Study of Integer Spin S = 1 in the Polar Magnet β-Ni(IO3)2
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
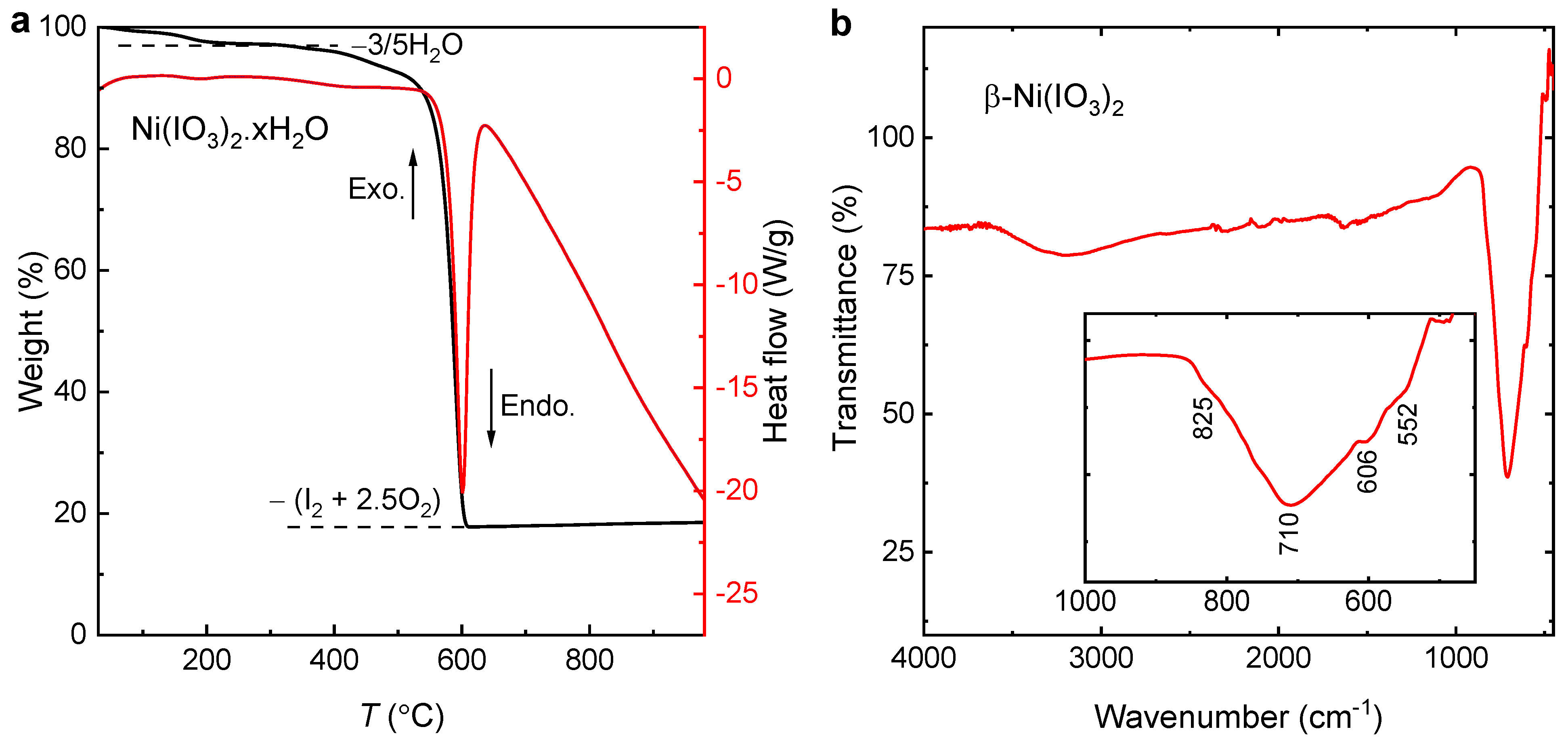
3.1. Crystal Structure
3.2. UV–Vis Spectra
3.3. Magnetization
3.4. Heat Capacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Spaldin, N.A.; Ramesh, R. Advances in magnetoelectric multiferroics. Nat. Mater. 2019, 18, 203–212. [Google Scholar] [CrossRef]
- Seki, S.; Yu, X.Z.; Ishiwata, S.; Tokura, Y. Observation of skyrmions in a multiferroic material. Science 2012, 336, 198. [Google Scholar] [CrossRef] [Green Version]
- Kanazawa, N.; Seki, S.; Tokura, Y. Noncentrosymmetric magnets hosting magnetic skyrmions. Adv. Mater. 2017, 29, 1603227. [Google Scholar] [CrossRef]
- Ruff, E.; Widmann, S.; Lunkenheimer, P.; Tsurkan, V.; Bordacs, S.; Kezsmarki, I.; Loidl, A. Multiferroicity and skyrmions carrying electric polarization in GaV4S8. Sci. Adv. 2015, 1, e1500916. [Google Scholar] [CrossRef] [Green Version]
- Araki, Y.; Sato, T.; Fujima, Y.; Abe, N.; Tokunaga, M.; Kimura, S.; Morikawa, D.; Ukleev, V.; Yamasaki, Y.; Tabata, C.; et al. Metamagnetic transitions and magnetoelectric responses in the chiral polar helimagnet Ni2InSbO6. Phys. Rev. B 2020, 102, 054409. [Google Scholar] [CrossRef]
- Oyeka, E.E.; Winiarski, M.J.; Sorolla, M., II; Taddei, K.M.; Scheie, A.; Tran, T.T. Spin and orbital effects on asymmetric exchange interaction in polar magnets: M(IO3)2 (M = Cu and Mn). Inorg. Chem. 2021, 60, 16544–16557. [Google Scholar] [CrossRef]
- Qian, Z.; Wu, H.; Yu, H.; Hu, Z.; Wang, J.; Wu, Y. Synthesis, structure, and characterization of d0 transition-metal iodate: BaTi(IO3)6·0.5H2O. Inorg. Chem. 2020, 59, 15430–15437. [Google Scholar] [CrossRef]
- Luo, M.; Liang, F.; Hao, X.; Lin, D.; Li, B.; Lin, Z.; Ye, N. Rational design of the nonlinear optical response in a tin iodate fluoride Sn(IO3)2F2. Chem. Mater. 2020, 32, 2615–2620. [Google Scholar] [CrossRef]
- Nguyen, S.D.; Halasyamani, P.S. Synthesis, structure, and characterization of new Li+-d0-lone-Pair-oxides: Noncentrosymmetric polar Li6(Mo2O5)3(SeO3)6 and Centrosymmetric Li2(MO3)(TeO3) (M = Mo6+ or W6+). Inorg. Chem. 2012, 51, 9529–9538. [Google Scholar] [CrossRef] [PubMed]
- Donakowski, M.D.; Gautier, R.; Yeon, J.; Moore, D.T.; Nino, J.C.; Halasyamani, P.S.; Poeppelmeier, K.R. The role of polar, lamdba (Λ)-shaped building units in noncentrosymmetric inorganic structures. J. Am. Chem. Soc. 2012, 134, 7679–7689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-H.; Yeon, J.; Halasyamani, P.S. Noncentrosymmetric polar oxide material, Pb3SeO5: Synthesis, characterization, electronic structure calculations, and structure-property relationships. Chem. Mater. 2009, 21, 5335–5342. [Google Scholar] [CrossRef]
- Nguyen, S.D.; Yeon, J.; Kim, S.-H.; Halasyamani, P.S. BiO(IO3): A new polar iodate that exhibits an aurivillius-type (Bi2O2)2+ layer and a large SHG response. J. Am. Chem. Soc. 2011, 133, 12422–12425. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-Y.; Kim, S.-H.; Halasyamani, P.S.; Ok, K.M. Alignment of lone pairs in a new polar material: Synthesis, characterization, and functional properties of Li2Ti(IO3)6. J. Am. Chem. Soc. 2009, 131, 2426–2427. [Google Scholar] [CrossRef]
- Ok, K.M. Toward the rational design of novel noncentrosymmetric materials: Factors influencing the framework structures. Acc. Chem. Res. 2016, 49, 2774–2785. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, C.-L.; Zhang, X.-H.; Li, B.-X.; Yang, B.-P.; Mao, J.-G. CsVO2F(IO3): An excellent SHG material faturing an unprecedented 3D [VO2F(IO3)]− anionic framework. Angew. Chem. Int. Ed. 2020, 59, 5381–5384. [Google Scholar] [CrossRef]
- Oyeka, E.E.; Winiarski, M.J.; Błachowski, A.; Taddei, K.M.; Scheie, A.; Tran, T.T. Potential skyrmion host Fe(IO3)3: Connecting stereoactive lone-pair electron effects to the Dzyaloshinskii-Moriya interaction. Chem. Mater. 2021, 33, 4661–4671. [Google Scholar] [CrossRef]
- Kurumaji, T.; Nakajima, T.; Ukleev, V.; Feoktystov, A.; Arima, T.H.; Kakurai, K.; Tokura, Y. Neel-type skyrmion lattice in the tetragonal polar magnet VOSe2O5. Phys. Rev. Lett. 2017, 119, 237201. [Google Scholar] [CrossRef] [Green Version]
- Buyers, W.J.L.; Morra, R.M.; Armstrong, R.L.; Hogan, M.J.; Gerlach, P.; Hirakawa, K. Experimental evidence for the Haldane gap in a spin-1 nearly isotropic, antiferromagnetic chain. Phys. Rev. Lett. 1986, 56, 371–374. [Google Scholar] [CrossRef]
- Cubitt, T.S.; Perez-Garcia, D.; Wolf, M.M. Undecidability of the spectral gap. Nature 2015, 528, 207–211. [Google Scholar] [CrossRef] [Green Version]
- Kelly, Z.A.; Tran, T.T.; McQueen, T.M. Nonpolar-to-polar trimerization transitions in the S = 1 Kagomé magnet Na2Ti3Cl8. Inorg. Chem. 2019, 58, 11941–11948. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.K.; Lake, B.; Islam, A.T.M.N.; Klemke, B.; Faulhaber, E.; Law, J.M. Field-induced magnetic ordering and single-ion anisotropy in the quasi-one-dimensional Haldane chain compound SrNi2V2O8: A single-crystal investigation. Phys. Rev. B 2013, 87, 224423. [Google Scholar] [CrossRef] [Green Version]
- Phanon, D.; Bentria, B.; Jeanneau, E.; Benbertal, D.; Mosset, A.; Gautier-Luneau, I. Crystal structure of M(IO3)2 metal iodates, twinned by pseudo-merohedry, with MII: MgII, MnII, CoII, NiII and ZnII. Z. Kristallogr. Cryst. Mater. 2006, 221, 635–642. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Pracht, G.; Lange, N.; Lutz, H.D. High-temperature Raman spectroscopic studies on nickel iodates. Thermochim. Acta 1997, 293, 13–24. [Google Scholar] [CrossRef]
- Ideue, T.; Kurumaji, T.; Ishiwata, S.; Tokura, Y. Giant thermal Hall effect in multiferroics. Nat. Mater. 2017, 16, 797–802. [Google Scholar] [CrossRef]
- Peng, L.; Takagi, R.; Koshibae, W.; Shibata, K.; Nakajima, K.; Arima, T.-h.; Nagaosa, N.; Seki, S.; Yu, X.; Tokura, Y. Controlled transformation of skyrmions and antiskyrmions in a non-centrosymmetric magnet. Nat. Nanotechnol. 2020, 15, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Halder, M.; Chacon, A.; Bauer, A.; Simeth, W.; Mühlbauer, S.; Berger, H.; Heinen, L.; Garst, M.; Rosch, A.; Pfleiderer, C. Thermodynamic evidence of a second skyrmion lattice phase and tilted conical phase in Cu2OSeO3. Phys. Rev. B 2018, 98, 144429. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Bernstein, J.L.; Elemans, J.B.A.A.; Verschoor, G.C. Paramagnetic Ni(IO3)2·2H2O. Crystal structure of the transition-metal iodates. I. J. Chem. Phys. 1973, 59, 2007–2018. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV-Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- Winiarski, M.J.; Tran, T.T.; Chamorro, J.R.; McQueen, T.M. (CsX)Cu5O2(PO4)2 (X = Cl, Br, I): A family of Cu2+ S = 1/2 compounds with capped-Kagome networks composed of OCu4 units. Inorg. Chem. 2019, 58, 4328–4336. [Google Scholar] [CrossRef]
- Fritsch, V.; Moreno, N.O.; Thompson, J.D.; Sarrao, J.L.; Bobev, S. Antiferromagnetic order and evolution of magnetic entropy in RE4Zn5Ge6 (RE = Y, Gd-Lu). J. Magn. Magn. Mater. 2005, 299, 87–93. [Google Scholar] [CrossRef]
- Fita, I.; Wisniewski, A.; Puzniak, R.; Iwanowski, P.; Markovich, V.; Kolesnik, S.; Dabrowski, B. Competing exchange bias and field-induced ferromagnetism in La-doped BaFeO3. Phys. Rev. B 2017, 95, 134428. [Google Scholar] [CrossRef]






| Refinement Parameter | Crystallographic Data |
|---|---|
| Chemical formula | Ni(IO3)2 |
| Color | Yellow |
| Shape | Block |
| Size (mm × mm × mm) | 0.02 × 0.02 × 0.02 |
| Formula weight (g/mol) | 408.51 |
| Temperature (K) | 300 |
| X-ray radiation | Mo Kα |
| Wavelength (λ, Å) | 0.71073 |
| Crystal system | Monoclinic |
| Space group | P21 |
| Z | 4 |
| a (Å) | 10.8067(4) |
| b (Å) | 5.1190(2) |
| c (Å) | 10.8151(4) |
| α = γ (°) | 90 |
| β (°) | 119.7950(10) |
| V (Å3) | 519.20(3) |
| ρcalc (g/cm3) | 5.226 |
| No. of reflections collected | 7093 |
| μ (mm−1) | 15.582 |
| 2θ (°) | 55 |
| GOF | 1.141 |
| Flack | 0.07(5) |
| R(F) a | 0.051 |
| Rw(Fo) b | 0.1046 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyeka, E.E.; Winiarski, M.J.; Tran, T.T. Study of Integer Spin S = 1 in the Polar Magnet β-Ni(IO3)2. Molecules 2021, 26, 7210. https://doi.org/10.3390/molecules26237210
Oyeka EE, Winiarski MJ, Tran TT. Study of Integer Spin S = 1 in the Polar Magnet β-Ni(IO3)2. Molecules. 2021; 26(23):7210. https://doi.org/10.3390/molecules26237210
Chicago/Turabian StyleOyeka, Ebube E., Michał J. Winiarski, and Thao T. Tran. 2021. "Study of Integer Spin S = 1 in the Polar Magnet β-Ni(IO3)2" Molecules 26, no. 23: 7210. https://doi.org/10.3390/molecules26237210
APA StyleOyeka, E. E., Winiarski, M. J., & Tran, T. T. (2021). Study of Integer Spin S = 1 in the Polar Magnet β-Ni(IO3)2. Molecules, 26(23), 7210. https://doi.org/10.3390/molecules26237210






