Towards an Improvement of Anticancer Activity of Benzyl Adenosine Analogs
Abstract
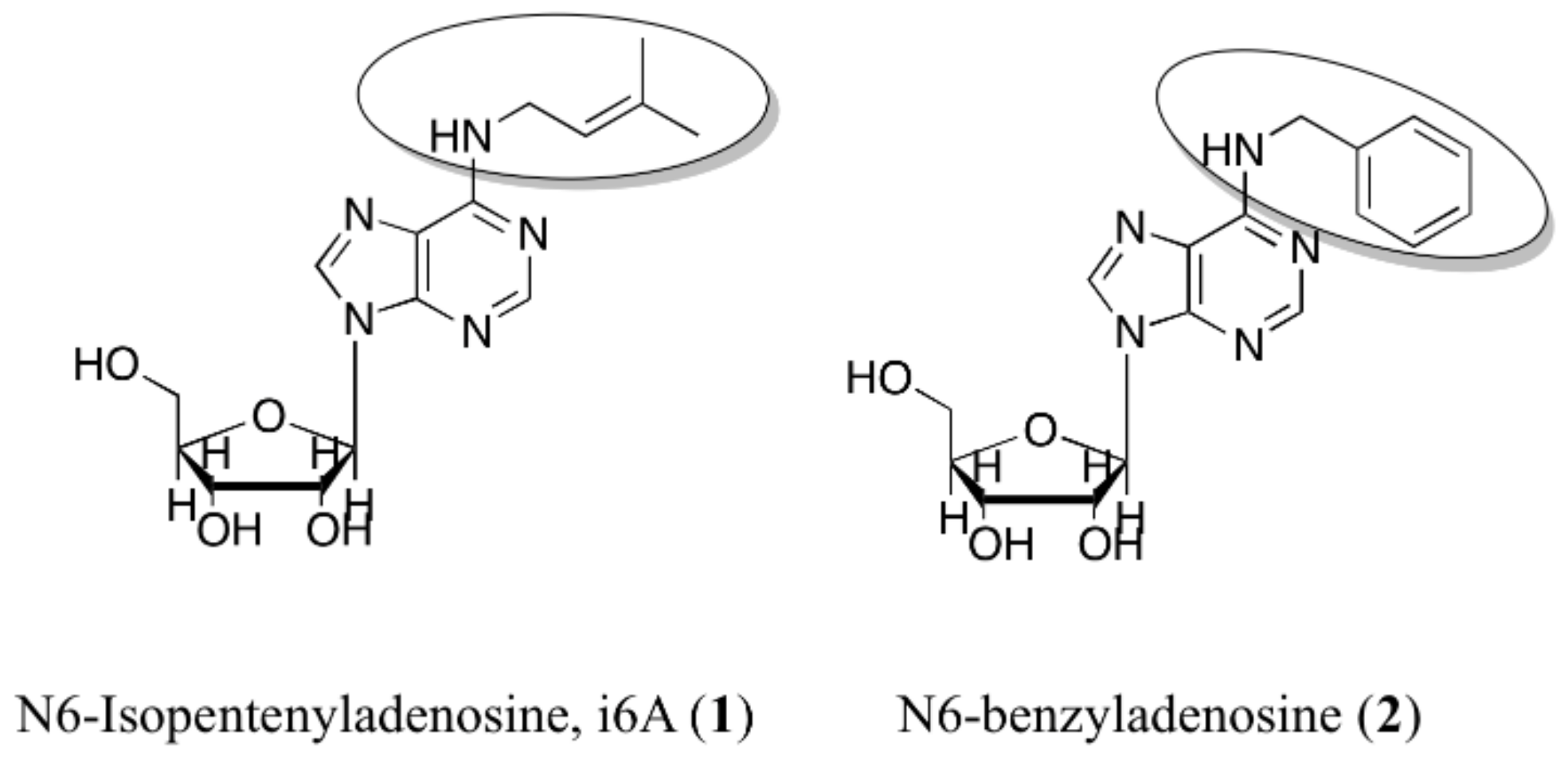
:1. Introduction
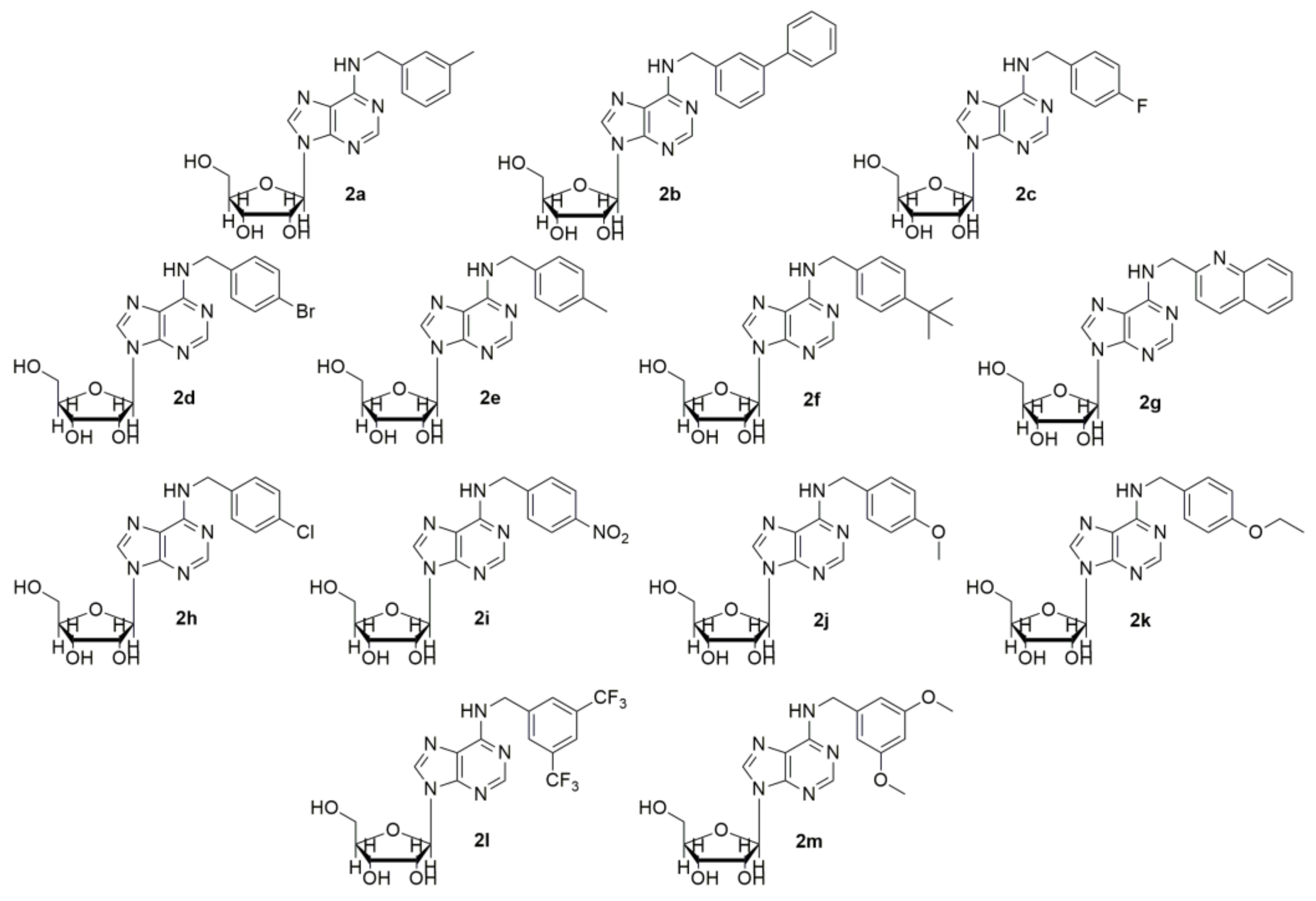
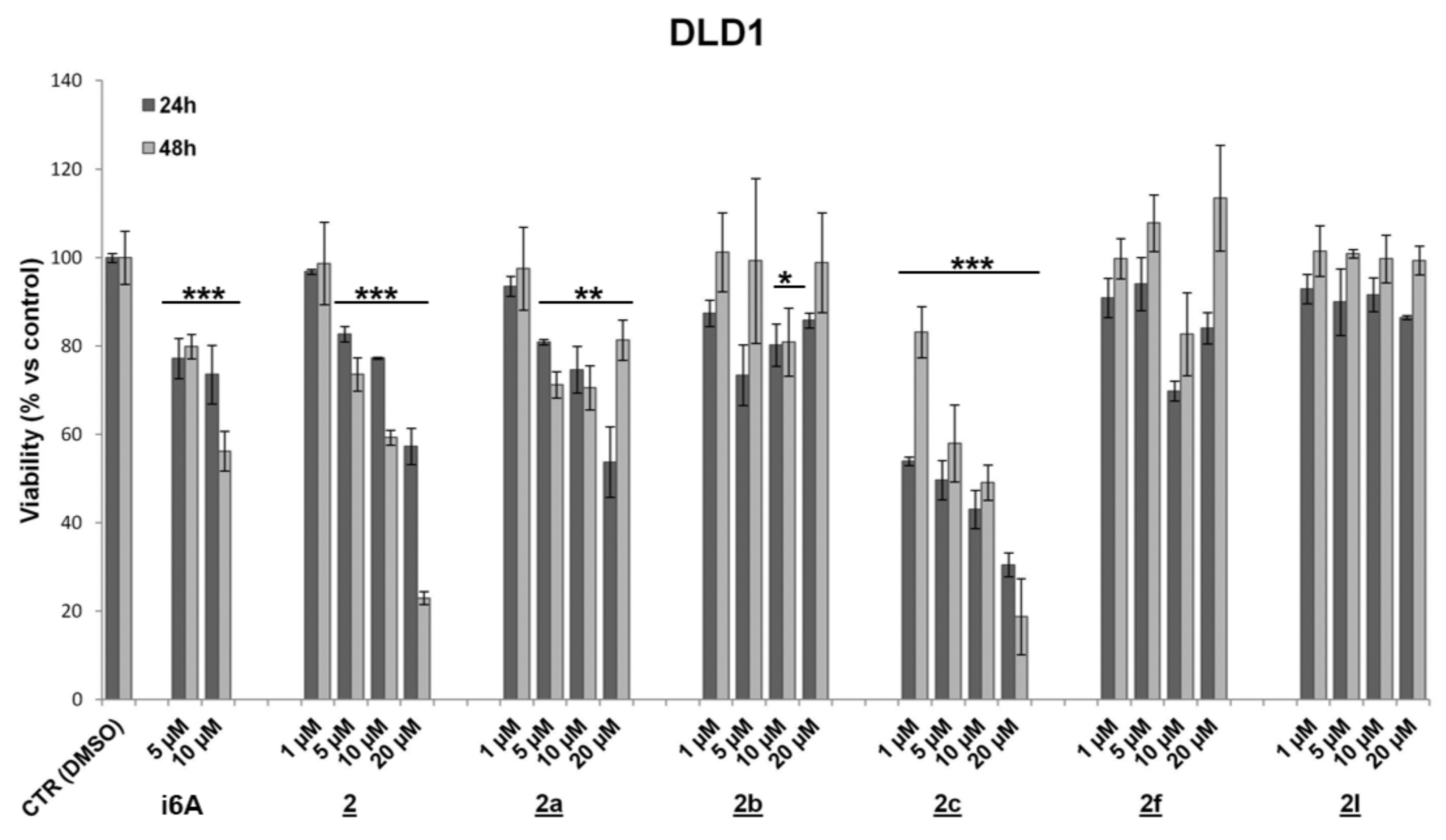
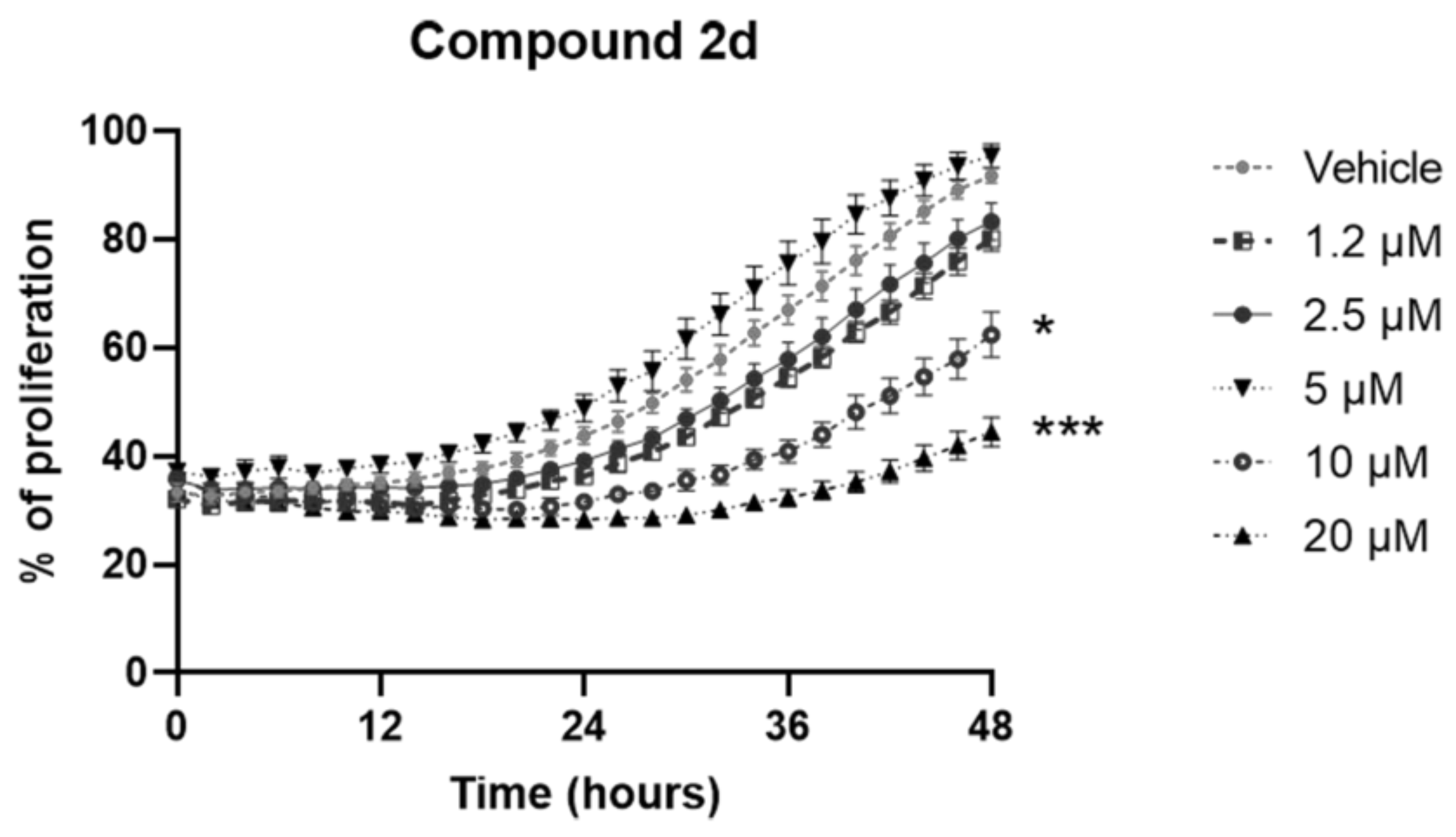
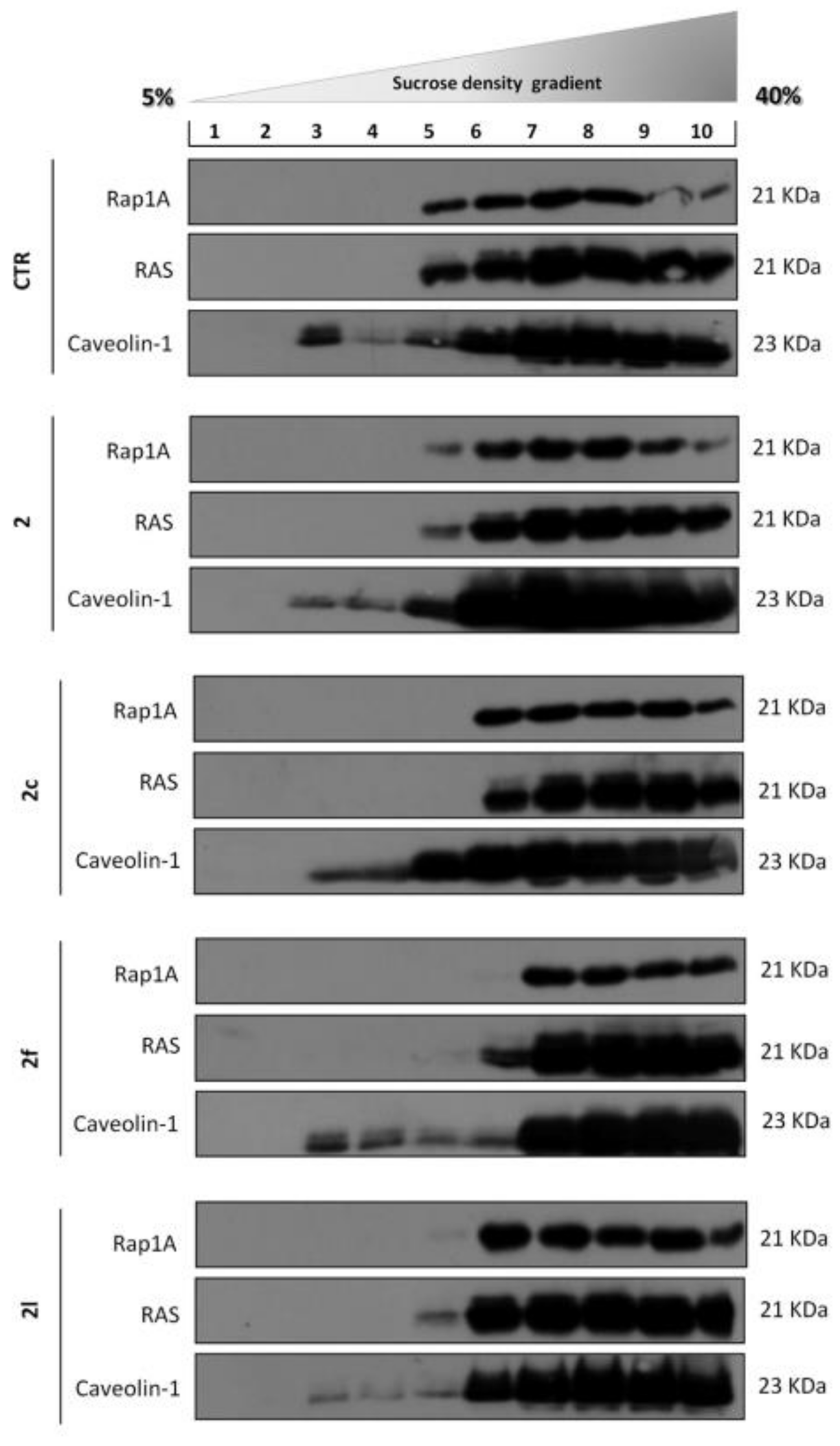
2. Results and Discussion
3. Materials and Methods
3.1. Reagents and Antibodies
3.2. Cell Cultures, Treatments and Cell Viability Assay
3.3. Cell Cultures, Treatments and Proliferation Assay
3.4. Sucrose Density Gradient
3.5. Western Blot Analysis
3.6. Chemistry
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Miller, C.O.; Skoog, F.; Von Saltza, M.H.; Strong, F. Kinetin, a cell division factor from deoxyribonucleic acid1. J. Am. Chem. Soc. 1955, 77, 1392. [Google Scholar] [CrossRef]
- Kersten, H. On the biological significance of modified nucleosides in tRNA. Prog. Nucleic Acid Res. Mol. Biol. 1984, 31, 59–114. [Google Scholar]
- Laten, H.M.; Zahareas-Doktor, S. Presence and source of free isopentenyladenosine in yeasts. Proc. Natl. Acad. Sci. USA 1985, 82, 1113–1115. [Google Scholar] [CrossRef] [Green Version]
- Schaller, G.E.; Bishopp, A.; Kieber, J.J. The yin-yang of hormones: Cytokinin and auxin interactions in plant development. Plant Cell 2015, 27, 44–63. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-m. Cytokinin biosynthesis and interconversion. Physiol. Plant. 1997, 101, 665–673. [Google Scholar] [CrossRef]
- Mok, M.C. Cytokinins and plant development—An overview. In Cytokinin: Chemistry, Activity and Function; Mok, D.W.S., Mokeds, M.C., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 155–166. [Google Scholar]
- Castiglioni, S.; Casati, S.; Ottria, R.; Ciuffreda, P.; AM Maier, J. N6-isopentenyladenosine and its analogue N6-benzyladenosine induce cell cycle arrest and apoptosis in bladder carcinoma T24 cells. Anti-Cancer Agents Med. Chem. 2013, 13, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Laezza, C.; Caruso, M.; Gentile, T.; Notarnicola, M.; Malfitano, A.; Di Matola, T.; Messa, C.; Gazzerro, P.; Bifulco, M. N6-isopentenyladenosine inhibits cell proliferation and induces apoptosis in a human colon cancer cell line DLD1. Int. J. Cancer 2014, 124, 1322–1329. [Google Scholar] [CrossRef]
- Laezza, C.; D’Alessandro, A.; Di Croce, L.; Picardi, P.; Ciaglia, E.; Pisanti, S.; Malfitano, A.M.; Comegna, M.; Faraonio, R.; Gazzerro, P. p53 regulates the mevalonate pathway in human glioblastoma multiforme. Bioorg. Chem. 2015, 6, e1909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisanti, S.; Picardi, P.; Ciaglia, E.; Margarucci, L.; Ronca, R.; Giacomini, A.; Malfitano, A.M.; Casapullo, A.; Laezza, C.; Gazzerro, P. Antiangiogenic effects of N6-isopentenyladenosine, an endogenous isoprenoid end product, mediated by AMPK activation. FASEB J. 2014, 28, 1132–1144. [Google Scholar] [CrossRef]
- Ciaglia, E.; Abate, M.; Laezza, C.; Pisanti, S.; Vitale, M.; Seneca, V.; Torelli, G.; Franceschelli, S.; Catapano, G.; Gazzerro, P. Antiglioma effects of N 6-isopentenyladenosine, an endogenous isoprenoid end product, through the downregulation of epidermal growth factor receptor. Int. J. Cancer 2017, 140, 959–972. [Google Scholar] [CrossRef] [Green Version]
- Eun, S.Y.; Kim, H.J.; Kang, E.S.; Kim, H.J.; Lee, J.H.; Chang, K.C.; Kim, J.-H.; Hong, S.-C.; Seo, H.G. Farnesyl diphosphate synthase attenuates paclitaxel-induced apoptotic cell death in human glioblastoma U87MG cells. Neurosci. Lett. 2010, 474, 115–120. [Google Scholar]
- Bifulco, M.; Malfitano, A.M.; Proto, M.C.; Santoro, A.; Caruso, M.G.; Laezza, C. Biological and pharmacological roles of N6-isopentenyladenosine: An emerging anticancer drug. Anti-Cancer Agents Med. Chem. 2008, 8, 200–204. [Google Scholar] [CrossRef]
- Laezza, C.; Notarnicola, M.; Caruso, M.G.; Messa, C.; Macchia, M.; Bertini, S.; Minutolo, F.; Portella, G.; Fiorentino, L.; Stingo, S.; et al. N6-isopentenyladenosine arrests tumor cell proliferation by inhibiting farnesyl diphosphate synthase and protein prenylation. FASEB J. 2006, 20, 412–418. [Google Scholar] [CrossRef]
- Štarha, P.; Popa, I.; Trávníček, Z.; Vančo, J. N6-Benzyladenosine derivatives as novel N-donor ligands of platinum (II) dichlorido complexes. Molecules 2013, 18, 6990–7003. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, K.; Popa, I.; Zatloukal, M.; Lenobel, R.; Hradecká, D.; Vojtesek, B.; Uldrijan, S.; Mlejnek, P.; Werbrouck, S.; Strnad, M. Substitution Derivatives of N6-benzyladenosine, Methods of Their Preparation, Their Use for Preparation of Drugs, Cosmetic Preparations and Growth Regulators, Pharmaceutical Preparations, Cosmetic Preparations and Growth Regulators Containing These Compounds. U.S. Patent 8,119,614, 21 February 2012. [Google Scholar]
- Dolezel, P.; Koudelkova, P.; Mlejnek, P. Halogenation of N6-benzyladenosine decreases its cytotoxicity in human leukemia cells. Toxicol. Vitr. 2010, 24, 2079–2083. [Google Scholar] [CrossRef]
- Ishii, Y.; Sakai, S.; Honma, Y. Cytokinin-induced differentiation of human myeloid leukemia HL-60 cells is associated with the formation of nucleotides, but not with incorporation into DNA or RNA. Biochim. Et Biophys. Acta -Mol. Cell Res. 2003, 1643, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Ishii, Y.; Hori, Y.; Sakai, S.; Honma, Y. Control of differentiation and apoptosis of human myeloid leukemia cells by cytokinins and cytokinin nucleosides, plant redifferentiation-inducing hormones. Cell Growth Differ. -Publ. Am. Assoc. Cancer Res. 2002, 13, 19–26. [Google Scholar]
- Mlejnek, P. Caspase inhibition and N6-benzyladenosine-induced apoptosis in HL-60 cells. J. Cell. Biochem. 2001, 83, 678–689. [Google Scholar] [CrossRef]
- Voller, J.; Zatloukal, M.; Lenobel, R.; Doležal, K.; Béreš, T.; Kryštof, V.; Spíchal, L.; Niemann, P.; Džubák, P.; Hajdúch, M. Anticancer activity of natural cytokinins: A structure–activity relationship study. Phytochemistry 2010, 71, 1350–1359. [Google Scholar] [CrossRef]
- Voller, J.; Béres, T.; Zatloukal, M.; Kaminski, P.A.; Niemann, P.; Doležal, K.; Džubák, P.; Hajdúch, M.; Strnad, M. The natural cytokinin 2OH3MeOBAR induces cell death by a mechanism that is different from that of the “classical” cytokinin ribosides. Phytochemistry 2017, 136, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Mlejnek, P.; Doležel, P. Apoptosis induced by N6-substituted derivatives of adenosine is related to intracellular accumulation of corresponding mononucleotides in HL-60 cells. Toxicol. Vitr. 2005, 19, 985–990. [Google Scholar] [CrossRef]
- Hertz, N.T.; Berthet, A.; Sos, M.L.; Thorn, K.S.; Burlingame, A.L.; Nakamura, K.; Shokat, K.M. A neo-substrate that amplifies catalytic activity of parkinson’s-disease-related kinase PINK1. Cell 2013, 154, 737–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, M.M.; Seegobin, M.; Kisiala, A.; Noble, A.; Brunetti, C.; Emery, R.N. Phytohormone metabolism in human cells: Cytokinins are taken up and interconverted in HeLa cell culture. FASEB BioAdv. 2019, 1, 320–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacchettini, J.C.; Poulter, C.D. Creating isoprenoid diversity. Science 1997, 277, 1788–1789. [Google Scholar] [CrossRef] [PubMed]
- Szkopińska, A.; Płochocka, D. Farnesyl diphosphate synthase; regulation of product specificity. Acta Biochim. Pol. 2005, 52, 45–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhar, M.K.; Koul, A.; Kaul, S. Farnesyl pyrophosphate synthase: A key enzyme in isoprenoid biosynthetic pathway and potential molecular target for drug development. New Biotechnol. 2013, 30, 114–123. [Google Scholar] [CrossRef]
- Ciaglia, E.; Pisanti, S.; Picardi, P.; Laezza, C.; Malfitano, A.M.; D’Alessandro, A.; Gazzerro, P.; Vitale, M.; Carbone, E.; Bifulco, M. N6-isopentenyladenosine, an endogenous isoprenoid end product, directly affects cytotoxic and regulatory functions of human NK cells through FDPS modulation. J. Leukoc. Biol. 2013, 94, 1207–1219. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Li, X.; Xia, Y.; Yu, Z.; Cai, N.; Malwal, S.R.; Han, X.; Oldfield, E.; Zhang, Y. Farnesyl pyrophosphate synthase as a target for drug development: Discovery of natural-product-derived inhibitors and their activity in pancreatic cancer cells. J. Med. Chem. 2019, 62, 10867–10896. [Google Scholar] [CrossRef] [PubMed]
- Scrima, M.; Lauro, G.; Grimaldi, M.; Di Marino, S.; Tosco, A.; Picardi, P.; Gazzerro, P.; Riccio, R.; Novellino, E.; Bifulco, M. Structural Evidence of N 6-Isopentenyladenosine as a New Ligand of Farnesyl Pyrophosphate Synthase. J. Med. Chem. 2014, 57, 7798–7803. [Google Scholar] [CrossRef] [PubMed]
- Ciaglia, E.; Grimaldi, M.; Abate, M.; Scrima, M.; Rodriquez, M.; Laezza, C.; Ranieri, R.; Pisanti, S.; Ciuffreda, P.; Manera, C. The isoprenoid derivative N6-benzyladenosine CM223 exerts antitumor effects in glioma patient-derived primary cells through the mevalonate pathway. Br. J. Pharmacol. 2017, 174, 2287–2301. [Google Scholar] [CrossRef] [Green Version]
- Grimaldi, M.; Randino, R.; Ciaglia, E.; Scrima, M.; Buonocore, M.; Stillitano, I.; Abate, M.; Covelli, V.; Tosco, A.; Gazzerro, P.; et al. NMR for screening and a biochemical assay: Identification of new FPPS inhibitors exerting anticancer activity. Bioorg. Chem. 2020, 98, 103449. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.; Meyer, B. Characterization of ligand binding by saturation transfer difference NMR spectroscopy. Angew. Chem. Int. Ed. 1999, 38, 1784–1788. [Google Scholar] [CrossRef]
- Mayer, M.; Meyer, B. Group epitope mapping by saturation transfer difference NMR to identify segments of a ligand in direct contact with a protein receptor. J. Am. Chem. Soc. 2001, 123, 6108–6117. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, D.K.; Bae, S.-H.; Gwak, H.; Jeon, J.H.; Kim, J.K.; Lee, B.I.; You, H.J.; Shin, D.H.; Kim, Y.-H. Farnesyl diphosphate synthase is important for the maintenance of glioblastoma stemness. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, F.; Sun, Q.; Patel, D.; Stommel, J.M. Cholesterol metabolism: A potential therapeutic target in glioblastoma. Cancers 2019, 11, 146. [Google Scholar] [CrossRef] [Green Version]
- Abate, M.; Laezza, C.; Pisanti, S.; Torelli, G.; Seneca, V.; Catapano, G.; Montella, F.; Ranieri, R.; Notarnicola, M.; Gazzerro, P. Deregulated expression and activity of Farnesyl Diphosphate Synthase (FDPS) in Glioblastoma. Sci. Rep. 2017, 7, 14123. [Google Scholar] [CrossRef] [Green Version]
- Berndt, N.; Hamilton, A.D.; Sebti, S.M. Targeting protein prenylation for cancer therapy. Nat. Rev. Cancer 2011, 11, 775–791. [Google Scholar] [CrossRef] [Green Version]
- Gazzerro, P.; Malfitano, A.M.; Proto, M.C.; Santoro, A.; Pisanti, S.; Caruso, M.G.; Notarnicola, M.; Messa, C.; Laezza, C.; Misso, G. Synergistic inhibition of human colon cancer cell growth by the cannabinoid CB1 receptor antagonist rimonabant and oxaliplatin. Oncol. Rep. 2010, 23, 171–175. [Google Scholar]
- Kim, Y.A.; Sharon, A.; Chu, C.K.; Rais, R.H.; Al Safarjalani, O.N.; Naguib, F.N.; el Kouni, M.H. Synthesis, biological evaluation and molecular modeling studies of N6-benzyladenosine analogues as potential anti-toxoplasma agents. Biochem. Pharmacol. 2007, 73, 1558–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravn, J.; Qvortrup, K.; Rosenbohm, C.; Koch, T. Design, synthesis, and biological evaluation of LNA nucleosides as adenosine A3 receptor ligands. Bioorg. Med. Chem. 2007, 15, 5440–5447. [Google Scholar] [CrossRef]
- Fujii, T.; Saito, T. Purines. XXVI. The Dimroth Rearrangement of 9-Substituted 1-Methyladenines: Accelerating Effect of a β-D-Ribofuranosyl Group at the 9-Position. Chem. Pharm. Bull. 1985, 33, 3635–3644. [Google Scholar] [CrossRef] [Green Version]
- Ottria, R.; Casati, S.; Baldoli, E.; Maier, J.A.; Ciuffreda, P. N6-Alkyladenosines: Synthesis and evaluation of in vitro anticancer activity. Bioorg. Med. Chem. 2010, 18, 8396–8402. [Google Scholar] [CrossRef] [PubMed]
- Randino, R.; Cini, E.; D’Ursi, A.M.; Novellino, E.; Rodriquez, M. Facile Baeyer–Villiger oxidation of cyclic ketones: Conventional versus microwave-assisted approach. Tetrahedron Lett. 2015, 56, 5723–5726. [Google Scholar] [CrossRef]
- de la Hoz, A.; Diaz-Ortiz, A.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef] [PubMed]
- GP Prism. GraphPad Software; GP Prism: San Diego, CA, USA, 1994. [Google Scholar]


| Compound (10 μM) | Cell Viability (%) ± SD | Cell Proliferation (%) ± SD | ||||
|---|---|---|---|---|---|---|
| DLD-1 | HCT116 | MC38 | ||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| 1 | 73.6 ± 6.6 | 56.2 ± 4.5 *** | 73.0 ± 2.6 | 43.2 ± 5.1 *** | 88.5 ± 4.4 | 99.8 ± 0.2 |
| 2 | 77.3 ± 0.3 | 59.3 ± 1.7 *** | 63.5 ± 5.9 | 50.2 ± 0.5 *** | 73.7 ± 5.9 | 98.0 ± 0.7 ** |
| 2a | 74.6 ± 5.3 | 70.6 ± 5.0 ** | 88.9 ± 6.1 | 70.1 ± 13.9 * | 93.6 ± 3.1 * | 99.9 ± 0.03 |
| 2b | 80.2 ± 4.8 | 80.9 ± 7.7 * | 95.1 ± 6.6 | 78.1 ± 12.8 | 53.3 ± 5.0 * | 98.5 ± 1.2 |
| 2c | 43.1 ± 4.4 | 49.1 ± 4.0 *** | 69.0 ± 0.5 | 37.1 ± 2.7 *** | 69.1 ± 6.0 | 99.3 ± 0.5 |
| 2d | 79.9 ± 1.8 | 72.3 ± 14.1 * | 127.8 ± 0.8 | 96.9 ± 8.7 | 31.5 ± 1.7 ** | 62.3 ± 7.3 * |
| 2e | 80.7 ± 2.5 | 113.6 ± 3.5 * | 70.0 ± 4.7 | 95.1 ± 1.5 | 67.5 ± 3.6 | 99.9 ± 0.1 |
| 2f | 69.8 ± 2.3 | 82.7 ± 9.4 | 120.2 ± 2.1 | 103.9 ± 4.3 | 67.9 ± 6.9 | 98.5 ± 0.8 |
| 2g | 53.9 ± 1.8 | 89.3 ± 3.5 | 108.7 ± 2.5 | 97.8 ± 5.8 | 64.6 ± 6.1 * | 99.1 ± 1.4 |
| 2h | 90.9 ± 5.5 | 90.4 ± 2.9 | 65.9 ± 26.6 | 92.9 ± 1.1 | 90.0 ± 5.9 | 99.9 ± 0.03 |
| 2i | 75.1 ± 1.2 | 72.0 ± 12.9 * | 52.0 ± 13.3 | 72.9 ± 18.2 | 84.0 ± 5.4 * | 99.9 ± 0.1 |
| 2j | 84.3 ± 4.4 | 101.2 ± 1.3 | 60.4 ± 19.1 | 80.0 ± 3.1 ** | 43.1 ± 3.5 * | 75.7 ± 4.6 * |
| 2k | 69.9 ± 4.5 | 80.9 ± 13.3 | 91.8 ± 7.7 | 98.1 ± 3.5 | 87.9 ± 8.9 * | 99.9 ± 0.01 * |
| 2l | 91.6 ± 3.8 | 99.8 ± 5.4 | 58.9 ± 2.5 | 65.1 ± 2.3 *** | 64.6 ± 1.4 | 99.8 ± 0.01 |
| 2m | 87.9 ± 0.7 | 73.3 ± 8.1 * | 88.0 ± 17.9 | 92.2 ± 7.4 | 97.7 ± 0.9 | 100 ± 0.0004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Covelli, V.; Grimaldi, M.; Randino, R.; Firoznezhad, M.; Proto, M.C.; Simone, V.D.; Matteoli, G.; Gazzerro, P.; Bifulco, M.; D’Ursi, A.M.; et al. Towards an Improvement of Anticancer Activity of Benzyl Adenosine Analogs. Molecules 2021, 26, 7146. https://doi.org/10.3390/molecules26237146
Covelli V, Grimaldi M, Randino R, Firoznezhad M, Proto MC, Simone VD, Matteoli G, Gazzerro P, Bifulco M, D’Ursi AM, et al. Towards an Improvement of Anticancer Activity of Benzyl Adenosine Analogs. Molecules. 2021; 26(23):7146. https://doi.org/10.3390/molecules26237146
Chicago/Turabian StyleCovelli, Verdiana, Manuela Grimaldi, Rosario Randino, Mohammad Firoznezhad, Maria Chiara Proto, Veronica De Simone, Gianluca Matteoli, Patrizia Gazzerro, Maurizio Bifulco, Anna Maria D’Ursi, and et al. 2021. "Towards an Improvement of Anticancer Activity of Benzyl Adenosine Analogs" Molecules 26, no. 23: 7146. https://doi.org/10.3390/molecules26237146
APA StyleCovelli, V., Grimaldi, M., Randino, R., Firoznezhad, M., Proto, M. C., Simone, V. D., Matteoli, G., Gazzerro, P., Bifulco, M., D’Ursi, A. M., & Rodriquez, M. (2021). Towards an Improvement of Anticancer Activity of Benzyl Adenosine Analogs. Molecules, 26(23), 7146. https://doi.org/10.3390/molecules26237146










