Long-Term Evaluation of Poly(lactic acid) (PLA) Implants in a Horse: An Experimental Pilot Study
Abstract
:1. Introduction
2. Results
2.1. Clinical Evaluation
2.2. Plasma Fibrinogen
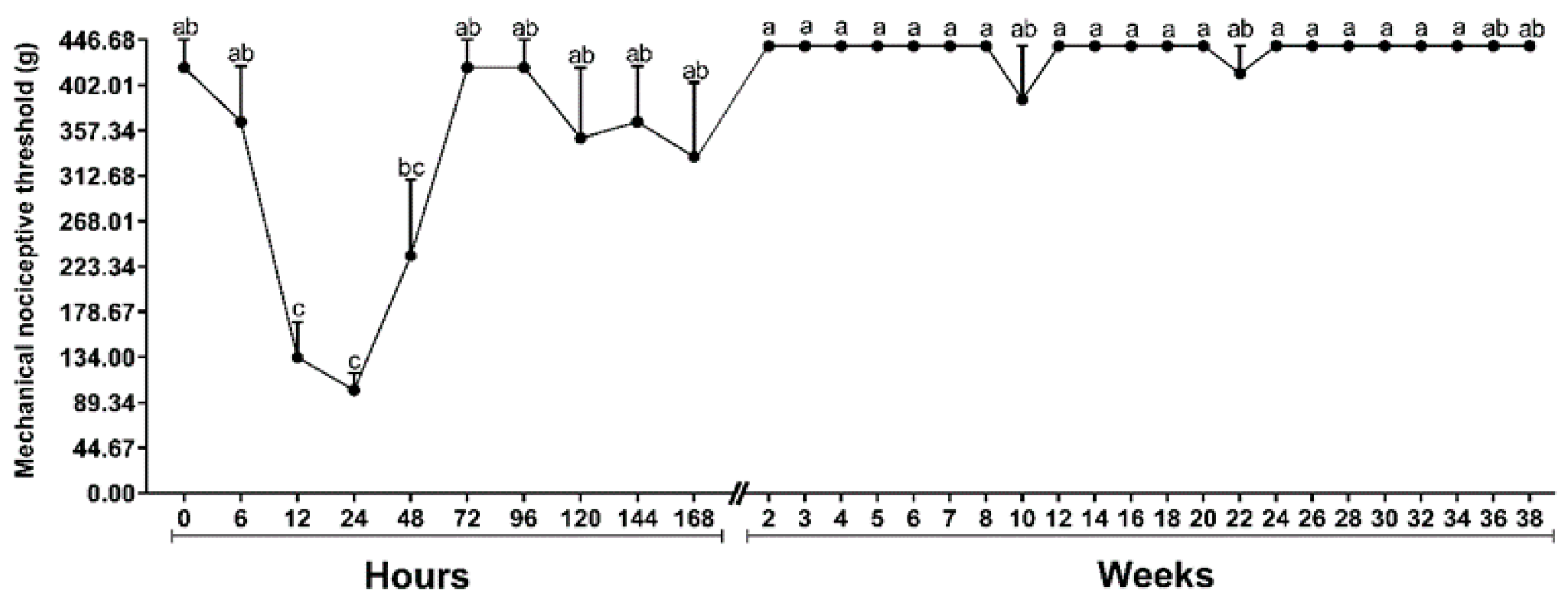
2.3. Mechanical Nociceptive Threshold (MNT)
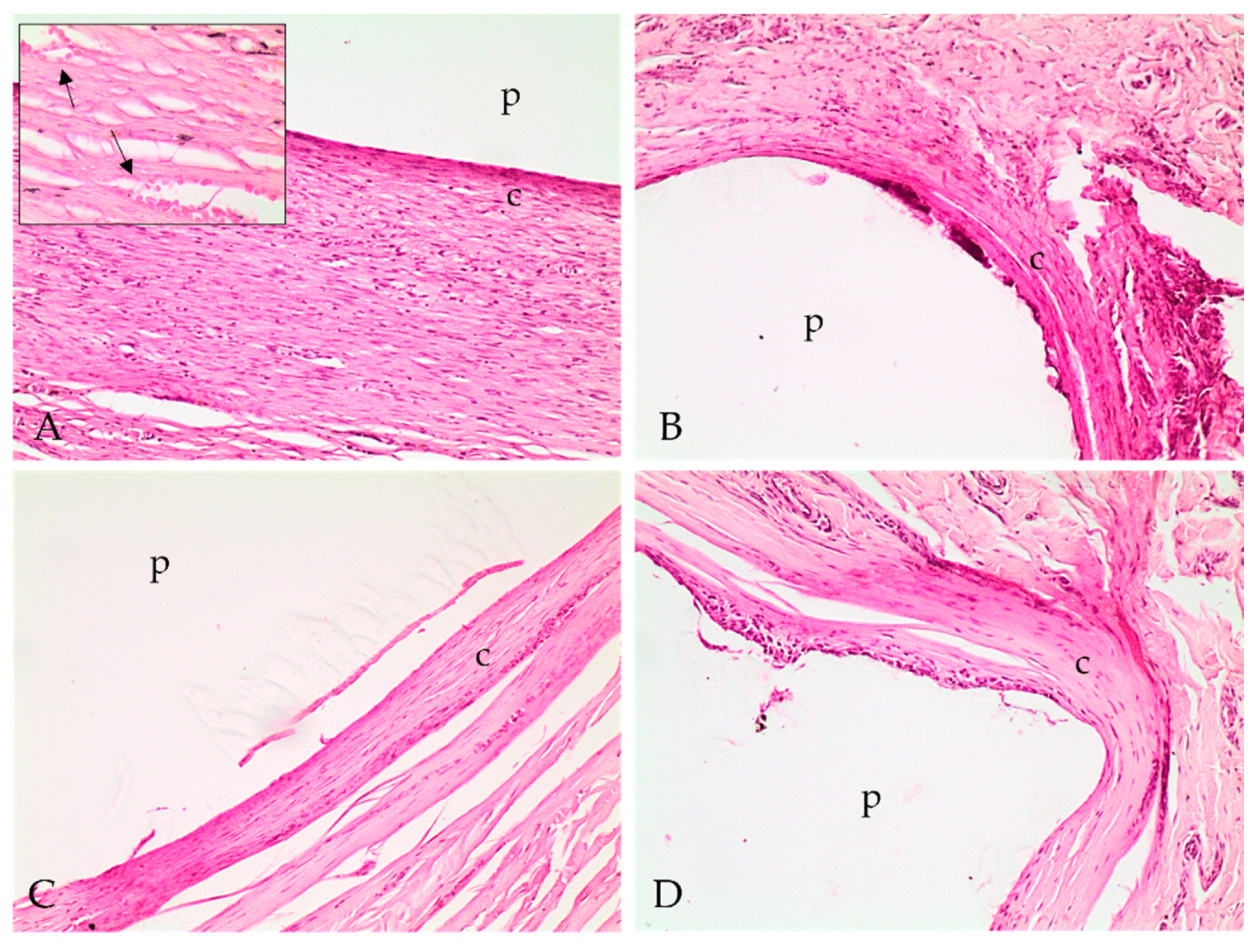
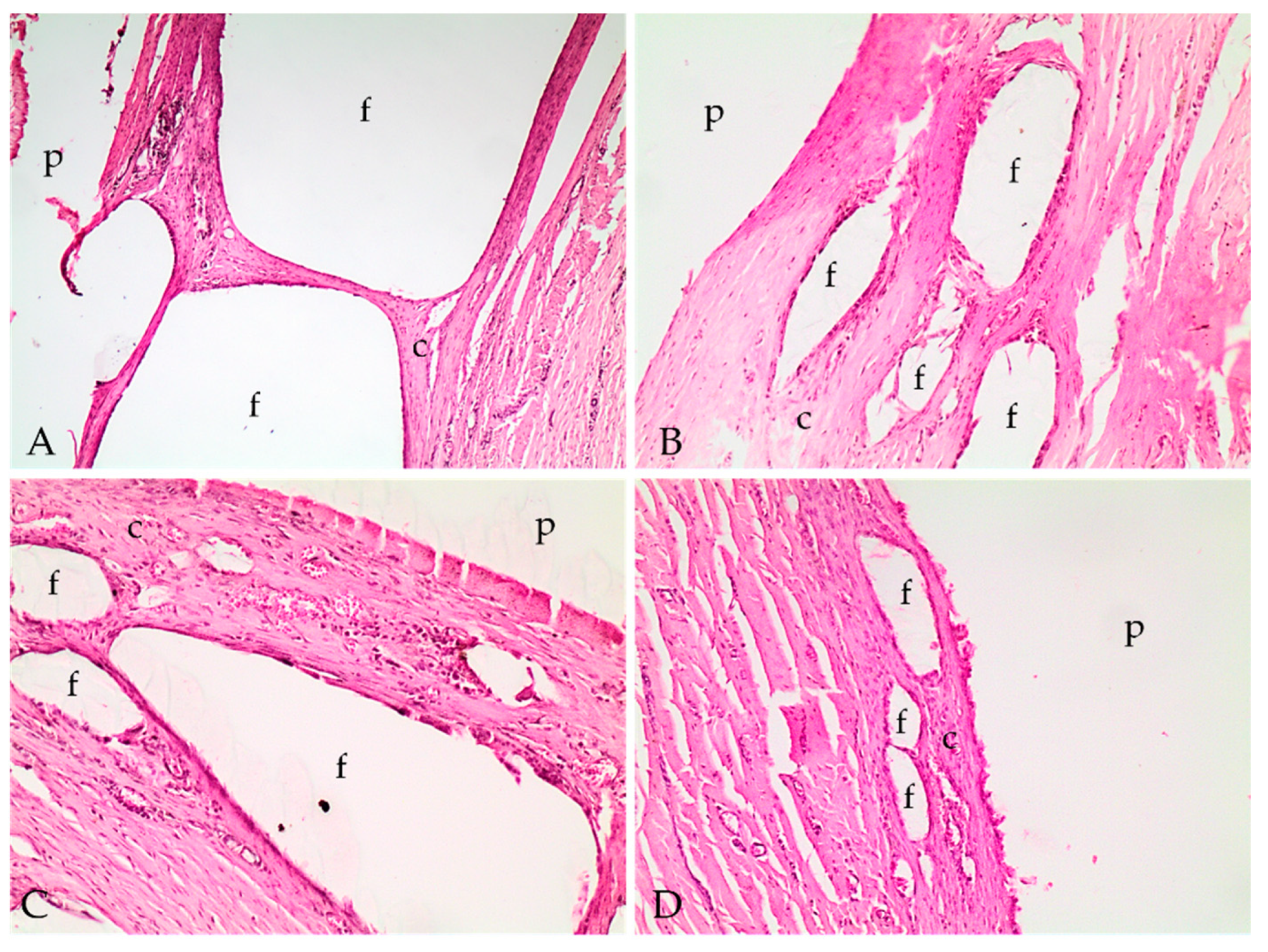
2.4. Histopathological Analysis
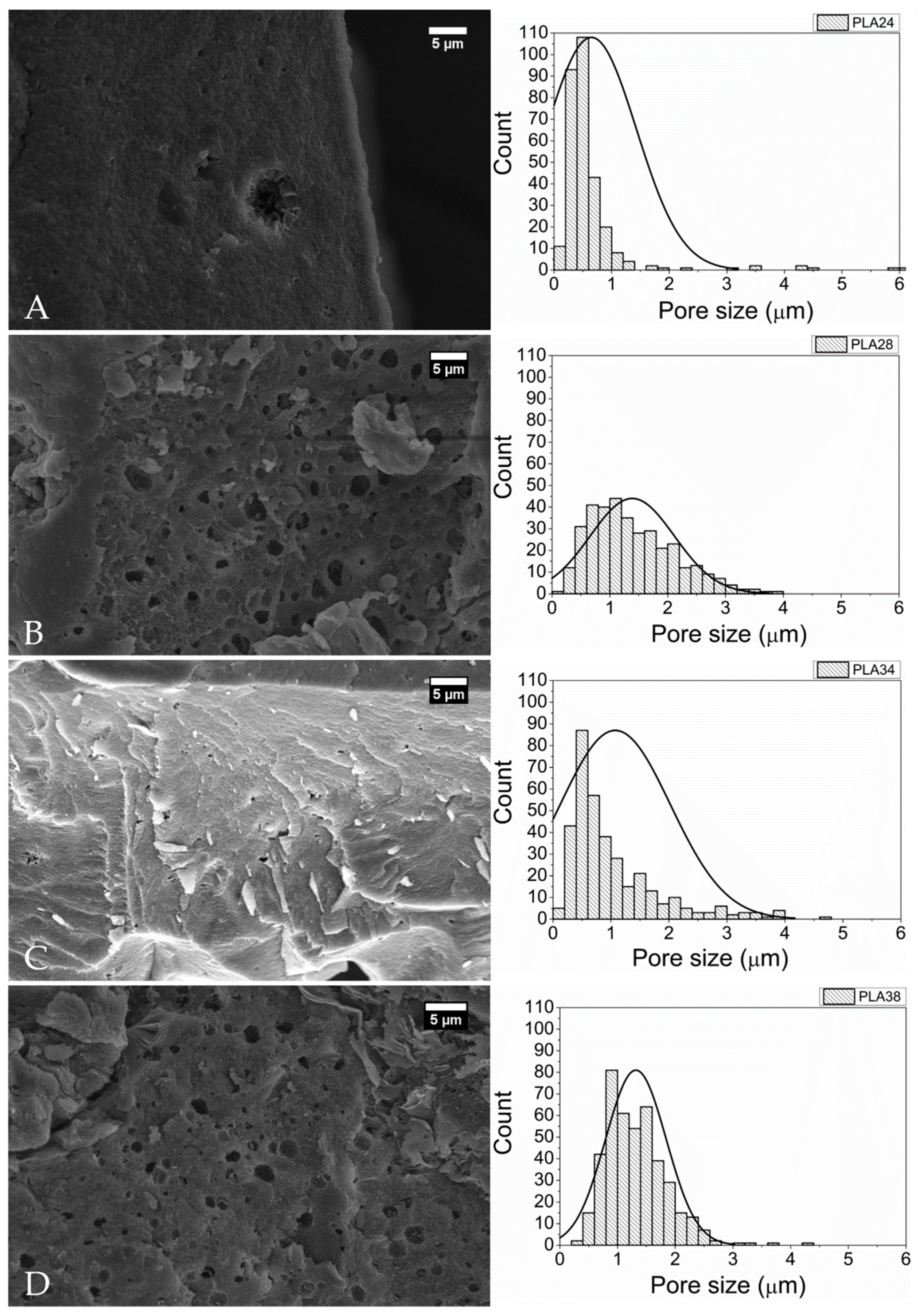
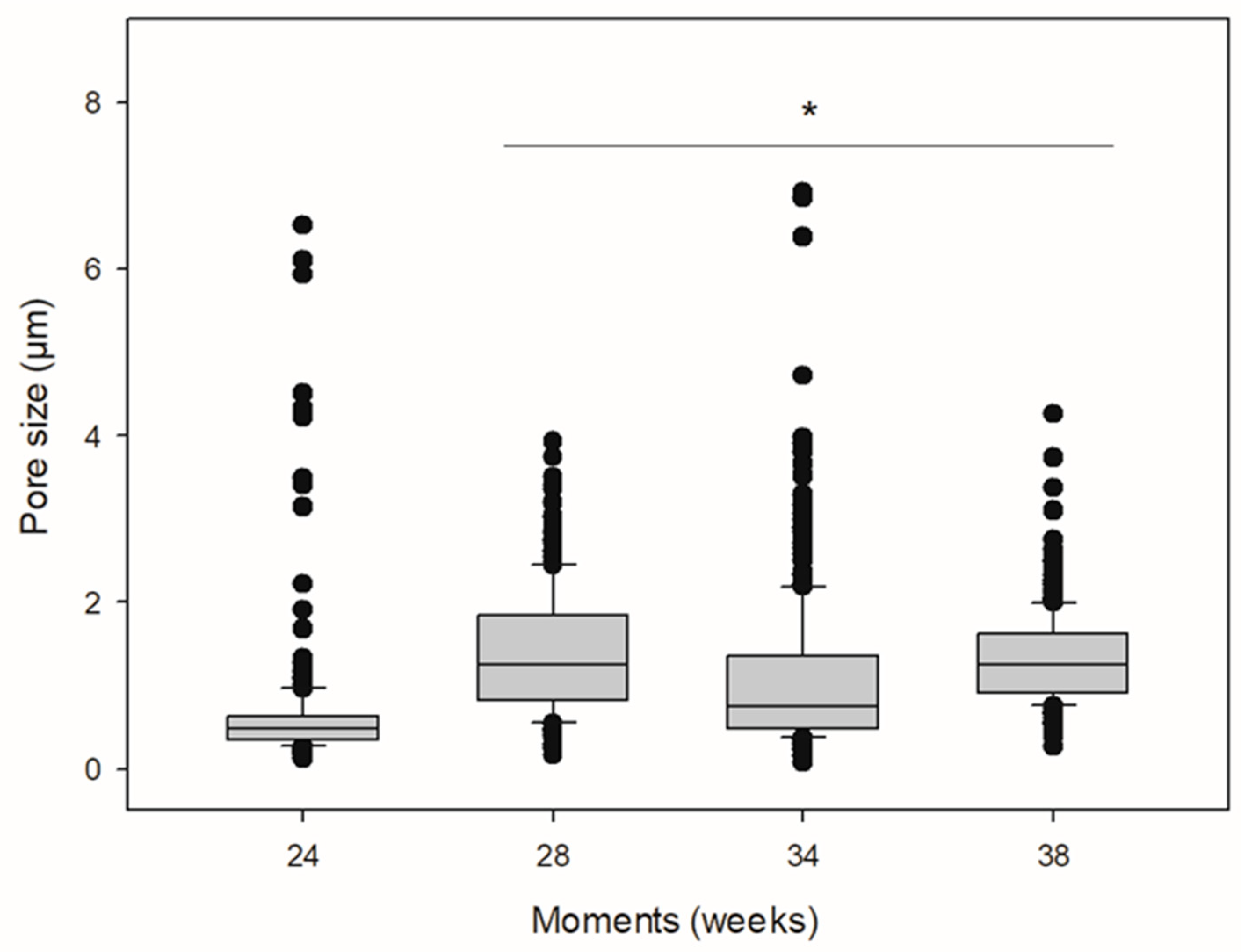
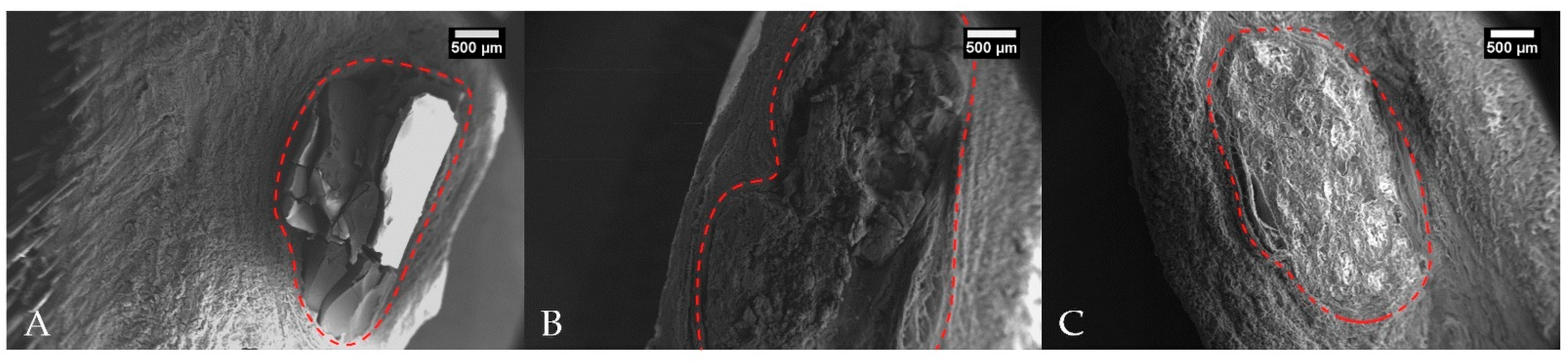
2.5. Scanning Electron Microscopy (SEM)
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Material Preparation
4.3. Animals
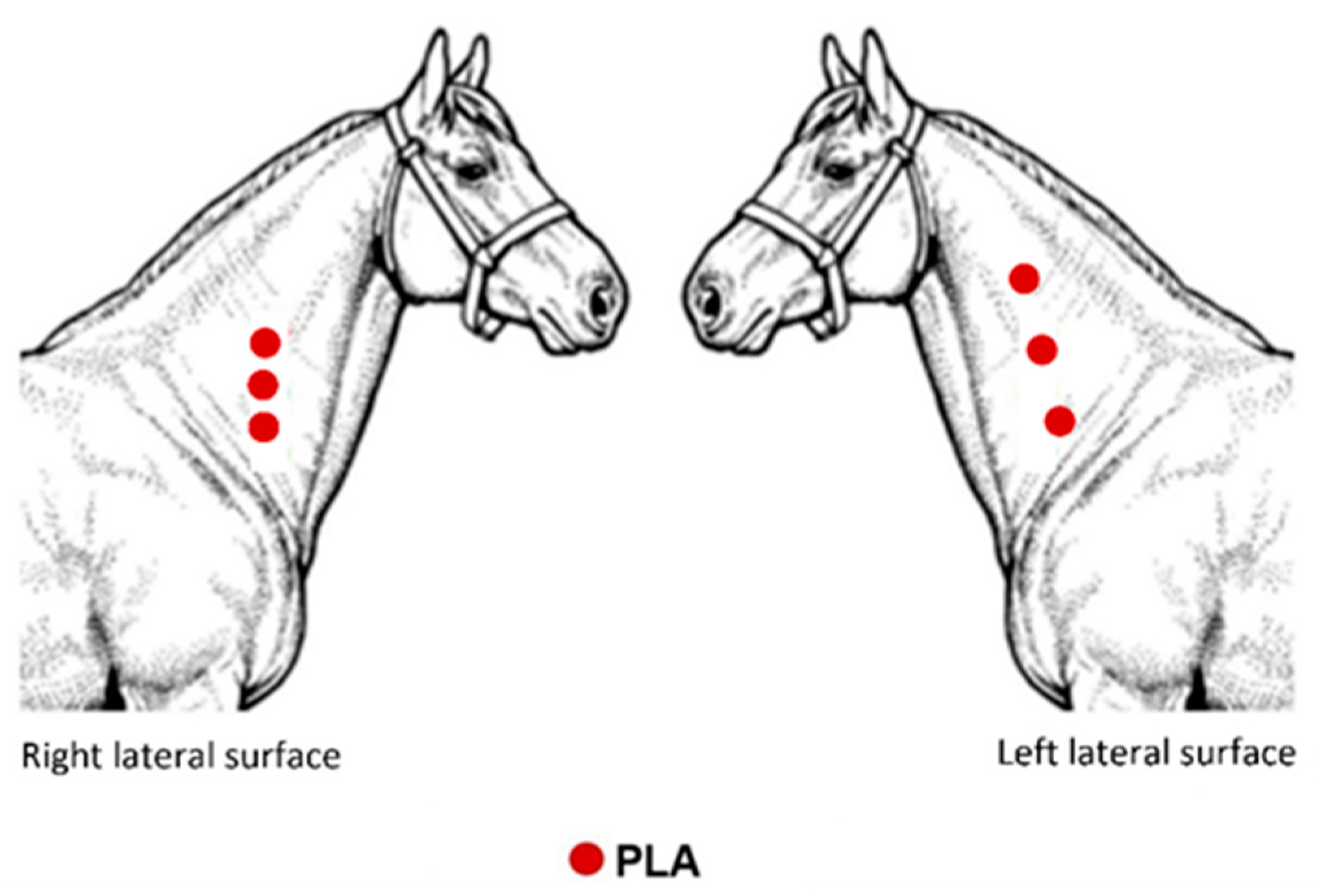
4.4. Polymers Implantation
4.5. Evaluation Methods
4.5.1. Clinical Evaluation
4.5.2. Plasma Fibrinogen (PF)
4.5.3. Mechanical Nociceptive Threshold (MNT)
4.5.4. Collection of Biopsy Specimens
4.5.5. Histopathological Analysis
4.5.6. Scanning Electron Microscopy (SEM)
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Maciel-Filho, R. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Abdeljawad, M.B.; Carette, X.; Argentati, C.; Martino, S.; Gonon, M.-F.; Odent, J.; Morena, F.; Mincheva, R.; Raquez, J.-M. Interfacial compatibilization into PLA/Mg composites for improved in vitro bioactivity and stem cell adhesion. Molecules 2021, 26, 5944. [Google Scholar] [CrossRef]
- Sharma, S.; Sudhakara, P.; Singh, J.; Ilyas, R.A.; Asyraf, M.R.M.; Razman, M.R. Critical review of biodegradable and bioactive polymer composites for bone tissue engineering and drug delivery applications. Polymers 2021, 13, 2623. [Google Scholar] [CrossRef] [PubMed]
- Saini, P.; Arora, M.; Ravi Kumar, M.N.V. Poly(lactic acid) blends in biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hu, H.; Wang, X.; Yu, X.; Zhou, W.; Peng, S. Super tough poly(lactic acid) blends: A comprehensive review. RSC Adv. 2020, 10, 13316. [Google Scholar] [CrossRef] [Green Version]
- Finotti, P.F.M.; Costa, L.C.; Capote, T.S.O.; Scarel-Caminaga, R.M.; Chinelatto, M.A. Immiscible poly(lactic acid)/poly(ε-caprolactone) for temporary implants: Compatibility and cytotoxicity. J. Mech. Behav. Biomed. Mater. 2017, 68, 155–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Xue, Y.; Yu, B.; Wang, L.; Zhou, C.; Ma, Y. A review of the recent developments in the bioproduction of polylactic acid and its precursors optically pure lactic acids. Molecules 2021, 26, 6446. [Google Scholar] [CrossRef] [PubMed]
- Ciambelli, G.S.; Perez, M.O.; Siqueira, G.V.; Candella, M.A.; Motta, A.C.; Duarte, M.A.T.; Alberto-Rincon, M.C.; Duek, E.A.R. Characterization of poly (L-co-D, L Lactic Acid) and a study of polymer-tissue interaction in subcutaneous implants in wistar rats. Mater. Res. 2013, 16, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Conde, G.; Carvalho, J.R.G.; Dias, P.P.; Moranza, H.G.; Montanhim, G.L.; Ribeiro, J.O.; Chinelatto, M.A.; Moraes, P.C.; Taboga, S.R.; Bertolo, P.H.L.; et al. In vivo biocompatibility and biodegradability of poly(lactic acid)/poly(ε-caprolactone) blend compatibilized with poly(ε-caprolactone-b-tetrahydrofuran) in Wistar rats. Biomed. Phys. Eng. Express 2021, 7, 035005. [Google Scholar] [CrossRef]
- Chvapil, M.; Pfister, T.; Escalada, S.; Ludwig, J.; Peacock, E.E. Dynamics of the healing of skin wounds in the horse as compared with the rat. Exp. Mol. Pathol. 1979, 30, 349–359. [Google Scholar] [CrossRef]
- Theoret, C. Physiology of wound healing. In Equine Wound Management, 3rd ed.; Theoret, C., Schumacher, J., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 1–13. [Google Scholar]
- FAO—Food and Agriculture Organization of the United Nations. 2019. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 23 August 2021).
- Golafshan, N.; Vorndran, E.; Zaharievski, S.; Brommer, H.; Kadumudi, F.B.; Dolatshahi-Pirouz, A.; Gbureck, W.; van Weeren, R.; Castilho, M.; Malda, J. Tough magnesium phosphate-based 3D-printed implants induce bone regeneration in an equine defect model. Biomaterials 2020, 261, 120302. [Google Scholar] [CrossRef]
- Pyles, M.D.; Alves, A.L.G.; Hussni, C.A.; Thomassian, A.; Nicoletti, J.L.M.; Watanabe, M.J. Parafusos biaobsorvíveis na reparação de fraturas experimentais de sesamóides proximais em equinos. Cienc. Rural 2007, 37, 1367–1373. [Google Scholar] [CrossRef] [Green Version]
- Moreira, R.C.; Graaf, G.M.M.V.D.; Pereira, C.A.; Zoppa, A.L.D.V.D. Mechanical evaluation of bone gap filled with rigid formulations castor oil polyurethane and chitosan in horses. Cienc. Rural 2016, 46, 2182–2188. [Google Scholar] [CrossRef] [Green Version]
- Nóbrega, F.S.; Selim, M.B.; Arana-Chavez, V.E.; Correa, L.; Ferreira, M.P.; Zoppa, A.L.V. Histologic and immunohistochemical evaluation of biocompatibility of castor oil polyurethane polymer with calcium carbonate in equine bone tissue. Am. J. Vet. Res. 2017, 78, 1210–1214. [Google Scholar] [CrossRef]
- Selim, M.B.; Nóbrega, F.S.; Facó, L.L.; Hagen, S.C.F.; Zoppa, A.L.V.; Arana-Chavez, V.E.; Corrêa, L. Histological and radiographic evaluation of equine bone structure after implantation of castor oil polymer. Vet. Comp. Orthop. Traumatol. 2018, 31, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Rumbaugh, M.L.; Burba, D.J.; Tetens, J.; Oliver, J.L.; Williams, J.; Hosgood, G.; LeBlanc, C.J. Effects of intra-articular injection of liquid silicone polymer in the equine middle carpal joint. In Proceedings of the 50th Annual Convention of the American Association of Equine Practitioners, Denver, CO, USA, 4–8 December 2004; American Association of Equine Practitioners: Lexington, KY, USA, 2004. [Google Scholar]
- Barnewitz, D.; Endres, M.; Krüger, I.; Becker, A.; Zimmermann, J.; Wilke, I.; Ringe, J.; Sittinger, M.; Kaps, C. Treatment of articular cartilage defects in horses with polymer-based cartilage tissue engineering grafts. Biomaterials 2006, 27, 2882–2889. [Google Scholar] [CrossRef] [PubMed]
- Albert, R.; Vásárhelyi, G.; Bodó, G.; Kenyeres, A.; Wolf, E.; Papp, T.; Terdik, T.; Módis, L.; Felszeghy, S. A computer-assisted microscopic analysis of bone tissue developed inside a polyactive polymer implanted into an equine articular surface. Histol. Histopathol. 2012, 27, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Petit, A.; Redout, E.M.; Van de Lest, C.H.; Grauw, J.C.; Müller, B.; Meyboom, R.; van Midwoud, P.; Vermonden, T.; Hennink, W.E.; van Weeren, P.R. Sustained intra-articular release of celecoxib from in situ forming gels made of acetyl-capped PCLA-PGE-PCLA triblock copolymers in horses. Biomaterials 2015, 53, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.R.G.; Conde, G.; Antonioli, M.L.; Dias, P.P.; Vasconcelos, R.O.; Taboga, S.R.; Canola, P.A.; Chinelatto, M.A.; Pereira, G.T.; Ferraz, G.C. Biocompatibility and biodegradation of poly (lactic acid) (PLA) and an immiscible PLA/poly (ε-caprolactone) (PCL) blend compatibilized by poly (ε-caprolactone-b-tetrahydrofuran) implanted in horses. Polym. J. 2020, 52, 629–643. [Google Scholar] [CrossRef]
- Byars, T.D.; Gonda, K.C. Equine history, physical examination, records, and recognizing abuse or neglect in patients. In Large Animal Internal Medicine, 5th ed.; Smith, B.P., Ed.; Elsevier: Riverport Lane, MO, USA, 2015; pp. 13–20. [Google Scholar]
- Kaneko, J.J.; Harvey, J.W.; Bruss, M.L. Clinical Biochemistry of Domestic Animals, 6th ed.; Elsevier: Oxford, UK, 2008; p. 884. [Google Scholar]
- Johns, J.L. Alterations in blood proteins. In Large Animal Internal Medicine, 5th ed.; Smith, B.P., Ed.; Elsevier: Riverport Lane, MO, USA, 2015; pp. 386–392. [Google Scholar]
- Gregory, N.S.; Harris, A.L.; Robinson, C.R.; Dougherty, P.M.; Fuchs, P.N.; Sluka, K.A. An overview of animal models of pain: Disease models and outcome measures. J. Pain 2013, 14, 1255–1269. [Google Scholar] [CrossRef]
- Rédua, M.A.; Valadão, C.A.A.; Duque, J.C.; Balestrero, L.T. The pre-emptive effect of epidural ketamine on wound sensitivity in horses tested by using Von Frey filaments. Vet. Anaesth. Analg. 2002, 29, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Kastellorizios, M.; Papadimitrakopoulos, F.; Burgess, D.J. Prevention of foreign body reaction in a pre-clinical large animal model. J. Control. Release 2015, 202, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Ringler, D.J. Inflamação e reparo. In Patologia Veterinária, 6th ed.; Jones, T.C., Hunt, R.D., King, N.W., Eds.; Editora Manole Ltda: Barueri, Brasil, 2000; pp. 119–166. [Google Scholar]
- De Jong, W.H.; Bergsma, J.E.; Robinson, J.E.; Bos, R.R.M. Tissue response to partially in vitro predegraded poly-L-lactide implants. Biomaterials 2005, 26, 1781–1791. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Morais, J.M.; Papadimitrakopoulos, F.; Burgess, D.J. Biomaterials/tissue interactions: Possible solutions to overcome foreign body response. AAPS J. 2010, 12, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. Sci. 2018, 340, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Zin, M.R.M.; Mahendrasingam, A.; Konkel, C.; Narayanan, T. Effect of D-isomer content on strain-induced crystallization behaviour of Poly(lactic acid) polymer under high speed uniaxial drawing. Polymer 2021, 216, 123422. [Google Scholar] [CrossRef]
- Tschakaloff, A.; Losken, H.W.; von Oepen, R.; Michaeli, W.; Moritz, O.; Mooney, M.P.; Losken, A. Degradation kinetics of biodegradable DL-polylactic acid biodegradable implants depending on the site of implantation. Int. J. Oral. Maxillofac. Surg. 1994, 23, 443–445. [Google Scholar] [CrossRef]
- Dias, P.P.; Chinelatto, M.A. Effect of poly(ε-caprolactone-b-tetrahydrofuran) triblock copolymer concentration on morphological, termal and mechanical properties of immiscible PLA/PCL blends. J. Renew. Mater. 2019, 7, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Clauss, A. Gerinnugsphysiologische schnellmethode zur bestimmung des fibrinogens. Acta. Haematol. 1957, 17, 237–246. [Google Scholar] [CrossRef]


| Polymer | Capsule Characterization | Infiltrate/ Inflammation | Cellular Growth | Phagocytosis | Angioplasia |
|---|---|---|---|---|---|
| PLA24 | 1 | 1 | 1 | 2 | 1 |
| PLA24F | 2 | 1 | 3 | 1 | 2 |
| PLA28 | 2 | 2 | 2 | 1 | 1 |
| PLA34 | 2 | 2 | 3 | 1 | 2 |
| PLA38 | 3 | 3 | 4 | 3 | 2 |
| PLA57 | Ns | Ns | Ns | Ns | Ns |
| Polymer | Pore Median Diameter (μm) (IQR) |
|---|---|
| PLA24 | 0.477 (0.354–0.624) |
| PLA28 | 1.251 (0.815–1.846) * |
| PLA34 | 0.751 (0.487–1.353) * |
| PLA38 | 1.254 (0.914–1.618) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, J.R.G.; Conde, G.; Antonioli, M.L.; Santana, C.H.; Littiere, T.O.; Dias, P.P.; Chinelatto, M.A.; Canola, P.A.; Zara, F.J.; Ferraz, G.C. Long-Term Evaluation of Poly(lactic acid) (PLA) Implants in a Horse: An Experimental Pilot Study. Molecules 2021, 26, 7224. https://doi.org/10.3390/molecules26237224
Carvalho JRG, Conde G, Antonioli ML, Santana CH, Littiere TO, Dias PP, Chinelatto MA, Canola PA, Zara FJ, Ferraz GC. Long-Term Evaluation of Poly(lactic acid) (PLA) Implants in a Horse: An Experimental Pilot Study. Molecules. 2021; 26(23):7224. https://doi.org/10.3390/molecules26237224
Chicago/Turabian StyleCarvalho, Júlia Ribeiro Garcia, Gabriel Conde, Marina Lansarini Antonioli, Clarissa Helena Santana, Thayssa Oliveira Littiere, Paula Patrocínio Dias, Marcelo Aparecido Chinelatto, Paulo Aléscio Canola, Fernando José. Zara, and Guilherme Camargo Ferraz. 2021. "Long-Term Evaluation of Poly(lactic acid) (PLA) Implants in a Horse: An Experimental Pilot Study" Molecules 26, no. 23: 7224. https://doi.org/10.3390/molecules26237224
APA StyleCarvalho, J. R. G., Conde, G., Antonioli, M. L., Santana, C. H., Littiere, T. O., Dias, P. P., Chinelatto, M. A., Canola, P. A., Zara, F. J., & Ferraz, G. C. (2021). Long-Term Evaluation of Poly(lactic acid) (PLA) Implants in a Horse: An Experimental Pilot Study. Molecules, 26(23), 7224. https://doi.org/10.3390/molecules26237224








