Headspace Volatiles and Endogenous Extracts of Prunus mume Cultivars with Different Aroma Types
Abstract
1. Introduction
2. Results
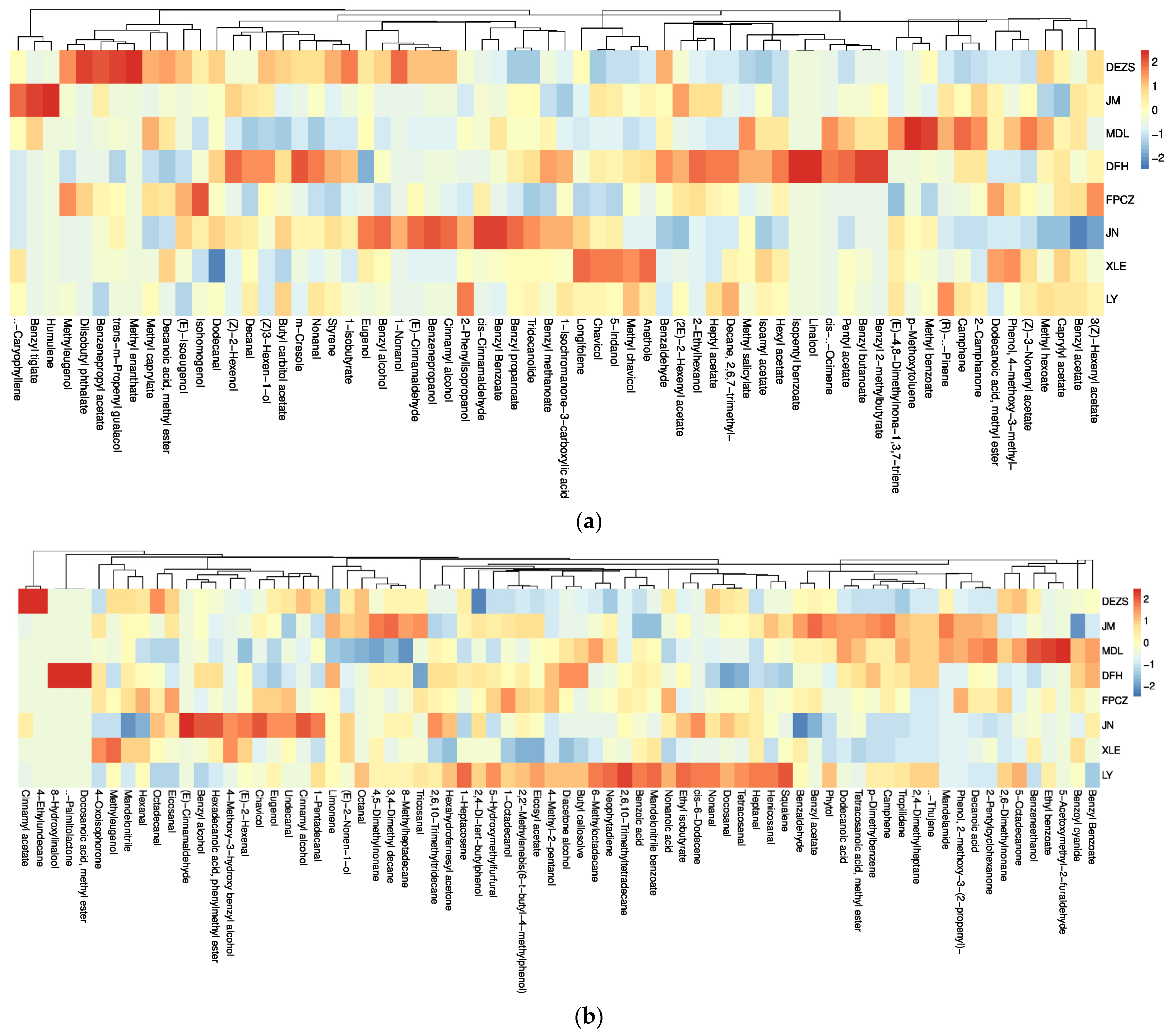
2.1. Identification and Relative Content Analysis of the Composition of the P. mume Cultivars with Four Aroma Types
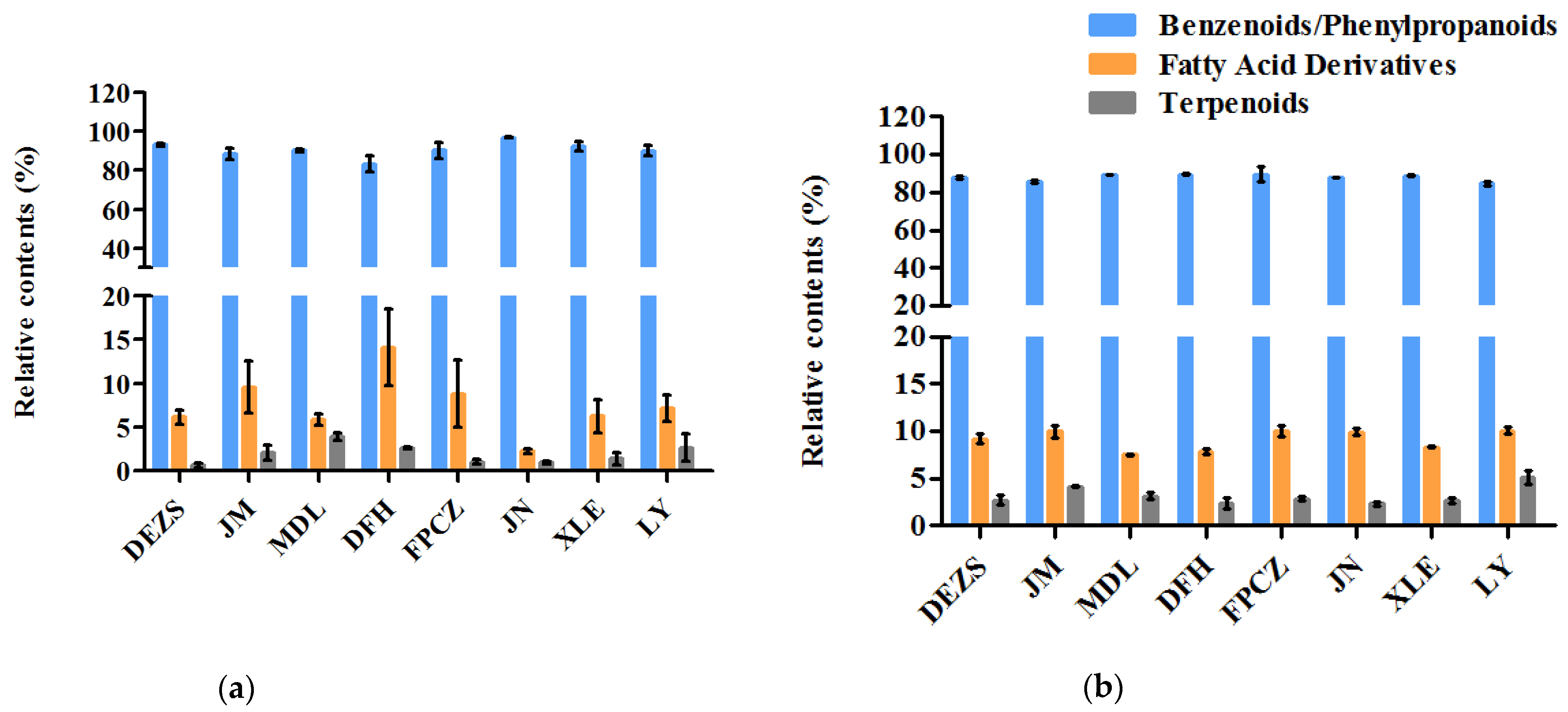
2.2. The Volatilization Rate of the Main Components of the P. mume Cultivars with Four Aroma Types
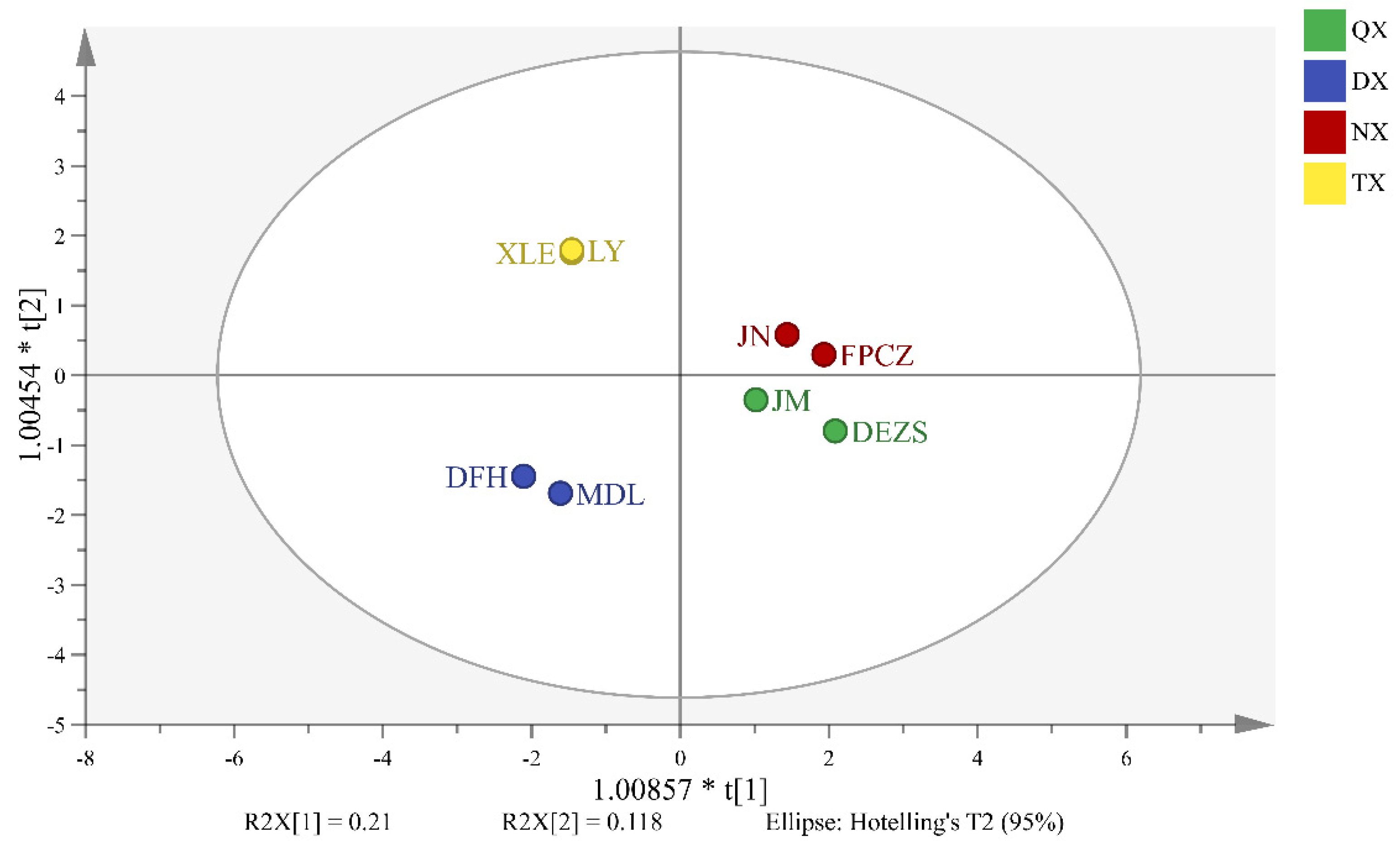
2.3. Analysis of Differential Headspace Volatiles of P. mume Cultivars with Four Aroma Types
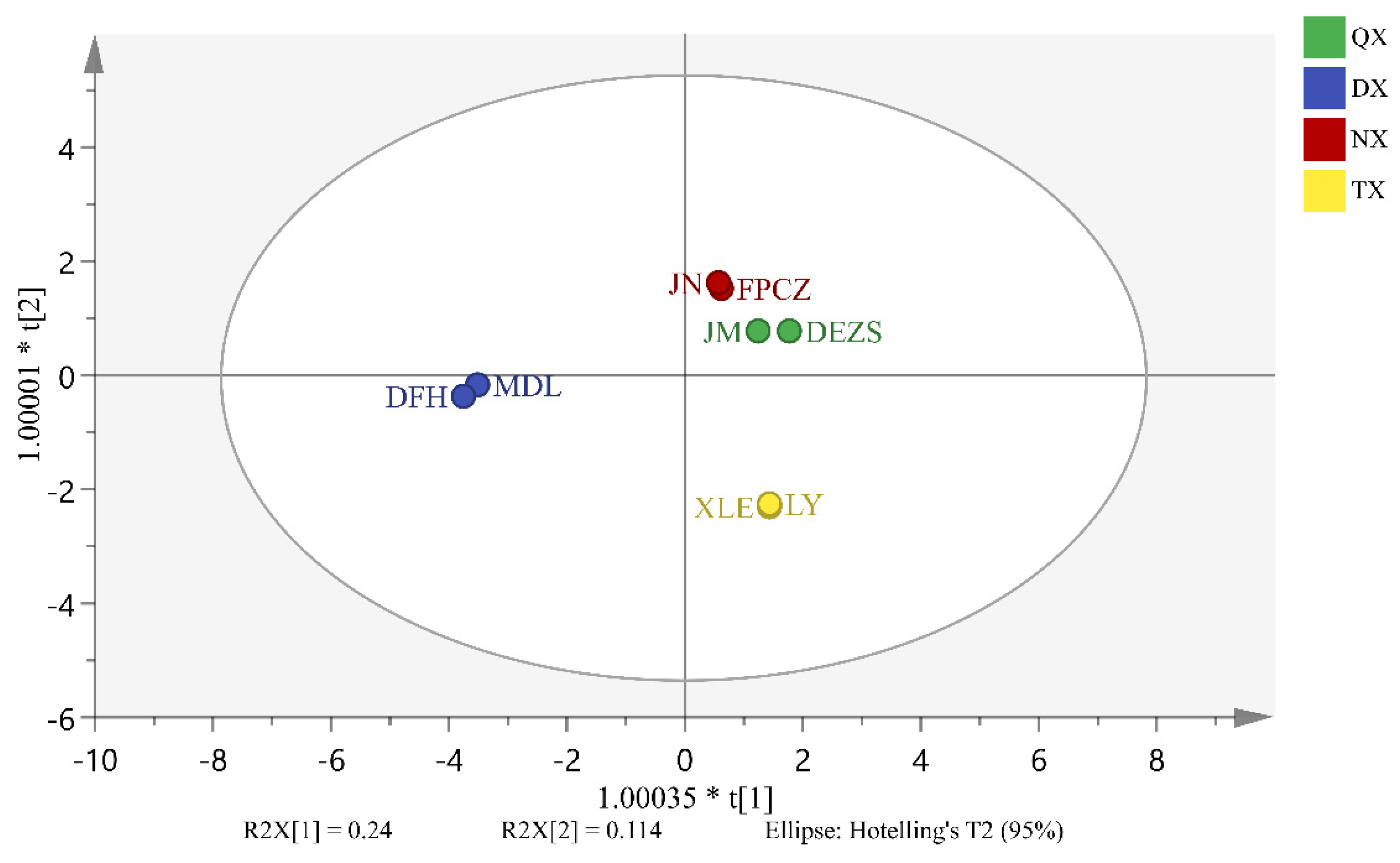
2.4. Analysis of Differential Endogenous Extracts of P. mume Cultivars with Four Aroma Types
3. Discussion
3.1. The Similarities and Differences among the Components of Four Aroma Types
3.2. Biomarkers Which Have Made Great Contribution to the Four Aroma Types
4. Materials and Methods
4.1. Plant Materials
4.2. Instruments and Reagents
4.3. SPME Collection and Solvent Extraction
4.4. GC-MS Analysis
4.5. Odor Activity Values (OAVs)
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Shi, S.; Duan, G.; Li, D.; Wu, J.; Liu, X.; Hong, B.; Yi, M.; Zhang, Z. Two-dimensional analysis provides molecular insight into flower scent of Lilium ‘Siberia’. Sci. Rep. 2018, 8, 5352. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, Y.; Zhao, Y.; Liu, C.; Bao, J. Identification of Floral Scent Profiles in Bearded Irises. Molecules 2019, 24, 1773. [Google Scholar] [CrossRef]
- Kondo, M.; Oyama-Okubo, N.; Ando, T.; Marchesi, E.; Nakayama, M. Floral scent diversity is differently expressed in emitted and endogenous components in Petunia axillaris lines. Ann. Bot. 2006, 98, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Li, J.; Li, X.; Yin, H. Composition analysis of floral scent within genus Camellia uncovers substantial interspecific variations. Sci. Hortic. 2019, 250, 207–213. [Google Scholar] [CrossRef]
- Han, Y.; Wang, H.; Wang, X.; Li, K.; Dong, M.; Li, Y.; Zhu, Q.; Shang, F. Mechanism of floral scent production in Osmanthus fragrans and the production and regulation of its key floral constituents, beta-ionone and linalool. Hortic. Res. 2019, 6, 106. [Google Scholar] [CrossRef]
- Fu, X.; Zhou, Y.; Zeng, L.; Dong, F.; Mei, X.; Liao, Y.; Watanabe, N.; Yang, Z. Analytical method for metabolites involved in biosynthesis of plant volatile compounds. RSC Adv. 2017, 7, 19363–19372. [Google Scholar] [CrossRef]
- Hao, R.; Du, D.; Wang, T.; Yang, W.; Wang, J.; Zhang, Q. A comparative analysis of characteristic floral scent compounds in Prunus mume and related species. Biosci. Biotechnol. Biochem. 2014, 78, 1640–1647. [Google Scholar] [CrossRef]
- Barman, M.; Mitra, A. Temporal relationship between emitted and endogenous floral scent volatiles in summer- and winter-blooming Jasminum species. Physiol. Plant 2019, 166, 946–959. [Google Scholar] [CrossRef]
- Kishimoto, K.; Nakayama, M.; Yagi, M.; Onozaki, T.; Oyama-Okubo, N. Evaluation of Wild Dianthus Species as Genetic Resources for Fragrant Carnation Breeding Based on Their Floral Scent Composition. J. Jpn. Soc. Hortic. Sci. 2011, 80, 175–181. [Google Scholar] [CrossRef]
- Hao, R.J.; Zhang, Q.; Yang, W.R.; Wang, J.; Cheng, T.R.; Pan, H.T.; Zhang, Q.X. Emitted and endogenous floral scent compounds of Prunus mume and hybrids. Biochem. Syst. Ecol. 2014, 54, 23–30. [Google Scholar] [CrossRef]
- Scalliet, G.; Piola, F.; Douady, C.J.; Réty, S.; Raymond, O.; Baudino, S.; Bordji, K.; Bendahmane, M.; Dumas, C.; Mark Cock, J.; et al. Scent evolution in Chinese roses. Proc. Natl. Acad. Sci. USA 2008, 105, 5927–5932. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, W.; Zhang, D.; Wang, G.; Cao, F. Flowering Stage and Daytime Affect Scent Emission of Malus ioensis “Prairie Rose”. Molecules 2019, 24, 2356. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, T.; Fan, Q.; Qi, X.; Fei, Z.; Fang, W.; Jiang, J.; Chen, F.; Chen, S. Identification of Floral Scent in Chrysanthemum Cultivars and Wild Relatives by Gas Chromatography-Mass Spectrometry. Molecules 2015, 20, 5346–5359. [Google Scholar] [CrossRef]
- Ferreira, O.O.; Cruz, J.N.D.; Franco, C.D.J.P.; Silva, S.G.; Costa, W.A.D.; Oliveira, M.S.D.; Andrade, E.H.D.A. First Report on Yield and Chemical Composition of Essential Oil Extracted from Myrcia eximia DC (Myrtaceae) from the Brazilian Amazon. Molecules 2020, 25, 783. [Google Scholar] [CrossRef]
- Oliveira, M.S.D.; Cruz, J.N.D.; Costa, W.A.D.; Silva, S.G.; Junior, R.N.D.C. Molecules Chemical Composition, Antimicrobial Properties of Siparuna guianensis Essential Oil and a Molecular Docking and Dynamics Molecular Study of its Major Chemical Constituent. Molecules 2020, 25, 3852. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Ding, A.; Zhang, T.; Luo, L.; Wang, J.; Cheng, T.; Zhang, Q. Expansion of PmBEAT genes in the Prunus mume genome induces characteristic floral scent production. Hortic. Res. 2019, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Bao, F.; Yang, Y.; Hu, L.; Ding, A.; Ding, A.; Wang, J.; Cheng, T.; Zhang, Q. A Comparative Analysis of Floral Scent Compounds in Intraspecific Cultivars of Prunus mume with Different Corolla Colours. Molecules 2019, 25, 145. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. China Mei Flower (Prunus mume) Cultivars in Colour; China Forestry Publishing House: Beijing, China, 2010. [Google Scholar]
- Zhao, Y.Q.; Pan, H.T.; Zhang, Q.X.; Sun, M.; Pan, B.C. Studies on the Volatile Constituents from Cultivars of Prunus mume. J. Trop. Subtrop. Bot. 2010, 18, 310–315. [Google Scholar]
- Zhao, Y.Q.; Pan, H.T.; Zhang, Q.X.; Pan, B.C.; Cai, M. Dynamics of fragrant compounds from Prunus mume flowers. J. Beijing For. Univ. 2010, 32, 201–206. [Google Scholar]
- Zhao, Y.Q.; Pan, H.T.; Zhang, Q.X.; Pan, B.C.; Cai, M. Analysis of volatile compounds from Prunus mume flowers. Guihaia 2011, 31, 554–558. [Google Scholar]
- Zhang, T.; Huo, T.; Ding, A.; Hao, R.; Wang, J.; Cheng, T.; Bao, F.; Zhang, Q. Genome-wide identification, characterization, expression and enzyme activity analysis of coniferyl alcohol acetyltransferase genes involved in eugenol biosynthesis in Prunus mume. PLoS ONE 2019, 14, e0223974. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, C.; Cheng, B.; Han, Y.; Zhang, Q. Studies on the volatile compounds in flower extracts of Rosa odorata and R. chinensis. Ind. Crop. Prod. 2020, 146, 112143. [Google Scholar] [CrossRef]
- Patrick, R.M.; Huang, X.Q.; Dudareva, N.; Li, Y. Dynamic histone acetylation in floral volatile synthesis and emission in petunia flowers. J. Exp. Bot. 2021, 72, 3704–3722. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, H.; Wan, Y.; Li, T.; Liu, X.; Sun, Z.; Li, Z. Volatile Organic Compounds Emissions from Luculia pinceana Flower and Its Changes at Different Stages of Flower Development. Molecules 2016, 21, 531. [Google Scholar] [CrossRef] [PubMed]
- Barman, M.; Kotamreddy, J.; Agarwal, A.; Mitra, A. Enhanced emission of linalool from floral scent volatile bouquet in Jasminum auriculatum variants developed via gamma irradiation. Ind. Crop. Prod. 2020, 152, 112545. [Google Scholar] [CrossRef]
- Page, P.; Favre, A.; Schiestl, F.P.; Karrenberg, S. Do Flower Color and Floral Scent of Silene Species affect Host Preference of Hadena bicruris, a Seed-Eating Pollinator, under Field Conditions? PLoS ONE 2014, 9, e98755. [Google Scholar] [CrossRef]
- Hu, Z.H.; Shen, Y.B.; Luo, Y.Q.; Shen, F.Y.; Gao, H.B.; Gao, R.F. Aldehyde volatiles emitted in succession from mechanically damaged leaves of poplar cuttings. J. Plant Biol. 2008, 51, 269–275. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, J.; Zheng, J.; Leng, P.; Li, X.; Hu, Z.; Liu, J.; Meng, X. Analysis of floral scent emitted from Syringa plants. J. For. Res. 2016, 27, 273–281. [Google Scholar] [CrossRef]
- De Oliveira, M.S.; da Silva, V.M.P.; Freitas, L.C.; Silva, S.G.; Cruz, J.N.; Andrade, E.H.d.A. Extraction Yield, Chemical Composition, Preliminary Toxicity of Bignonia nocturna (Barb.Rodr.) L.G. Lohmann (Bignoniaceae) Essential Oil and In silico Evaluation of the interaction. Chem. Biodivers. 2021, 18, e2000982. [Google Scholar]
- Adebesin, F.; Widhalm, J.R.; Boachon, B.; Lefevre, F.; Pierman, B.; Lynch, J.H.; Alam, I.; Junqueira, B.; Benke, R.; Ray, S. Emission of volatile organic compounds from petunia flowers is facilitated by an ABC transporter. Science 2017, 356, 1386–1388. [Google Scholar] [CrossRef] [PubMed]
- Rh, A.; Sy, A.; Zz, A.; Yz, A.; Jc, A.; Chen, Q.B. Identification and specific expression patterns in flower organs of ABCG genes related to floral scent from Prunus mume. Sci. Hortic. 2021, 288, 110218. [Google Scholar]
- Hu, D.; Guo, J.; Li, T.; Zhao, M.; Zou, T.; Song, H.; Alim, A. Comparison and Identification of the Aroma-Active Compounds in the Root of Angelica dahurica. Molecules 2019, 24, 4352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Mi, J.; Zhang, M.; Wang, Y.; Jiang, Z.; Hu, P. GC-MS Profiling of Volatile Components in Different Fermentation Products of Cordyceps Sinensis Mycelia. Molecules 2017, 22, 1800. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Gulati, A. Fractionation and identification of minor and aroma-active constituents in Kangra orthodox black tea. Food Chem. 2015, 167, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Zarzo, M. Effect of Functional Group and Carbon Chain Length on the Odor Detection Threshold of Aliphatic Compounds. Sensors 2012, 12, 4105–4112. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.Y.; Zhang, L.P.; Wang, C.J.; Li, B.; Wang, J.P.; Wang, Y. Analysis of Volatile Components in Three Peony Petals by HS-SPME-GC/MS. Sci. Technol. Food Ind. 2021, 42, 294–302. [Google Scholar]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Li, X.Y.; Wu, J.K.; Wang, H.J.; Li, M.Y.; Shen, Y.H.; Liu, J.Z.; Zhang, L.B. Characterization of Volatiles Changes in Chinese Dwarf Cherry Fruit during Its Development. Sci. China Chem. 2021, 1964–1980. [Google Scholar]
- Oyama-Okubo, N.; Tsuji, T. Analysis of Floral Scent Compounds and Classification by Scent Quality in Tulip Cultivars. J. Jpn Soc. Hortic. Sci. 2013, 82, 344–353. [Google Scholar] [CrossRef]
- Sabbatini, A.; Jurnatan, Y.; Fraatz, M.A.; Govori, S.; Haziri, A.; Millaku, F.; Zorn, H.; Zhang, Y. Aroma characterization of a wild plant (Sanguisorba albanica) from Kosovo using multiple headspace solid phase microextraction combined with gas chromatography-mass spectrometry-olfactometry. Food Res. Int. 2019, 120, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Motooka, R.; Usami, A.; Nakahashi, H.; Koutari, S.; Nakaya, S.; Shimizu, R.; Tsuji, K.; Marumoto, S.; Miyazawa, M. Characteristic odor components of essential oils from Eurya japonica. J. Oleo. Sci. 2015, 64, 577–584. [Google Scholar] [CrossRef] [PubMed][Green Version]


| Strong-Scented vs. Light-Scented | Strong-Scented vs. Sweet-Scented | Fresh-Scented vs. Light-Scented | Fresh-Scented vs. Strong-Scented | Fresh-Scented vs. Sweet-Scented | Sweet-Scented vs. Light-Scented |
|---|---|---|---|---|---|
| (E)-cinnamaldehyde | (E)-cinnamaldehyde | methyl salicylate | cis-cinnamaldehyde | (Z)3-hexen-1-ol | benzyl butanoate |
| camphene | (Z)3-hexen-1-ol | camphene | cinnamyl alcohol | (Z)-2-hexenol | pentyl acetate |
| (E)-cinnamaldehyde | m-cresole | ||||
| (Z)3-hexen-1-ol |
| Strong-Scented vs. Light-Scented | Strong-Scented vs. Sweet-Scented | Fresh-Scented Vs. Light-Scented | Fresh-Scented vs. Strong-Scented | Fresh-Scented vs. Sweet-Scented | Sweet-Scented vs. Light-Scented |
|---|---|---|---|---|---|
| tetracosanoic acid, methyl ester | tetracosanoic acid, methyl ester | tetracosanal | 1-heptacosene | mandelonitrile benzoate | tetracosanoic acid, methyl ester |
| tetracosanal | 1-heptacosene | cinnamyl acetate | hexadecanoic acid, phenylmethyl ester | benzoyl cyanide | benzyl benzoate |
| chavicol | benzoyl cyanide | tricosanal | tricosanal | squalene | |
| cinnamyl alcohol | benzyl benzoate | chavicol | tetracosanoic acid, methyl ester | tetracosanal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wu, Y.; Zhu, H.; Zhang, H.; Xu, J.; Fu, Q.; Bao, M.; Zhang, J. Headspace Volatiles and Endogenous Extracts of Prunus mume Cultivars with Different Aroma Types. Molecules 2021, 26, 7256. https://doi.org/10.3390/molecules26237256
Wang X, Wu Y, Zhu H, Zhang H, Xu J, Fu Q, Bao M, Zhang J. Headspace Volatiles and Endogenous Extracts of Prunus mume Cultivars with Different Aroma Types. Molecules. 2021; 26(23):7256. https://doi.org/10.3390/molecules26237256
Chicago/Turabian StyleWang, Xueqin, Yanyan Wu, Huanhuan Zhu, Hongyan Zhang, Juan Xu, Qiang Fu, Manzhu Bao, and Jie Zhang. 2021. "Headspace Volatiles and Endogenous Extracts of Prunus mume Cultivars with Different Aroma Types" Molecules 26, no. 23: 7256. https://doi.org/10.3390/molecules26237256
APA StyleWang, X., Wu, Y., Zhu, H., Zhang, H., Xu, J., Fu, Q., Bao, M., & Zhang, J. (2021). Headspace Volatiles and Endogenous Extracts of Prunus mume Cultivars with Different Aroma Types. Molecules, 26(23), 7256. https://doi.org/10.3390/molecules26237256








