 6.04. 2017. Available online: https://www.ozm.cz/explosives-performance-tests/thermochemical-computer-code-explo5/ (accessed on 28 November 2021).
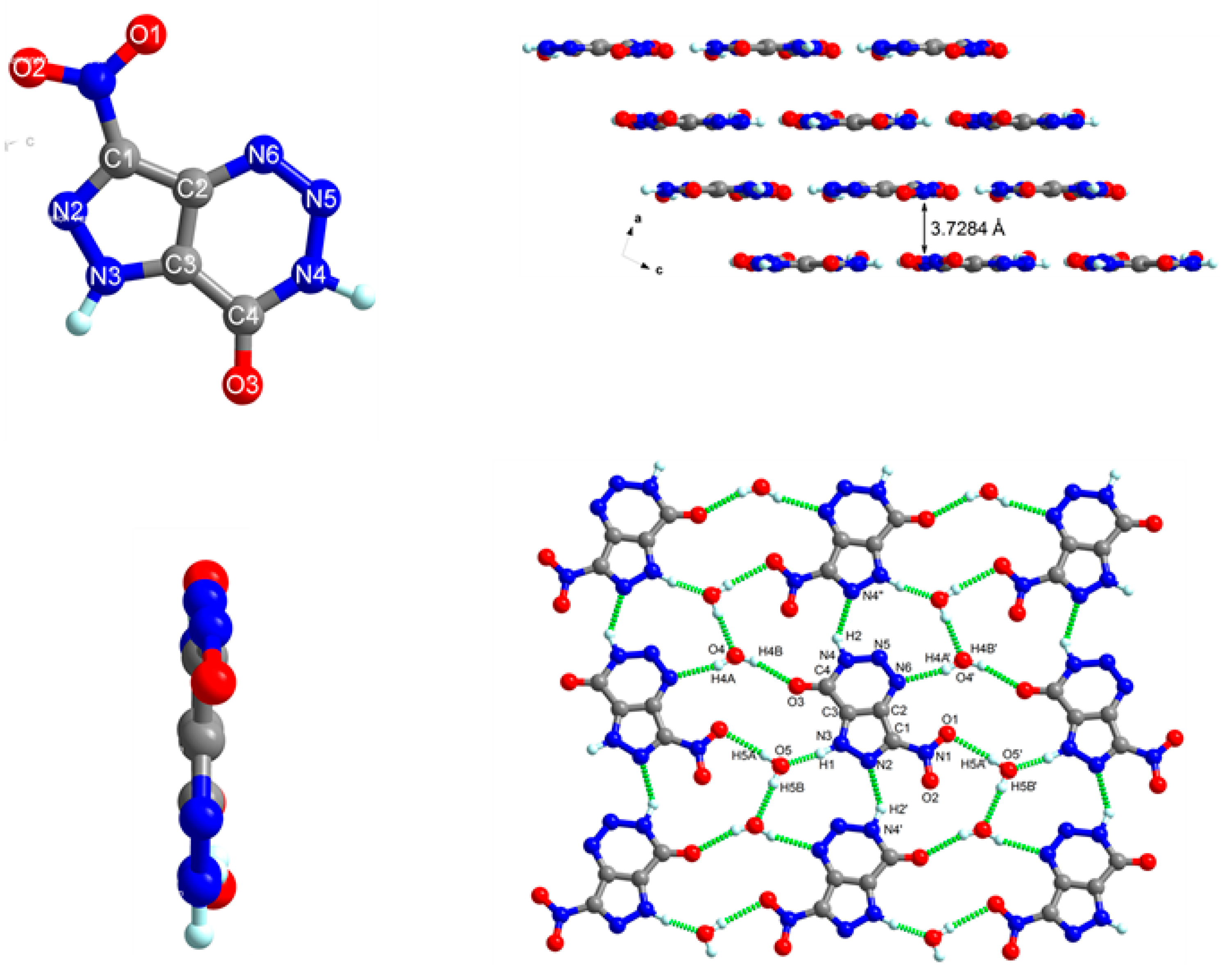

| Compound | NPTO∙2H2O |
|---|---|
| Formula | C4H6N6O5 |
| Formula weight | 218.15 |
| T (K) | 296(2) |
| λ (Å) | 0.71073 |
| Crystal system | Monoclinic |
| Space group | P2(1)/n |
| a (Å) | 6.608(3) |
| b (Å) | 9.330(4) |
| c (Å) | 13.998(6) |
| Volume (Å3) | 855.7(7) |
| Z | 4 |
| Dc (g/cm3) | 1.693 |
| F (000) | 448 |
| θ range/(°) | 2.63 to 25.09 |
| Reflections collected/unique | 4177/1519 [R(int) = 0.0623] |
| Refinement method | Full-matrix least-squares on F2 |
| GOF on F2 | 1.002 |
| Final R indexes (I > 2σ(I)) | R1 = 0.0560, wR2 = 0.1254 |
| Final R indexes (all data) | R1 = 0.1058, wR2 = 0.1498 |
| Largest diff peak and hole (e Å−3) | 0.322 and −0.222 |
| GOF on F2 | 0.949 |
| CCDC number | 2082280 |
| Heating Rates β/(°C/min) | Tpi/(°C) | Ea/(kJ·mol−1) | lg(Aa/s−1) | Tp0/ (°C) | ΔH≠/ (KJ·mol−1) |
|---|---|---|---|---|---|
| 2.5 | 176.94 | 429.14 | 27.33 | 173.33 | 427.70 |
| 5 | 179.7 | ||||
| 10 | 183.0 | ||||
| 15 | 183.88 |
| Compound | ρa (g⋅cm−3) | ΔHf b (kj⋅mol−1) | Dc (m⋅s−1) | Pd (Gpa) | H50e (cm) | H50f (J) |
|---|---|---|---|---|---|---|
| ANPP | 1.678 | 283.28 | 7317 | 19.79 | 44.63 | 10.94 |
| DNPP | 1.758 | 290.44 | 8068 | 26.96 | 33.98 | 8.32 |
| NPTO | 1.752 | 217.13 | 7556 | 22.32 | 40.44 | 9.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, F.-Q.; Luo, Y.-F.; Zhang, J.-L.; Huo, H.; Wang, B.-Z. Synthesis of Energetic 7-Nitro-3,5-dihydro-4H-pyrazolo[4,3-d][1,2,3]triazin-4-one Based on a Novel Hofmann-Type Rearrangement. Molecules 2021, 26, 7319. https://doi.org/10.3390/molecules26237319
Bi F-Q, Luo Y-F, Zhang J-L, Huo H, Wang B-Z. Synthesis of Energetic 7-Nitro-3,5-dihydro-4H-pyrazolo[4,3-d][1,2,3]triazin-4-one Based on a Novel Hofmann-Type Rearrangement. Molecules. 2021; 26(23):7319. https://doi.org/10.3390/molecules26237319
Chicago/Turabian StyleBi, Fu-Qiang, Yi-Fen Luo, Jun-Lin Zhang, Huan Huo, and Bo-Zhou Wang. 2021. "Synthesis of Energetic 7-Nitro-3,5-dihydro-4H-pyrazolo[4,3-d][1,2,3]triazin-4-one Based on a Novel Hofmann-Type Rearrangement" Molecules 26, no. 23: 7319. https://doi.org/10.3390/molecules26237319
APA StyleBi, F.-Q., Luo, Y.-F., Zhang, J.-L., Huo, H., & Wang, B.-Z. (2021). Synthesis of Energetic 7-Nitro-3,5-dihydro-4H-pyrazolo[4,3-d][1,2,3]triazin-4-one Based on a Novel Hofmann-Type Rearrangement. Molecules, 26(23), 7319. https://doi.org/10.3390/molecules26237319





