Utilization of Rosmarinic and Ascorbic Acids for Maturation Culture Media in Order to Increase Sow Oocyte Quality Prior to IVF
Abstract
1. Introduction
2. Materials and Methods
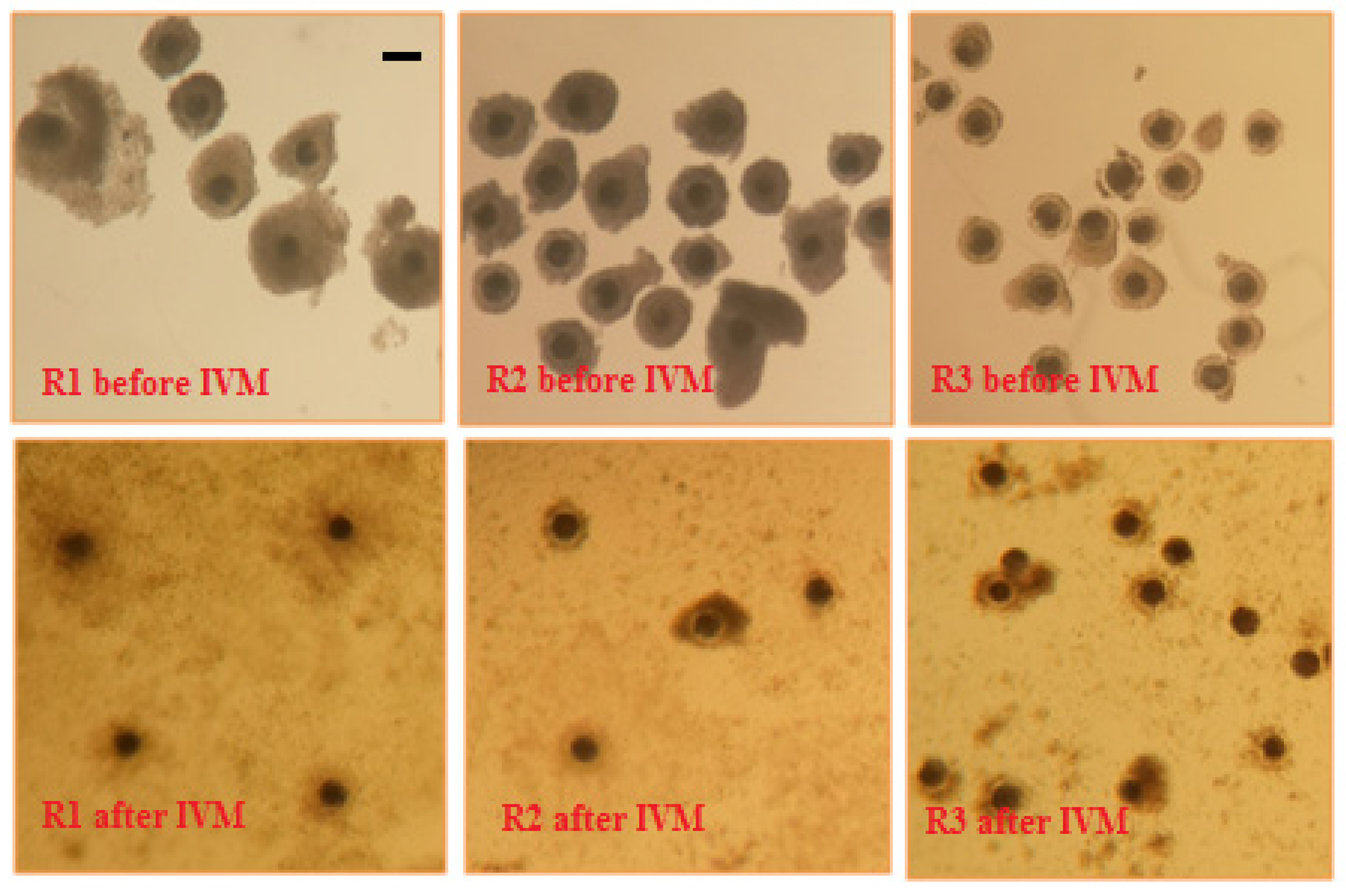
2.1. Collection of Sow Cumulus-Oocyte Complexes
2.2. In Vitro Maturation of Sow Cumulus-Oocyte Complexes
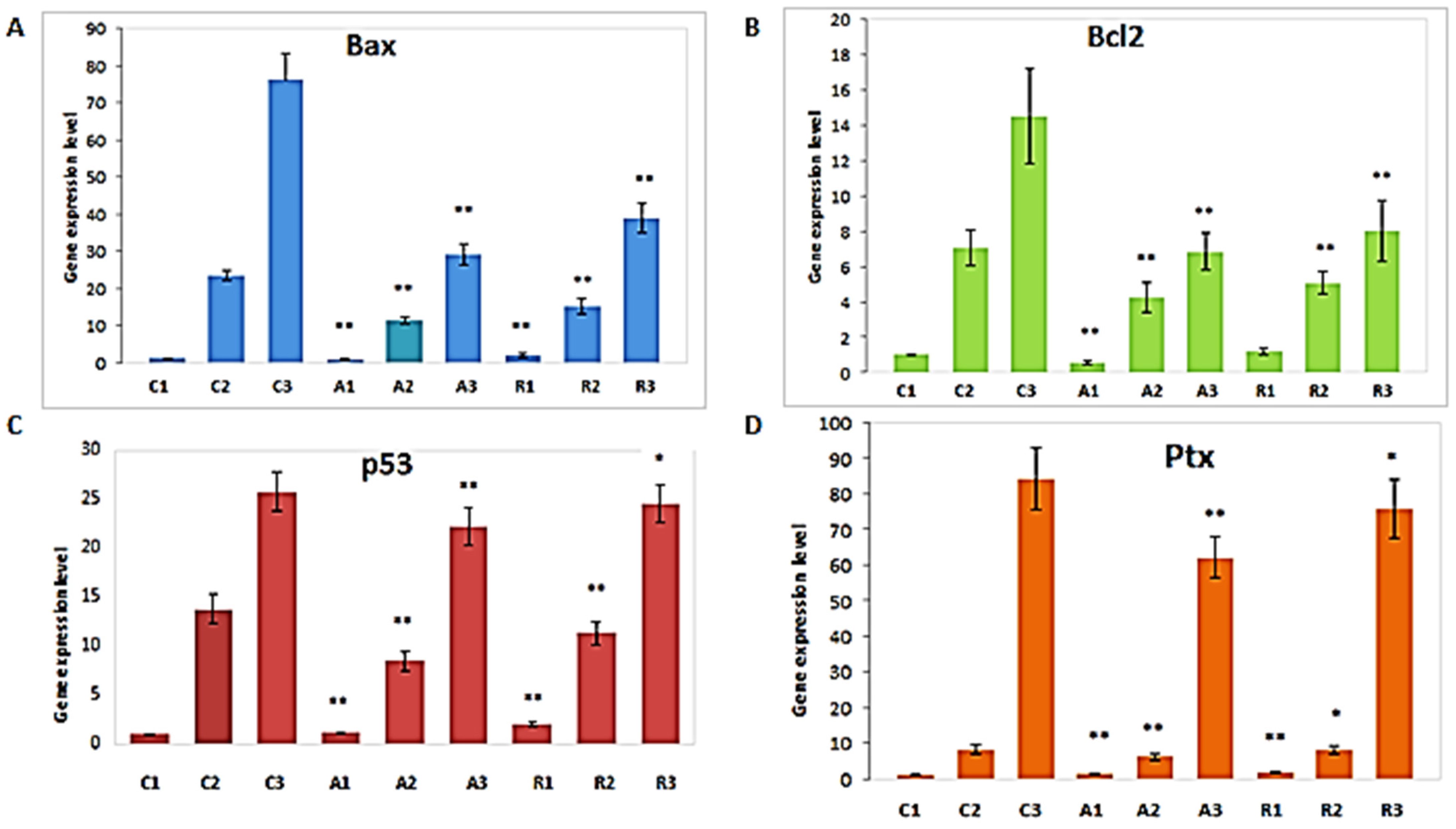
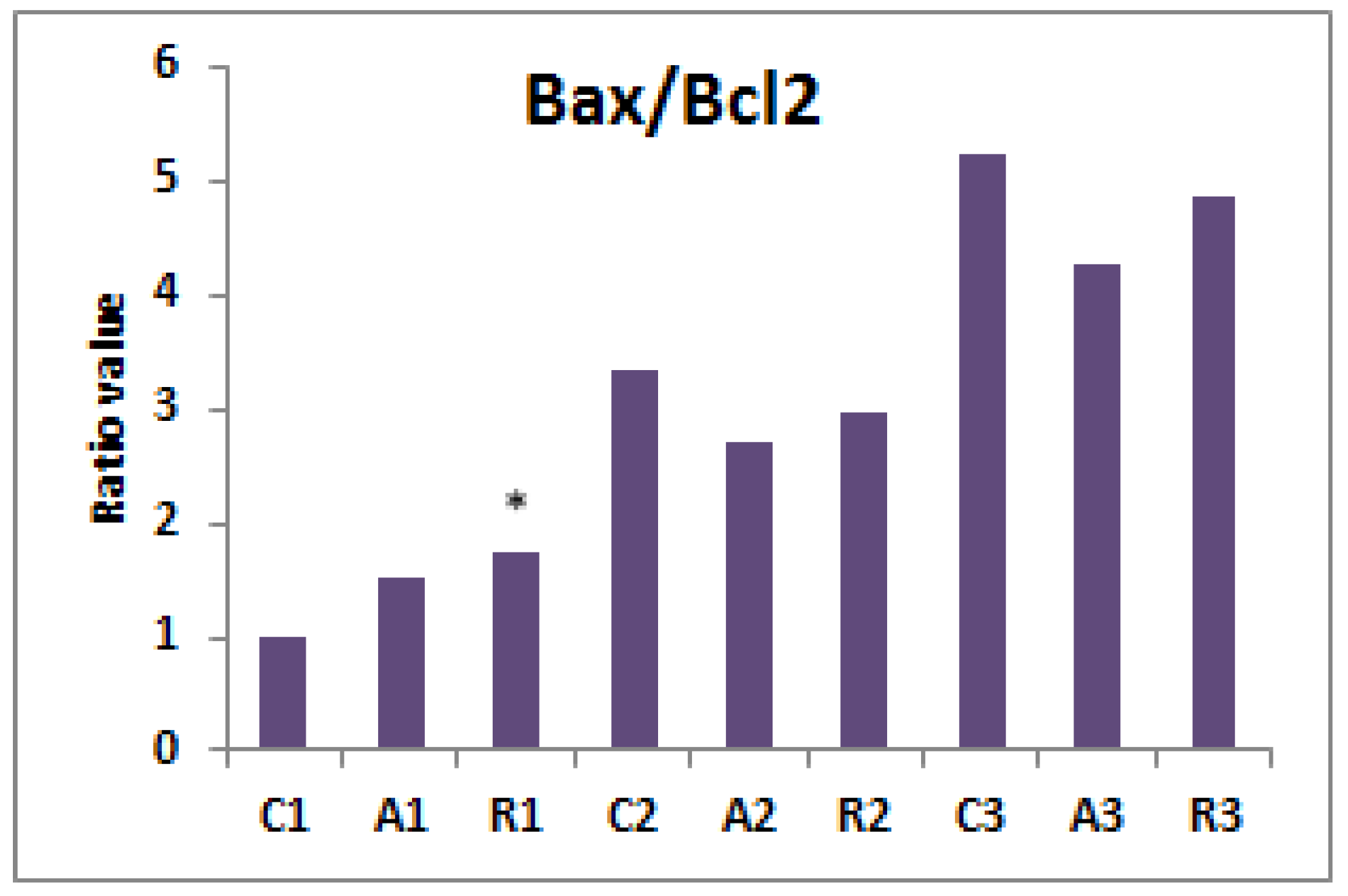
2.3. Molecular Analysis of In Vitro Maturated Sow Cumulus-Oocyte Complexes
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A


References
- Leeuw, A.V.W.-D. Ovum Pick Up and In Vitro Production in the bovine after use in several generations: A 2005 status. Theriogenology 2006, 65, 914–925. [Google Scholar] [CrossRef]
- Marc, S.; Cernescu, H.; Tulcan, C.; Hutu, I.; Boldura, O.; Otava, G.; Ratiu, A.; Keller, T.; Ungureanu, G.; Mircu, C. In vitro fertilization and related techniques. Rev. Rom. Med. Vet. 2017, 27, 17–22. [Google Scholar]
- Batista, R.I.T.P.; Moro, L.N.; Corbin, E.; Alminana, C.; Souza-Fabjan, J.M.G.; Freitas, V.J.D.F.; Mermillod, P. Combination of oviduct fluid and heparin to improve monospermic zygotes production during porcine in vitro fertilization. Theriogenology 2016, 86, 495–502. [Google Scholar] [CrossRef]
- Romar, R.; Funahashi, H.; Coy, P. In vitro fertilization in pigs: New molecules and protocols to consider in the forthcoming years. Theriogenology 2015, 85, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Zarcula, S.M.; Godja, G.; Huțu, I.; Tulcan, C.; Bonca, G.; Otavă, G.; Markovski, A. Comparison of morphological aspects and nuclear status of bovine COCs cultured in medium with/without sheep FSH. In Proceedings of the 3rd International Virtual Conference on Advanced Scientific Results, Virtual Meeting, 25–29 May 2015. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Agarwal, A.; Virk, G.; Cho, C.-L. Potential role of green tea catechins in the management of oxidative stress-associated infertility. Reprod. Biomed. Online 2017, 34, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Durairajanayagam, D.; Du Plessis, S.S. Utility of antioxidants during assisted reproductive techniques: An evidence based review. Reprod. Biol. Endocrinol. 2014, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-X.; Liu, Y.-H.; Liu, X.-M.; Wang, P.-C.; Liu, S.; Miao, J.-K.; Du, Z.-Q.; Yang, C.-X. Ascorbic acid induces global epigenetic reprogramming to promote meiotic maturation and developmental competence of porcine oocytes. Sci. Rep. 2018, 8, 6132. [Google Scholar] [CrossRef] [PubMed]
- Comizzoli, P.; Wildt, D.; Pukazhenthi, B. Overcoming poor in vitro nuclear maturation and developmental competence of domestic cat oocytes during the non-breeding season. Reproduction 2003, 126, 809–816. [Google Scholar] [CrossRef]
- El-Naby, A.-S.A.-H.H.; Mahmoud, K.M.; Sosa, G.A.; Abouel-Roos, M.E.; Ahmed, Y.F. Effect of using ascorbic acid and cysteamine supplementation on in-vitro development of buffalo embryos. Asian Pac. J. Reprod. 2017, 6, 85–88. [Google Scholar] [CrossRef]
- Sovernigo, T.C.; Adona, P.R.; Monzani, P.S.; Guemra, S.; Barros, F.; Lopes, F.G.; Leal, C. Effects of supplementation of medium with different antioxidants during in vitro maturation of bovine oocytes on subsequent embryo production. Reprod. Domest. Anim. 2017, 52, 561–569. [Google Scholar] [CrossRef]
- Pernes, A.J.; Miclea, I.; Zahan, M.; Orlovschi, D.; Codea, A.R. The influence of ascorbic acid on in vitro maturation of canine oocytes. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Anim. Sci. Biotechnol. 2016, 73, 230–234. [Google Scholar] [CrossRef][Green Version]
- Mittal, P.K.; Anand, M.; Madan, A.K.; Yadav, S.; Kumar, J. Antioxidative capacity of vitamin E, vitamin C and their combination in cryopreserved Bhadavari bull semen. Vet. World 2014, 7, 1127–1131. [Google Scholar] [CrossRef]
- Mangoli, E.; Talebi, A.R.; Anvari, M.; Taheri, F.; Vatanparast, M.; Rahiminia, T.; Hosseini, A. Vitamin C attenuates negative effects of vitrification on sperm parameters, chromatin quality, apoptosis and acrosome reaction in neat and prepared normozoospermic samples. Taiwan. J. Obstet. Gynecol. 2018, 57, 200–204. [Google Scholar] [CrossRef]
- Blaschke, K.; Ebata, K.T.; Karimi, M.M.; Zepeda-Martínez, J.A.; Goyal, P.; Mahapatra, S.; Tam, A.; Laird, D.J.; Hirst, M.; Rao, A.; et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature 2013, 500, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, H.; Liu, J.; Qi, J.; Wei, B.; Yang, J.; Liang, H.; Chen, Y.; Chen, J.; Wu, Y.; et al. H3K9 methylation is a barrier during somatic cell reprogramming into iPSCs. Nat. Genet. 2013, 45, 34–42. [Google Scholar] [CrossRef]
- Mingay, M.; Chaturvedi, A.; Bilenky, M.; Cao, Q.; Jackson, L.; Hui, T.; Moksa, M.; Heravi-Moussavi, A.; Humphries, R.K.; Heuser, M.; et al. Vitamin C-induced epigenomic remodelling in IDH1 mutant acute myeloid leukaemia. Leukemia 2018, 32, 11–20. [Google Scholar] [CrossRef]
- Zhao, M.; Hur, T.-Y.; No, J.; Nam, Y.; Kim, H.; Im, G.-S.; Lee, S. Ascorbic acid increases demethylation in somatic cell nuclear transfer embryos of the pig (Sus scrofa). Asian-Australas. J. Anim. Sci. 2017, 30, 944–949. [Google Scholar] [CrossRef]
- Mircu, C.; Boldura, O.; Tulcan, C.; Hutu, I. Evaluation of Bcl2 and Ptx3 genes expression in swine cumulus cell cultured in different media. In Proceedings of the 6th International Symposium on Animal Functional Genomics, Piacenza, Italy, 27–29 July 2015. [Google Scholar]
- Mircu, C.; Boldura, O.-M.; Ignatiadi, A.; Rațiu, A.M.; Hutu, I.; Sorina, P.; Tulcan, C.; Ahmadi, M.; Bonca, G.; Milovanov, C. Effect of Cysteine Supplementation on Sow Cumulus Cells and on Bcl2 Gene Expression during In Vitro Maturation. In Proceedings of the International Scientific Congress Soil and Food Resources for a Healthy Life—54th Annual meeting of Veterinary Medicine—“Towards a Global Health”, Faculty of Veterinary Medicine, Iasi, Romania, 22–23 October 2015; Series Veterinary Medicine. Volume 58, pp. 292–297. [Google Scholar] [CrossRef]
- Kim, G.-D.; Park, Y.S.; Jin, Y.-H.; Park, C.-S. Production and applications of rosmarinic acid and structurally related compounds. Appl. Microbiol. Biotechnol. 2015, 99, 2083–2092. [Google Scholar] [CrossRef]
- Pattananandecha, T.; Apichai, S.; Julsrigival, J.; Ungsurungsie, M.; Kawasaki, N.; Saenjum, C. Antioxidant Activity and Anti-Photoaging Effects on UVA-Irradiated Human Fibroblasts of Rosmarinic Acid Enriched Extract Prepared from Thunbergia laurifolia Leaves. Plants 2021, 10, 1648. [Google Scholar] [CrossRef]
- Touiss, I.; Ouahhoud, S.; Harnafi, M.; Khatib, S.; Bekkouch, O.; Amrani, S.; Harnafi, H. Toxicological Evaluation and Hepatoprotective Efficacy of Rosmarinic Acid-Rich Extract from Ocimum basilicum L. Evid.-Based Complement. Altern. Med. 2021, 2021, 6676998. [Google Scholar] [CrossRef] [PubMed]
- Marinho, S.; Illanes, M.; Ávila-Román, J.; Motilva, V.; Talero, E. Anti-Inflammatory Effects of Rosmarinic Acid-Loaded Nanovesicles in Acute Colitis through Modulation of NLRP3 Inflammasome. Biomolecules 2021, 11, 162. [Google Scholar] [CrossRef]
- Zaharescu, T.; Blanco, I. Stabilization Effects of Natural Compounds and Polyhedral Oligomeric Silsesquioxane Nanoparticles on the Accelerated Degradation of Ethylene-Propylene-Diene Monomer. Molecules 2021, 26, 4390. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Gupta, T.B.; Bronlund, J.; Kaur, L. The Potential of Rosemary as a Functional Ingredient for Meat Products—A Review. Food Rev. Int. 2021, 1–21. [Google Scholar] [CrossRef]
- Sierżant, K.; Korzeniowska, M.; Orda, J.; Wojdyło, A.; Gondret, F.; Półbrat, T. The Effect of Rosemary (Rosmarinus officinalis) and Blackcurrant Extracts (Ribes nigrum) Supplementation on Performance Indices and Oxidative Stability of Chicken Broiler Meat. Animals 2021, 11, 1155. [Google Scholar] [CrossRef] [PubMed]
- Olivares, A.; Alcaraz-Saura, M.; Achel, D.; Berná-Mestre, J.; Alcaraz, M. Radiation-Induced Bystander Effect: Loss of Radioprotective Capacity of Rosmarinic Acid In Vivo and In Vitro. Antioxidants 2021, 10, 231. [Google Scholar] [CrossRef]
- Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Anticancer potential of rosmarinic acid and its improved production through biotechnological interventions and functional genomics. Appl. Microbiol. Biotechnol. 2018, 102, 7775–7793. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, J.; Nie, X.W.; Li, Z.Y.; Wang, Y.M.; Liang, S.; Li, S. Rosmarinic acid treatment during porcine oocyte maturation attenuates oxidative stress and improves subsequent embryo development in vitro. PeerJ 2019, 7, e6930. [Google Scholar] [CrossRef]
- Olaciregui, M.; Luño, V.; Domingo, P.; González, N.; Gil, L. In vitro developmental ability of ovine oocytes following intracytoplasmic injection with freeze-dried spermatozoa. Sci. Rep. 2017, 7, 1096. [Google Scholar] [CrossRef]
- Luño, V.; Gil, L.; Olaciregui, M.; González, N.; Jerez, R.A.; de Blas, I. Rosmarinic acid improves function and in vitro fertilising ability of boar sperm after cryopreservation. Cryobiology 2014, 69, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.-J.; Wang, Y.-Y. Effects of rosmarinic acid on DNA integrity and H19 differentially methylated region methylation levels in human sperm preserved by freeze-drying. Reprod. Dev. Med. 2021, 5, 9–14. [Google Scholar] [CrossRef]
- Chakravarthi, V.P.; Kona, S.; Kumar, A.S.; Bhaskar, M.; Rao, V. Quantitative expression of antiapoptotic and proapoptotic genes in sheep ovarian follicles grown in vivo or cultured in vitro. Theriogenology 2015, 83, 590–595. [Google Scholar] [CrossRef]
- Rahim, B.; Jalal, S.; Yosef, N. Effect of cysteine supplementation on in vitro maturation of bovine oocyte. Afr. J. Biotechnol. 2011, 10, 15830–15833. [Google Scholar] [CrossRef]
- Hu, W.; Zheng, T.; Wang, J. Regulation of Fertility by the p53 Family Members. Genes Cancer 2011, 2, 420–430. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mulligan, B.; Hwang, J.-Y.; Kim, H.-M.; Oh, J.-N.; Choi, K.-H.; Lee, C.-K. Pro-apoptotic Effect of Pifithrin-α on Preimplantation Porcine In vitro Fertilized Embryo Development. Asian-Australas. J. Anim. Sci. 2012, 25, 1681–1690. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salustri, A.; Garlanda, C.; Hirsch, E.; De Acetis, M.; Maccagno, A.; Bottazzi, B.; Doni, A.; Bastone, A.; Mantovani, G.; Peccoz, P.B.; et al. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development 2004, 131, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Borjizadeh, A.; Ahmadi, H.; Daneshi, E.; Roshani, D.; Fathi, F.; Abdi, M.; Nasseri, S.; Abouzaripour, M. The effect of adding Rosmarinic and Ascorbic acids to vitrification media on fertilization rate of the mice oocyte: An experimental study. Int. J. Reprod. Biomed. 2019, 17, 195–200. [Google Scholar] [CrossRef]
- Jeon, R.-H.; Maeng, G.; Lee, W.; Kim, T.-H.; Lee, Y.-M.; Lee, J.-H.; Kumar, B.-M.; Lee, S.-L.; Rho, G.-J. Removal of cumulus cells before oocyte nuclear maturation enhances enucleation rates without affecting the developmental competence of porcine cloned embryos. Jpn. J. Vet. Res. 2012, 60, 191–203. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Mahanta, N.; Bhuyan, D.; Biswas, R.K.; Kumar, S.; Deka, B.C.; Dutta, D.J.; Das, A.; Dewry, R.K.; Mahanta, B.; Barman, D. In-vitro maturation of porcine oocyte: Effect of antioxidants. J. Cell Tissue Res. 2016, 16, 5685–5690. [Google Scholar]
- Sadeesh, E.M.; Shah, F.; Balhara, A.K.; Thirumaran, S.M.K.; Yadav, S.; Yadav, P.S. Effect of growth factor and antioxidant on in vitro maturation of oocytes and cleavage rates of in vitro produced Indian buffalo (Bubalus bubalis) embryos. Vet. Arh. 2014, 84, 459–474. [Google Scholar]
- Sohail, M.U.; Shahzad, A.H.; Iqbal, S.; Shabbir, M.Z.; Iqbal, Z.; Abbas, S.; Younus, M.; Nak, D.; Nak, Y.; Arshaad, T. Ascorbic acid inclusion in semen extender improves the post-thawed semen quality of Sahiwal cattle (Bos indicus). Pak. J. Zool. 2015, 47, 1571–1577. [Google Scholar]
- Tilly, J.L. Apoptosis and ovarian function. Rev. Reprod. 1996, 1, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.Y.; Kong, H.S.; Lee, S.S.; Choi, K.D.; Jeon, G.J.; Yang, B.K.; Lee, C.K.; Lee, H.K. Expression of the Antioxidant Enzyme and Apoptosis Genes in In Vitro Maturation/In Vitro Fertilization Porcine Embryos. Asian-Australas. J. Anim. Sci. 2004, 17, 33–38. [Google Scholar] [CrossRef]
- Hyttel, P.; Xu, K.P.; Smith, S.; Greve, T. Ultrastructure of in-vitro oocyte maturation in cattle. J. Reprod. Fertil. 1986, 78, 615–625. [Google Scholar] [CrossRef]
- Lonergan, P.; Fair, T.; Forde, N.; Rizos, D. Embryo development in dairy cattle. Theriogenology 2016, 86, 270–277. [Google Scholar] [CrossRef]
- Okabe, M. The cell biology of mammalian fertilization. Development 2013, 140, 4471–4479. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Brzezinska-Slebodzinska, E.; Madsen, F. Oxidative Stress, Antioxidants, and Animal Function. J. Dairy Sci. 1993, 76, 2812–2823. [Google Scholar] [CrossRef]
- Gupta, S.; Sekhon, L.; Kim, Y.; Agarwal, A. The Role of Oxidative Stress and Antioxidants in Assisted Reproduction. Curr. Women’s Health Rev. 2010, 6, 227–238. [Google Scholar] [CrossRef]
- Zhong, R.-Z.; Zhou, D.-W. Oxidative Stress and Role of Natural Plant Derived Antioxidants in Animal Reproduction. J. Integr. Agric. 2013, 12, 1826–1838. [Google Scholar] [CrossRef]
- Prasad, S.; Tiwari, M.; Pandey, A.N.; Shrivastav, T.G.; Chaube, S.K. Impact of stress on oocyte quality and reproductive outcome. J. Biomed. Sci. 2016, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Cho, H.-S.; Yu, I.-J. Effect of Bovine Follicular Fluid on Reactive Oxygen Species and Glutathione in Oocytes, Apoptosis and Apoptosis-Related Gene Expression of In Vitro-Produced Blastocysts. Reprod. Domest. Anim. 2014, 49, 370–377. [Google Scholar] [CrossRef]
- Hong, H.; Takahashi, K.; Ichisaka, T.; Aoi, T.; Kanagawa, O.; Nakagawa, M.; Okita, K.; Yamanaka, S. Suppression of induced pluripotent stem cell generation by the p53–p21 pathway. Nat. Cell Biol. 2009, 460, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Xie, B.; Li, J.; Huan, Y.; Huang, T.; Wei, R.; Lv, J.; Liu, S.; Liu, Z. Identification and Characterization of an Oocyte Factor Required for Porcine Nuclear Reprogramming. J. Biol. Chem. 2014, 289, 6960–6968. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.; Nagy, R.; El-Asheeri, A.K.; Eid, L.N. Developmental and molecular responses of buffalo (Bubalus bubalis) cumulus–oocyte complex maturedin vitrounder heat shock conditions. Zygote 2018, 26, 177–190. [Google Scholar] [CrossRef]
- Camaioni, A.; Klinger, F.G.; Campagnolo, L.; Salustri, A. The Influence of Pentraxin 3 on the Ovarian Function and Its Impact on Fertility. Front. Immunol. 2018, 9, 2808. [Google Scholar] [CrossRef] [PubMed]
- Magrini, E.; Mantovani, A.; Garlanda, C. The Dual Complexity of PTX3 in Health and Disease: A Balancing Act? Trends Mol. Med. 2016, 22, 497–510. [Google Scholar] [CrossRef]
- Huang, X.; Hao, C.; Shen, X.; Zhang, Y.; Liu, X. RUNX2, GPX3 and PTX3 gene expression profiling in cumulus cells are reflective oocyte/embryo competence and potentially reliable predictors of embryo developmental competence in PCOS patients. Reprod. Biol. Endocrinol. 2013, 11, 109. [Google Scholar] [CrossRef]
- Huang, Z.; Pang, Y.; Hao, H.; Du, W.; Zhao, X.; Zhu, H. Effects of epigallocatechin-3-gallate on bovine oocytes matured in vitro. Asian-Australas. J. Anim. Sci. 2018, 31, 1420–1430. [Google Scholar] [CrossRef]
- Tian, X.; Wang, F.; He, C.; Zhang, L.; Tan, D.; Reiter, R.J.; Xu, J.; Ji, P.; Liu, G. Beneficial effects of melatonin on bovine oocytes maturation: A mechanistic approach. J. Pineal Res. 2014, 57, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Park, S.-Y.; Kim, J.-W.; Yang, S.-G.; Kim, M.-J.; Jegal, H.-G.; Kim, I.-S.; Choo, Y.-K.; Koo, D.-B. Melatonin improves oocyte maturation and mitochondrial functions by reducing bisphenol a-derived superoxide in porcine oocytes in vitro. Int. J. Mol. Sci. 2018, 19, 3422. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boldura, O.-M.; Marc, S.; Otava, G.; Hutu, I.; Balta, C.; Tulcan, C.; Mircu, C. Utilization of Rosmarinic and Ascorbic Acids for Maturation Culture Media in Order to Increase Sow Oocyte Quality Prior to IVF. Molecules 2021, 26, 7215. https://doi.org/10.3390/molecules26237215
Boldura O-M, Marc S, Otava G, Hutu I, Balta C, Tulcan C, Mircu C. Utilization of Rosmarinic and Ascorbic Acids for Maturation Culture Media in Order to Increase Sow Oocyte Quality Prior to IVF. Molecules. 2021; 26(23):7215. https://doi.org/10.3390/molecules26237215
Chicago/Turabian StyleBoldura, Oana-Maria, Simona Marc, Gabriel Otava, Ioan Hutu, Cornel Balta, Camelia Tulcan, and Calin Mircu. 2021. "Utilization of Rosmarinic and Ascorbic Acids for Maturation Culture Media in Order to Increase Sow Oocyte Quality Prior to IVF" Molecules 26, no. 23: 7215. https://doi.org/10.3390/molecules26237215
APA StyleBoldura, O.-M., Marc, S., Otava, G., Hutu, I., Balta, C., Tulcan, C., & Mircu, C. (2021). Utilization of Rosmarinic and Ascorbic Acids for Maturation Culture Media in Order to Increase Sow Oocyte Quality Prior to IVF. Molecules, 26(23), 7215. https://doi.org/10.3390/molecules26237215








