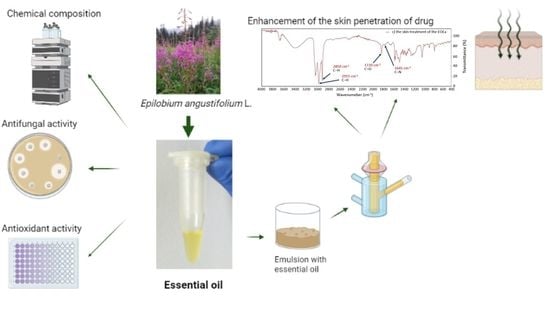
Epilobium angustifolium L. Essential Oil—Biological Activity and Enhancement of the Skin Penetration of Drugs—In Vitro Study
Abstract
:1. Introduction
2. Results
2.1. Essential Oil with E. angustifolium L. (EOEa)
2.2. Antioxidant Activity
2.3. Antifungal Activity
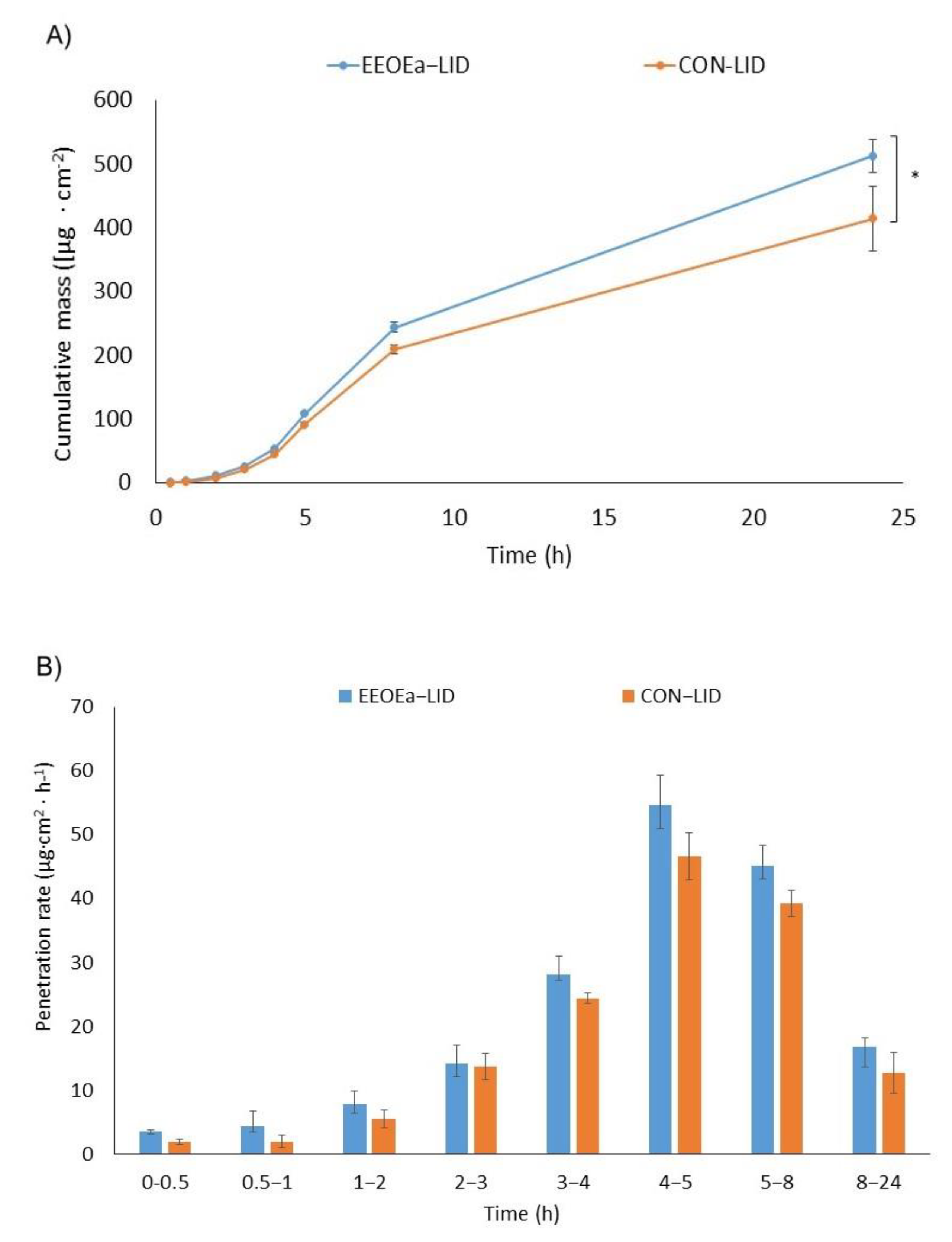
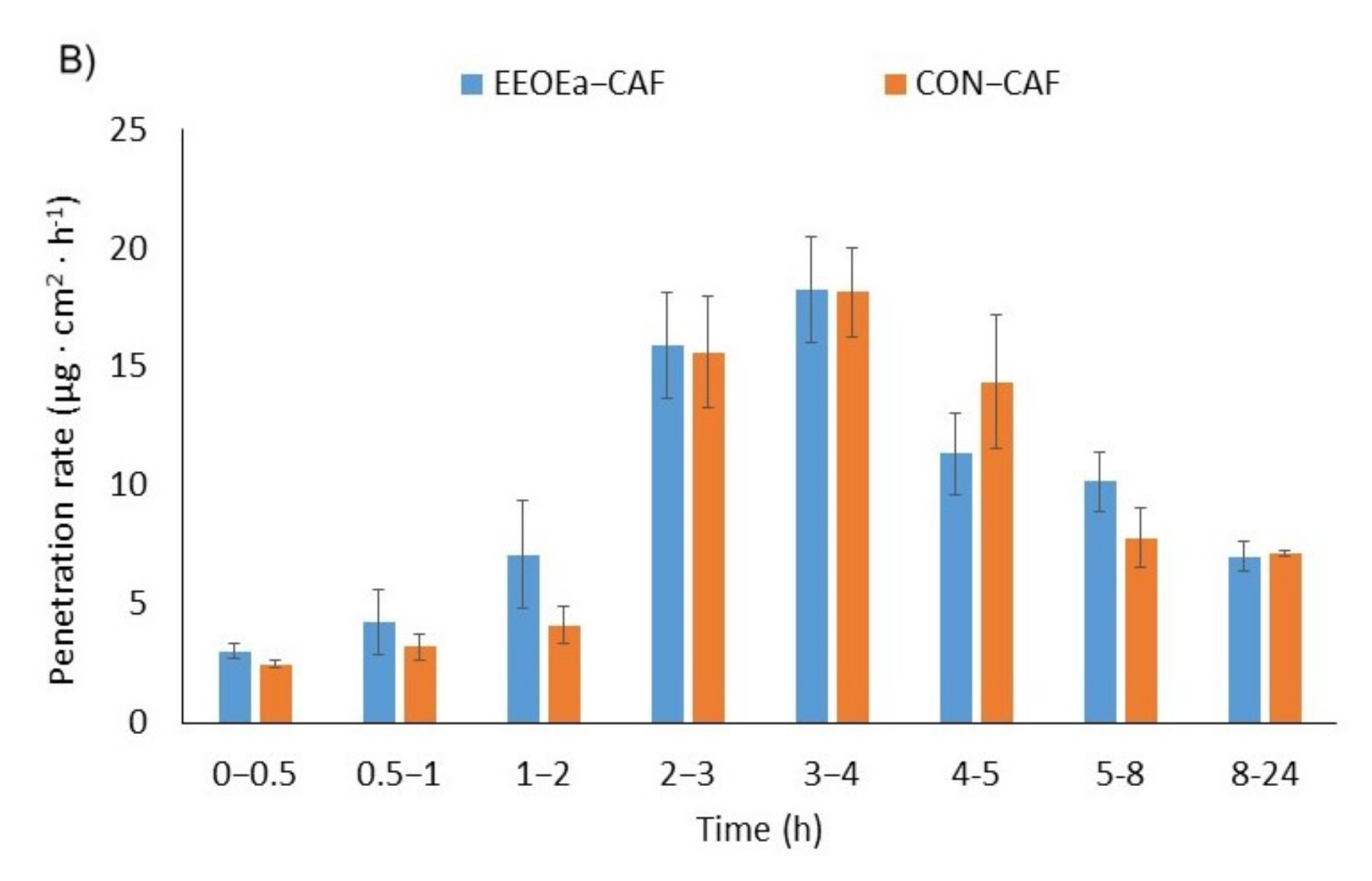
2.4. In Vitro Skin Penetration Studies
2.5. The Effect of EOEa on the Penetration of Studied Compounds
2.5.1. GC-MS of Skin and Acceptor Fluid after 24-h Penetration
2.5.2. The FTIR Spectra of Skin before and after EOEa Application
3. Discussion
3.1. Isolation and Composition of EOEa
3.2. Antioxidant Activity of EOEa
3.3. Antifungal Activity of EOEa
3.4. Skin Penetration
3.4.1. The Penetration of Active Compounds of EOEa and Their Accumulation in the Skin—GC-MS Analysis
3.4.2. FTIR of Skins after EOEa Application on the Skin
4. Materials and Methods
4.1. Chemicals
4.2. Plant Materials
4.3. Distillation of Essential Oil
4.4. Antioxidant Activity EOEa
4.5. Antifungal Activity
4.6. In Vitro Skin Permeation Studies
4.7. Preparation of the Emulsion
4.8. Stability of Preparations
4.9. GC-MS Analysis
4.10. FTIR-ATR Analysis
4.11. HPLC Analyses
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dacrema, M.; Sommella, E.; Santarcangelo, C.; Bruno, B.; Marano, M.G.; Insolia, V.; Saviano, A.; Campiglia, P.; Stornaiuolo, M.; Daglia, M. Metabolic Profiling, in Vitro Bioaccessibility and in Vivo Bioavailability of a Commercial Bioactive Epilobium angustifolium L. Extract. Biomed. Pharmacother. 2020, 131, 110670. [Google Scholar] [CrossRef]
- Kalle, R.; Belichenko, O.; Kuznetsova, N.; Kolosova, V.; Prakofjewa, J.; Stryamets, N.; Mattalia, G.; Šarka, P.; Simanova, A.; Prūse, B.; et al. Gaining Momentum: Popularization of Epilobium angustifolium as Food and Recreational Tea on the Eastern Edge of Europe. Appetite 2020, 150, 104638. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Zagórska-Dziok, M.; Ossowicz-Rupniewska, P.; Makuch, E.; Duchnik, W.; Kucharski, Ł.; Adamiak-Giera, U.; Prowans, P.; Czapla, N.; Bargiel, P.; et al. Epilobium angustifolium L. Extracts as Valuable Ingredients in Cosmetic and Dermatological Products. Molecules 2021, 26, 3456. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Klimowicz, A.; Duchnik, W.; Kucharski, Ł.; Florkowska, K.; Muzykiewicz, A.; Wira, D.; Zielonkabrzezicka, J.; Siedłowska, A.; Nadarzewska, K. Application of Green-Extraction Technique to Evaluate of Antioxidative Capacity of Wild Population of Fireweed (Epilobium angustifolium ). Herba Pol. 2019, 65, 18–30. [Google Scholar] [CrossRef]
- Lasinskas, M.; Jariene, E.; Vaitkeviciene, N.; Hallmann, E.; Najman, K. Effect of Different Durations of Solid-Phase Fermentation for Fireweed (Chamerion Angustifolium (L.) Holub) Leaves on the Content of Polyphenols and Antioxidant Activity In Vitro. Molecules 2020, 25, 1011. [Google Scholar] [CrossRef] [Green Version]
- Kowalczyk, D.; Szymanowska, U.; Skrzypek, T.; Bartkowiak, A.; Materska, M.; Łupina, K. Release of Fireweed Extract (Epilobium angustifolium L.) from Corn Starch- and Methylcellulose-Based Films—A Comparative Study. Food Hydrocoll. 2021, 120, 106887. [Google Scholar] [CrossRef]
- Ferrante, C.; Chiavaroli, A.; Angelini, P.; Venanzoni, R.; Angeles Flores, G.; Brunetti, L.; Petrucci, M.; Politi, M.; Menghini, L.; Leone, S.; et al. Phenolic Content and Antimicrobial and Anti-Inflammatory Effects of Solidago Virga-Aurea, Phyllanthus niruri, Epilobium angustifolium, Peumus boldus, and Ononis spinosa Extracts. Antibiotics 2020, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Cybulska, K.; Makuch, E.; Kucharski, Ł.; Różewicka-Czabańska, M.; Prowans, P.; Czapla, N.; Bargiel, P.; Petriczko, J.; Klimowicz, A. In Vitro Human Skin Penetration, Antioxidant and Antimicrobial Activity of Ethanol-Water Extract of Fireweed (Epilobium angustifolium L.). Molecules 2021, 26, 329. [Google Scholar] [CrossRef]
- Kosalec, I.; Zovko, M.; Sankovic, K.; Kremer, D.; Pepeljnjak, S. Antioxidant and Antimicrobial Activity of Willow Herb (Epilobium angustifolium L.). Planta Med. 2008, 74, PA43. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; de Martino, L.; Coppola, R.; de Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Adamczak, A.; Dreger, M.; Seidler-Łożykowska, K.; Wielgus, K. Fireweed (Epilobium angustifolium L.): Botany, Phytochemistry and Traditional Uses. A Review. Herba Pol. 2019, 65, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Schepetkin, I.A.; Ramstead, A.G.; Kirpotina, L.N.; Voyich, J.M.; Jutila, M.A.; Quinn, M.T. Therapeutic Potential of Polyphenols from Epilobium angustifolium (Fireweed): Polyphenols from Fireweed. Phytother. Res. 2016, 30, 1287–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karakaya, S.; Süntar, I.; Yakinci, O.F.; Sytar, O.; Ceribasi, S.; Dursunoglu, B.; Ozbek, H.; Guvenalp, Z. In Vivo Bioactivity Assessment on Epilobium Species: A Particular Focus on Epilobium angustifolium and Its Components on Enzymes Connected with the Healing Process. J. Ethnopharmacol. 2020, 262, 113207. [Google Scholar] [CrossRef] [PubMed]
- Shikov, A.N.; Poltanov, E.A.; Dorman, H.J.D.; Makarov, V.G.; Tikhonov, V.P.; Hiltunen, R. Chemical Composition and in Vitro Antioxidant Evaluation of Commercial Water-Soluble Willow Herb (Epilobium angustifolium L.) Extracts. J. Agric. Food Chem. 2006, 54, 3617–3624. [Google Scholar] [CrossRef] [PubMed]
- Bajer, T.; Šilha, D.; Ventura, K.; Bajerová, P. Composition and Antimicrobial Activity of the Essential Oil, Distilled Aromatic Water and Herbal Infusion from Epilobium Parviflorum Schreb. Ind. Crops Prod. 2017, 100, 95–105. [Google Scholar] [CrossRef]
- Zeng, Q.Y.; Wu, J.; Lin, P.C. Chemical Composition and Antimicrobial Activity of the Essential Oil from Epilobium angustifolium. Chem. Nat. Compd. 2016, 52, 1113–1115. [Google Scholar] [CrossRef]
- Paduch, R.; Kandefer-Szerszeń, M.; Trytek, M.; Fiedurek, J. Terpenes: Substances Useful in Human Healthcare. Arch. Immunol. Ther. Exp. 2007, 55, 315. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wu, Y.; Zhang, H.; Liu, P.; Yao, J.; Yao, P.; Chen, J.; Duan, J. Development of Essential Oils as Skin Permeation Enhancers: Penetration Enhancement Effect and Mechanism of Action. Pharm. Biol. 2017, 55, 1592–1600. [Google Scholar] [CrossRef] [Green Version]
- Ahad, A.; Aqil, M.; Ali, A. The Application of Anethole, Menthone, and Eugenol in Transdermal Penetration of Valsartan: Enhancement and Mechanistic Investigation. Pharm. Biol. 2016, 54, 1042–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janus, E.; Ossowicz, P.; Klebeko, J.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Klimowicz, A. Enhancement of Ibuprofen Solubility and Skin Permeation by Conjugation with l-Valine Alkyl Esters. RSC Adv. 2020, 10, 7570–7584. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Quan, P.; Liu, X.; Wang, M.; Fang, L. Novel Chemical Permeation Enhancers for Transdermal Drug Delivery. Asian J. Pharm. Sci. 2014, 9, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Zillich, O.V.; Schweiggert-Weisz, U.; Hasenkopf, K.; Eisner, P.; Kerscher, M. Release and in Vitro Skin Permeation of Polyphenols from Cosmetic Emulsions. Int. J. Cosmet. Sci. 2013, 35, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, L.; Gabbanini, S.; Berlini, E.; Lucchi, E.; Beltramini, C.; Bertarelli, Y.L. Lemon (Citrus Limon, Burm.f.) Essential Oil Enhances the Trans-Epidermal Release of Lipid- (A, E) and Water- (B6, C) Soluble Vitamins from Topical Emulsions in Reconstructed Human Epidermis: Lemon EO as Vitamins’ Skin-Penetration Enhancer. Int. J. Cosmet. Sci. 2012, 34, 347–356. [Google Scholar] [CrossRef]
- Shen, Q.; Li, W.; Li, W. The Effect of Clove Oil on the Transdermal Delivery of Ibuprofen in the Rabbit by In Vitro and In Vivo Methods. Drug Dev. Ind. Pharm. 2007, 33, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Wu, Q.; Mao, Y.; Wang, Q.; An, J.; Chen, Y.; Wang, W.; Zhao, B.; Liu, N.; Zhang, Y. Cytotoxicity and Enhancement Activity of Essential Oil from Zanthoxylum Bungeanum Maxim. as a Natural Transdermal Penetration Enhancer. J. Zhejiang Univ. Sci. B 2014, 15, 153–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpanen, T.J.; Conway, B.R.; Worthington, T.; Hilton, A.C.; Elliott, T.S.; Lambert, P.A. Enhanced Chlorhexidine Skin Penetration with Eucalyptus Oil. BMC Infect. Dis. 2010, 10, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osanloo, M.; Arish, J.; Sereshti, H. Developed Methods for the Preparation of Electrospun Nanofibers Containing Plant-Derived Oil or Essential Oil: A Systematic Review. Polym. Bull. 2020, 77, 6085–6104. [Google Scholar] [CrossRef]
- Eghmazi, E.; Akhgar, M.R.; Kariminik, A. Chemical Constituents and Antibacterial Activity of the Essential Oil from Epilobium Hirsutum. J. Biodivers. Environ. Sci. 2015, 7, 338–344. [Google Scholar]
- Kaškonienė, V.; Stankevičius, M.; Drevinskas, T.; Akuneca, I.; Kaškonas, P.; Bimbiraitė-Survilienė, K.; Maruška, A.; Ragažinskienė, O.; Kornyšova, O.; Briedis, V.; et al. Evaluation of Phytochemical Composition of Fresh and Dried Raw Material of Introduced Chamerion Angustifolium L. Using Chromatographic, Spectrophotometric and Chemometric Techniques. Phytochemistry 2015, 115, 184–193. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-Caryophyllene and β-Caryophyllene Oxide-Natural Compounds of Anticancer and Analgesic Properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Nowak, K.; Ogonowski, J.; Jaworska, M.; Grzesik, K. Clove Oil—Properties and Applications. Chemik 2012, 66, 145–152. [Google Scholar]
- Malik, S.; de Mesquita, L.; Silva, C.; de Mesquita, J.; de Sá Rocha, E.; Bose, J.; Abiri, R.; de Maria Silva Figueiredo, P.; Costa-Júnior, L. Chemical Profile and Biological Activities of Essential Oil from Artemisia Vulgaris L. Cultivated in Brazil. Pharmaceuticals 2019, 12, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouyahya, A.; Et-Touys, A.; Abrini, J.; Talbaoui, A.; Fellah, H.; Bakri, Y.; Dakka, N. Lavandula Stoechas Essential Oil from Morocco as Novel Source of Antileishmanial, Antibacterial and Antioxidant Activities. Biocatal. Agric. Biotechnol. 2017, 12, 179–184. [Google Scholar] [CrossRef]
- Bouyahya, A.; Lagrouh, F.; El Omari, N.; Bourais, I.; El Jemli, M.; Marmouzi, I.; Salhi, N.; Faouzi, M.E.A.; Belmehdi, O.; Dakka, N.; et al. Essential Oils of Mentha Viridis Rich Phenolic Compounds Show Important Antioxidant, Antidiabetic, Dermatoprotective, Antidermatophyte and Antibacterial Properties. Biocatal. Agric. Biotechnol. 2020, 23, 101471. [Google Scholar] [CrossRef]
- Souza, C.F.; Baldissera, M.D.; de Silva, L.L.; Geihs, M.A.; Baldisserotto, B. Is Monoterpene Terpinen-4-Ol the Compound Responsible for the Anesthetic and Antioxidant Activity of Melaleuca Alternifolia Essential Oil (Tea Tree Oil) in Silver Catfish? Aquaculture 2018, 486, 217–223. [Google Scholar] [CrossRef]
- Kałwa, K.; Wilczyński, K.; Olesińska, K. Wpływ Warunków Przechowywania Suszonej Mięty Pieprzowej (Mentha Piperita L.) na Antyoksydacyjne Właściwości Otrzymanych Naparów Oraz Zawartość I Skład Olejku Eterycznego. Acta Sci. Pol. Tech. Agrar. 2018, 16, 13–22. [Google Scholar] [CrossRef]
- Bouyahya, A.; Belmehdi, O.; Abrini, J.; Dakka, N.; Bakri, Y. Chemical Composition of Mentha Suaveolens and Pinus Halepensis Essential Oils and Their Antibacterial and Antioxidant Activities. Asian Pac. J. Trop. Med. 2019, 12, 117. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Ziemlewska, A.; Bujak, T.; Nizioł-Łukaszewska, Z.; Hordyjewicz-Baran, Z. Cosmetic and Dermatological Properties of Selected Ayurvedic Plant Extracts. Molecules 2021, 26, 614. [Google Scholar] [CrossRef]
- Diniz do Nascimento, L.; de Moraes, A.A.B.; da Costa, K.S.; Pereira Galúcio, J.M.; Taube, P.S.; Costa, C.M.L.; Neves Cruz, J.; de Aguiar Andrade, E.H.; de Faria, L.J.G. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Bujak, T.; Ziemlewska, A.; Nizioł-Łukaszewska, Z. Positive Effect of Cannabis Sativa L. Herb Extracts on Skin Cells and Assessment of Cannabinoid-Based Hydrogels Properties. Molecules 2021, 26, 802. [Google Scholar] [CrossRef]
- Calleja, M.A.; Vieites, J.M.; Montero-Meterdez, T.; Torres, M.I.; Faus, M.J.; Gil, A.; Suárez, A. The Antioxidant Effect of β-Caryophyllene Protects Rat Liver from Carbon Tetrachloride-Induced Fibrosis by Inhibiting Hepatic Stellate Cell Activation. Br. J. Nutr. 2013, 109, 394–401. [Google Scholar] [CrossRef]
- Dahham, S.; Tabana, Y.; Iqbal, M.; Ahamed, M.; Ezzat, M.; Majid, A.; Majid, A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria Crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.-Q.; Zhou, S.-Y.; Mao, J.-X.; Liu, S.; Lan, X.-Z.; Liao, Z.-H.; Chen, M. HPLC-ESI-MS/MS Analysis of Phenolics and in Vitro Antioxidant Activity of Epilobium angustifolium L. Nat. Prod. Res. 2018, 32, 1432–1435. [Google Scholar] [CrossRef]
- Szwajgier, D.; Baranowska-Wójcik, E.; Kukula-Koch, W.; Kowalik, K.; Polak-Berecka, M.; Waśko, A. Evolution of the Anticholinesterase, Antioxidant, and Anti-Inflammatory Activity of Epilobium angustifolium L. Infusion during in Vitro Digestion. J. Funct. Foods 2021, 85, 104645. [Google Scholar] [CrossRef]
- Yanishlieva, N.V.; Marinova, E.M.; Gordon, M.H.; Raneva, V.G. Antioxidant Activity and Mechanism of Action of Thymol and Carvacrol in Two Lipid Systems. Food Chem. 1999, 64, 59–66. [Google Scholar] [CrossRef]
- Duarte, A.; Luís, Â.; Oleastro, M.; Domingues, F.C. Antioxidant Properties of Coriander Essential Oil and Linalool and Their Potential to Control Campylobacter spp. Food Control. 2016, 61, 115–122. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A. Antibacterial and Antioxidant Activity of Essential Oil Terpenes against Pathogenic and Spoilage-Forming Bacteria and Cell Structure-Activity Relationships Evaluated by SEM Microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid. Based Complement. Altern. Med. 2016, 2016, 1–21. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Kong, W.; Zhao, G.; Yang, M. Mechanisms of Antifungal and Anti-Aflatoxigenic Properties of Essential Oil Derived from Turmeric (Curcuma Longa L.) on Aspergillus Flavus. Food Chem. 2017, 220, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hasheminejad, N.; Khodaiyan, F.; Safari, M. Improving the Antifungal Activity of Clove Essential Oil Encapsulated by Chitosan Nanoparticles. Food Chem. 2019, 275, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ban, X.; Zeng, H.; He, J.; Chen, Y.; Wang, Y. The Mechanism of Antifungal Action of Essential Oil from Dill (Anethum Graveolens L.) on Aspergillus Flavus. PLoS ONE 2012, 7, e030147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleveland, T.E.; Yu, J.; Fedorova, N.; Bhatnagar, D.; Payne, G.A.; Nierman, W.C.; Bennett, J.W. Potential of Aspergillus Flavus Genomics for Applications in Biotechnology. Trends Biotechnol. 2009, 27, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Kılıç, G.; Seher, S. Antimicrobial, Antioxidant, Tyrosinase Activities and Volatile Compounds of the Essential Oil and Solvent Extract of Epilobium Hirsutum L. Growing in Turkey. Turk. J. Anal. Chem. 2020, 2, 87–94. [Google Scholar]
- Lima, I.O.; de Pereira, F.O.; de Oliveira, W.A.; de Lima, E.O.; Menezes, E.A.; Cunha, F.A.; de Diniz, M.F.F.M. Antifungal Activity and Mode of Action of Carvacrol against Candida Albicans Strains. J. Essent. Oil Res. 2013, 25, 138–142. [Google Scholar] [CrossRef]
- Nazir, A. A Review: Use of Plant Extracts and Their Phytochemical Constituents to Control Antibiotic Resistance in S. Aureus. PAB 2020, 9, 720–727. [Google Scholar] [CrossRef]
- Kawana, M.; Miyamoto, M.; Ohno, Y.; Kihara, A. Comparative Profiling and Comprehensive Quantification of Stratum Corneum Ceramides in Humans and Mice by LC/MS/MS. J. Lipid Res. 2020, 61, 884–895. [Google Scholar] [CrossRef] [Green Version]
- Ghavam, M.; Manconi, M.; Manca, M.L.; Bacchetta, G. Extraction of Essential Oil from Dracocephalum Kotschyi Boiss (Lamiaceae), Identification of Two Active Compounds and Evaluation of the Antimicrobial Properties. J. Ethnopharmacol. 2021, 267, 113513. [Google Scholar] [CrossRef]
- Ju, J.; Chen, X.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of Essential Oil as a Sustained Release Preparation in Food Packaging. Trends Food Sci. Technol. 2019, 92, 22–32. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant Activity of Essential Oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, R.G.; Sharma, S.; Narayan, R.P.; Koul, V. Effective Permeation of 2.5 and 5% Lidocaine Hydrochloride in Human Skin Using Iontophoresis Technique. Int. J. Dermatol. 2018, 57, 1335–1343. [Google Scholar] [CrossRef]
- Luo, L.; Lane, M.E. Topical and Transdermal Delivery of Caffeine. Int. J. Pharm. 2015, 490, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Dias, M. Topical Delivery of Caffeine from Some Commercial Formulations. Int. J. Pharm. 1999, 182, 41–47. [Google Scholar] [CrossRef]
- Sawant, P.D.; Luu, D.; Ye, R.; Buchta, R. Drug Release from Hydroethanolic Gels. Effect of Drug’s Lipophilicity (LogP), Polymer–Drug Interactions and Solvent Lipophilicity. Int. J. Pharm. 2010, 396, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Ossowicz, P.; Klebeko, J.; Janus, E.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Klimowicz, A. The Effect of Alcohols as Vehicles on the Percutaneous Absorption and Skin Retention of Ibuprofen Modified with l—Valine Alkyl Esters. RSC Adv. 2020, 10, 41727–41740. [Google Scholar] [CrossRef]
- Jacobi, U.; Kaiser, M.; Toll, R.; Mangelsdorf, S.; Audring, H.; Otberg, N.; Sterry, W.; Lademann, J. Porcine Ear Skin: An in Vitro Model for Human Skin. Skin Res. Technol. 2007, 13, 19–24. [Google Scholar] [CrossRef]
- Khiao In, M.; Richardson, K.C.; Loewa, A.; Hedtrich, S.; Kaessmeyer, S.; Plendl, J. Histological and Functional Comparisons of Four Anatomical Regions of Porcine Skin with Human Abdominal Skin. Anat. Histol. Embryol. 2019, 48, 207–217. [Google Scholar] [CrossRef]
- Song, Y.H.; Gwak, H.S.; Chun, I.K. The Effects of Terpenes on the Permeation of Lidocaine and Ofloxacin from Moisture-Activated Patches. Drug Deliv. 2009, 16, 75–81. [Google Scholar] [CrossRef]
- Sapra, B.; Jain, S.; Tiwary, A.K. Percutaneous Permeation Enhancement by Terpenes: Mechanistic View. AAPS J. 2008, 10, 120. [Google Scholar] [CrossRef] [Green Version]
- Cal, K.; Janicki, S.; Sznitowska, M. In Vitro Studies on Penetration of Terpenes from Matrix-Type Transdermal Systems through Human Skin. Int. J. Pharm. 2001, 224, 81–88. [Google Scholar] [CrossRef]
- Sales, A.; de Felipe, L.O.; Bicas, J.L. Production, Properties, and Applications of α-Terpineol. Food Bioprocess. Technol. 2020, 13, 1261–1279. [Google Scholar] [CrossRef]
- Khaleel, C.; Tabanca, N.; Buchbauer, G. α-Terpineol, a Natural Monoterpene: A Review of Its Biological Properties. Open Chem. 2018, 16, 349–361. [Google Scholar] [CrossRef]
- Cal, K. How Does the Type of Vehicle Influence the in Vitro Skin Absorption and Elimination Kinetics of Terpenes? Arch. Dermatol. Res. 2006, 297, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Amnuaikit, C.; Ikeuchi, I.; Ogawara, K.; Higaki, K.; Kimura, T. Skin Permeation of Propranolol from Polymeric Film Containing Terpene Enhancers for Transdermal Use. Int. J. Pharm. 2005, 289, 167–178. [Google Scholar] [CrossRef]
- Jain, A. Transdermal Drug Delivery of Imipramine Hydrochloride. I. Effect of Terpenes. J. Control. Release 2002, 79, 93–101. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Q.-D.; Wu, Y.-M.; Liu, P.; Yao, J.-H.; Lu, Q.; Zhang, H.; Duan, J.-A. Potential of Essential Oils as Penetration Enhancers for Transdermal Administration of Ibuprofen to Treat Dysmenorrhoea. Molecules 2015, 20, 18219–18236. [Google Scholar] [CrossRef] [Green Version]
- Valgas, C.; de Souza, S.M.; Smânia, E.F.A.; Smânia, A., Jr. Screening Methods to Determine Antibacterial Activity of Natural Products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Haq, A.; Michniak-Kohn, B. Effects of Solvents and Penetration Enhancers on Transdermal Delivery of Thymoquinone: Permeability and Skin Deposition Study. Drug Deliv. 2018, 25, 1943–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suñer-Carbó, J.; Calpena-Campmany, A.; Halbaut-Bellowa, L.; Clares-Naveros, B.; Rodriguez-Lagunas, M.; Barbolini, E.; Zamarbide-Losada, J.; Boix-Montañés, A. Biopharmaceutical Development of a Bifonazole Multiple Emulsion for Enhanced Epidermal Delivery. Pharmaceutics 2019, 11, 66. [Google Scholar] [CrossRef] [Green Version]
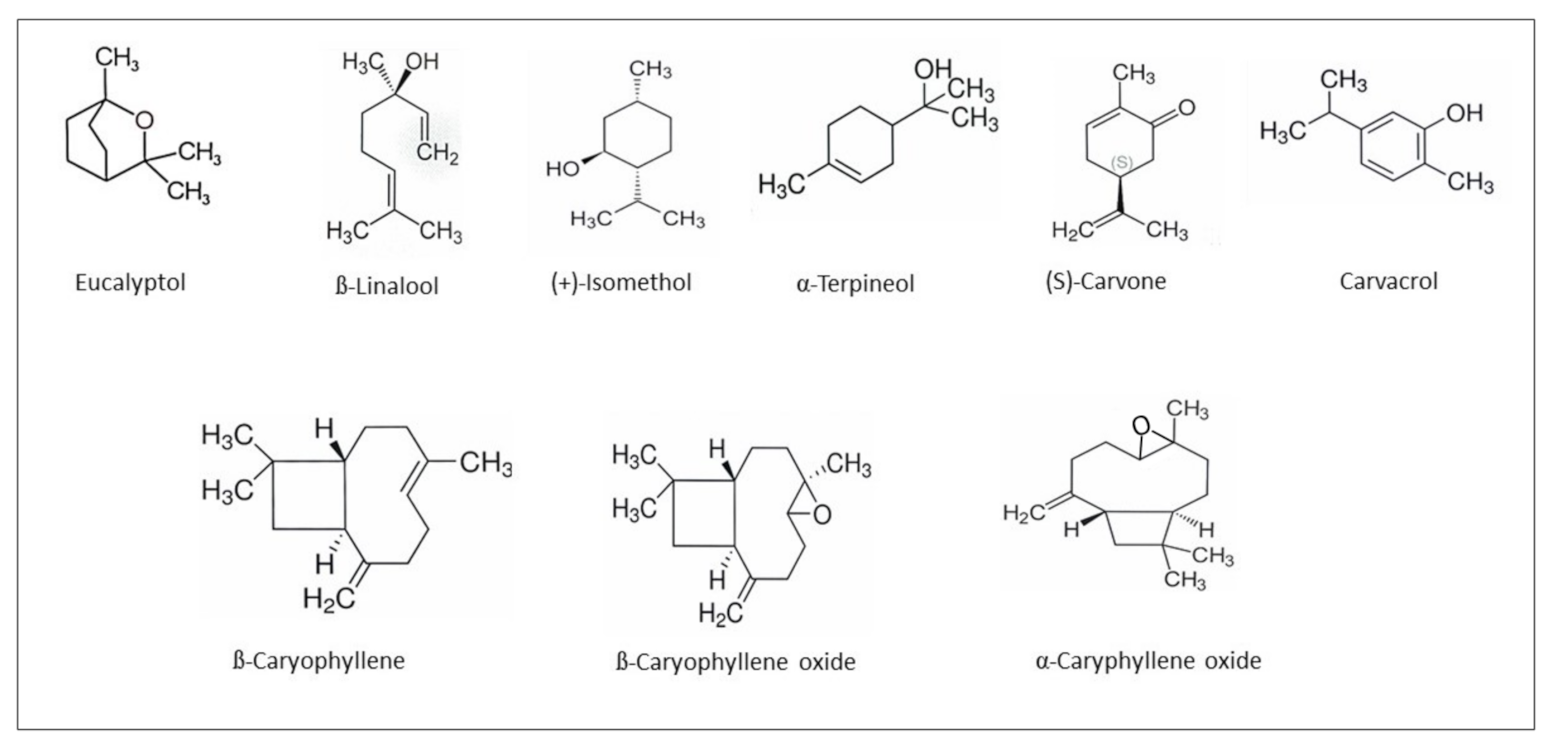


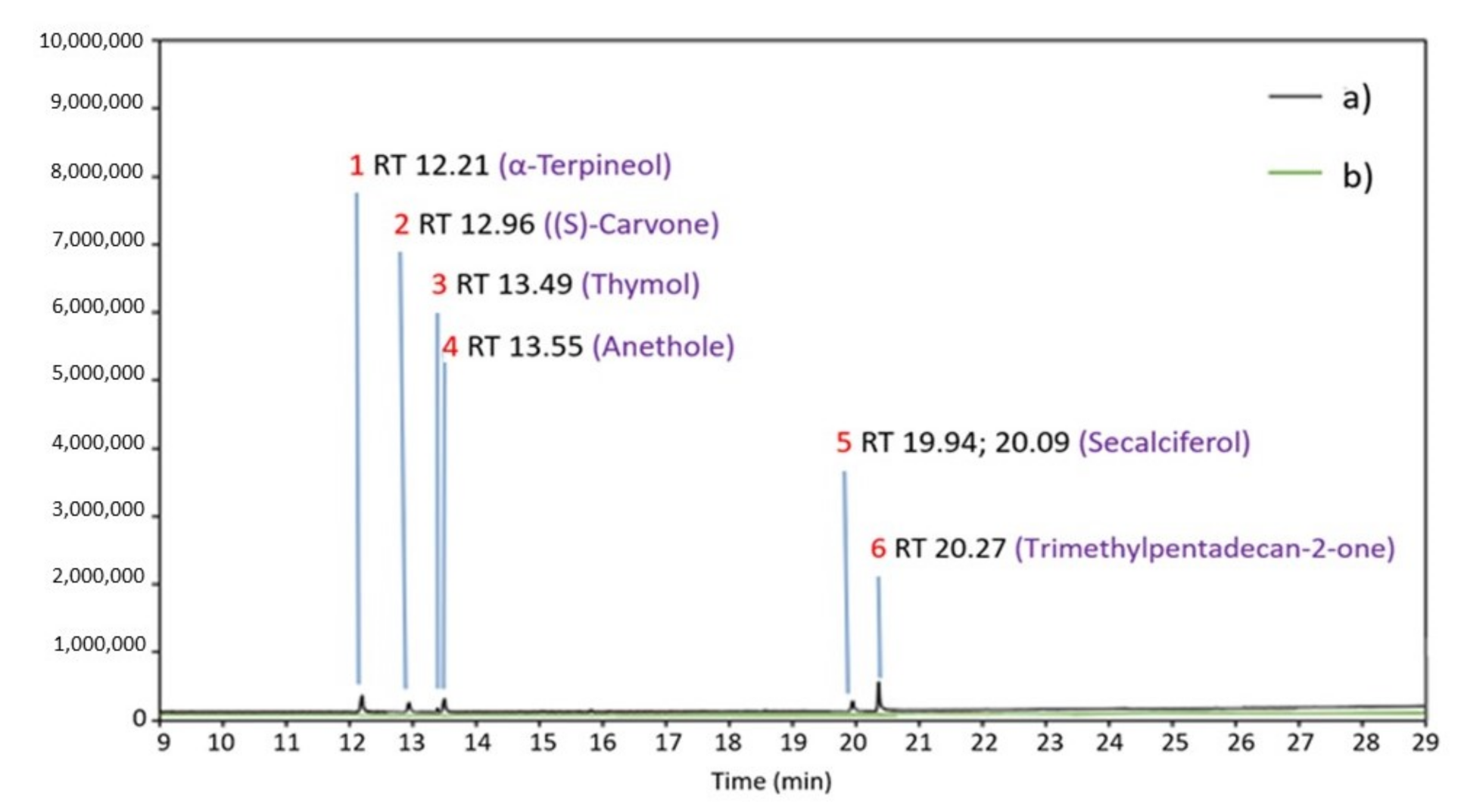

| No. | Retention Time (Min) | Compound Name | Area (%) |
|---|---|---|---|
| 1 | 9.57 | Eucalyptol | 3.20 ± 0.092 |
| 2 | 10.57 | β-Linalool | 4.54 ± 0.031 |
| 3 | 11.54 | Camphor | 3.90 ± 0.183 |
| 4 | 11.93 | (+)-Isomenthol | 2.42 ± 0.081 |
| 5 | 12.21 | α-Terpineol | 1.98 ± 0.072 |
| 6 | 12.96 | (S)-Carvone | 3.99 ± 0.132 |
| 7 | 13.49 | Thymol | 2.65 ± 0.177 |
| 8 | 13.55 | Anethole | 1.94 ± 0.095 |
| 9 | 13.65 | Carvacrol | 2.41 ± 0.202 |
| 10 | 14.39 | α-Terpinyl acetate | 0.53 ± 0.036 |
| 11 | 14.88 | δ-Cadinene | 0.61 ± 0.144 |
| 12 | 15.10 | Germacradien-4-ol | 1.67 ± 0.210 |
| 13 | 15.59 | β-Caryophyllene | 3.75 ± 0.234 |
| 14 | 16.23 | γ-Cadinene | 2.05 ± 0.094 |
| 15 | 16.75 | β-Cadinene | 2.11 ± 0.094 |
| 16 | 17.71 | α-Caryophyllene oxide | 8.57 ± 0.184 |
| 17 | 18.05 | α-Caryophyllene | 2.49 ± 0.397 |
| 18 | 18.35 | β-Caryophyllene oxide | 2.73 ± 0.091 |
| 19 | 18.50 | α-Cadinol | 2.60 ± 0.054 |
| 20 | 18.66 | β- Caryophyllene | 2.98 ± 0.489 |
| 21 | 19.94 | Secalciferol | 1.34 ± 0.099 |
| 20.09 | 1.35 ± 0.098 | ||
| 22 | 20.27 | Trimethylpentadecan-2-one | 0.54 ± 0.067 |
| 23 | 24.72 | 5-Methyldocosane | 14.95 ± 0.301 |
| 24 | 26.40 | Cosanes | 5.82 ± 0.304 |
| 27.52 | 6.75 ± 0.335 | ||
| 28.09 | 11.13 ± 0.779 |
| Antioxidant Activity | |
|---|---|
| DPPH [mg Trolox/g EOEa] | 2.445 ± 0.025 |
| RSA [%] | 78.021 ± 0.755 |
| Fungi | Concentration of EOEa (%) | |||
|---|---|---|---|---|
| 12.5 | 25 | 50 | 100 | |
| Aspergillus niger | 10.10 ± 1.00 a | 13.90 ± 1.80 b | 15.30 ± 1.50 c | 21.00 ± 2.20 d |
| Aspergillus ochraceus | 12.15 ± 1.50 a | 15.00 ± 1.50 ab | 19.10 ± 2.00 c | 28.30 ± 3.90 d |
| Aspergillus parasiticus | 9.00 ± 1.00 a | 12.45 ± 1.80 ab | 17.00 ± 2.60 c | 23.10 ± 2.60 d |
| Penicillium cyclopium | 7.80 ± 1.70 a | 9.00 ± 1.30 b | 10.00 ± 2.50 b | 17.10 ± 2.00 c |
| Compound | JSS, µg/cm2∙h | KP∙103, cm/h | LT, h | D∙104, cm2/h | Km | Q%24h |
|---|---|---|---|---|---|---|
| CON-IBU | 26.238 ± 1.104 a | 2.624 ± 0.110 a | 1.795 ± 0.306 a | 2.321 ± 0.032 a | 0.565 ± 0.132 a | 2.17 ± 0.218 a |
| EOEa-IBU | 35.162 ± 1.152 b | 3.414 ± 0.111 b | 1.762 ± 0.195 a | 2.365 ± 0.019 a | 0.722 ± 0.062 b | 2.87 ± 0.176 a |
| CON-CAF | 16.267 ± 0.397 a | 1.611 ± 0.039 a | 1.571 ± 0.077 b | 2.652 ± 0.013 a | 0.304 ± 0.008 a | 1.91 ± 0.124 a |
| EOEa-CAF | 15.507 ± 3.532 a | 1.520 ± 0.346 a | 1.262 ± 0.436 a | 3.302 ± 0.174 b | 0.230 ± 0.120 a | 1.95 ± 0.200 a |
| CON-LID | 35.578 ± 0.732 a | 3.454 ± 0.071 a | 2.508 ± 0.037 a | 1.662 ± 0.024 a | 1.039 ± 0.006 a | 4.02 ± 0.685 a |
| EOEa-LID | 41.439 ± 0.239 b | 3.985 ± 0.022 a | 2.479 ± 0.064 a | 1.681 ± 0.046 a | 1.185 ± 0.025 a | 4.93 ± 0.353 b |
| Ingredient | EOEa–IBU | CON–IBU | EOEa–LID | CON–LID | EOEa–CAF | CON–CAF |
|---|---|---|---|---|---|---|
| EOEa * | 0.1 | - | 0.1 | - | 0.1 | - |
| IBU * | 0.1 | 0.1 | - | - | - | - |
| LID * | - | - | 0.1 | 0.1 | - | - |
| CAFF * | - | - | - | - | 0.1 | 0.1 |
| Propylene glycol * | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Biobase * | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 |
| Grape seed oil * | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Beeswax * | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 |
| Water up to * | 10 | 10 | 10 | 10 | 10 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, A.; Duchnik, W.; Makuch, E.; Kucharski, Ł.; Ossowicz-Rupniewska, P.; Cybulska, K.; Sulikowski, T.; Moritz, M.; Klimowicz, A. Epilobium angustifolium L. Essential Oil—Biological Activity and Enhancement of the Skin Penetration of Drugs—In Vitro Study. Molecules 2021, 26, 7188. https://doi.org/10.3390/molecules26237188
Nowak A, Duchnik W, Makuch E, Kucharski Ł, Ossowicz-Rupniewska P, Cybulska K, Sulikowski T, Moritz M, Klimowicz A. Epilobium angustifolium L. Essential Oil—Biological Activity and Enhancement of the Skin Penetration of Drugs—In Vitro Study. Molecules. 2021; 26(23):7188. https://doi.org/10.3390/molecules26237188
Chicago/Turabian StyleNowak, Anna, Wiktoria Duchnik, Edyta Makuch, Łukasz Kucharski, Paula Ossowicz-Rupniewska, Krystyna Cybulska, Tadeusz Sulikowski, Michał Moritz, and Adam Klimowicz. 2021. "Epilobium angustifolium L. Essential Oil—Biological Activity and Enhancement of the Skin Penetration of Drugs—In Vitro Study" Molecules 26, no. 23: 7188. https://doi.org/10.3390/molecules26237188
APA StyleNowak, A., Duchnik, W., Makuch, E., Kucharski, Ł., Ossowicz-Rupniewska, P., Cybulska, K., Sulikowski, T., Moritz, M., & Klimowicz, A. (2021). Epilobium angustifolium L. Essential Oil—Biological Activity and Enhancement of the Skin Penetration of Drugs—In Vitro Study. Molecules, 26(23), 7188. https://doi.org/10.3390/molecules26237188