Production of Polyhydroxyalkanoates in Unsterilized Hyper-Saline Medium by Halophiles Using Waste Silkworm Excrement as Carbon Source
Abstract
:1. Introduction
2. Results
2.1. Isolation and Identification of the Endogenous Halotolerant Microorganisms in Silkworm Excrement
2.2. Screening of Endogenous PHAs Accumulating Strains in Silkworm Excrement
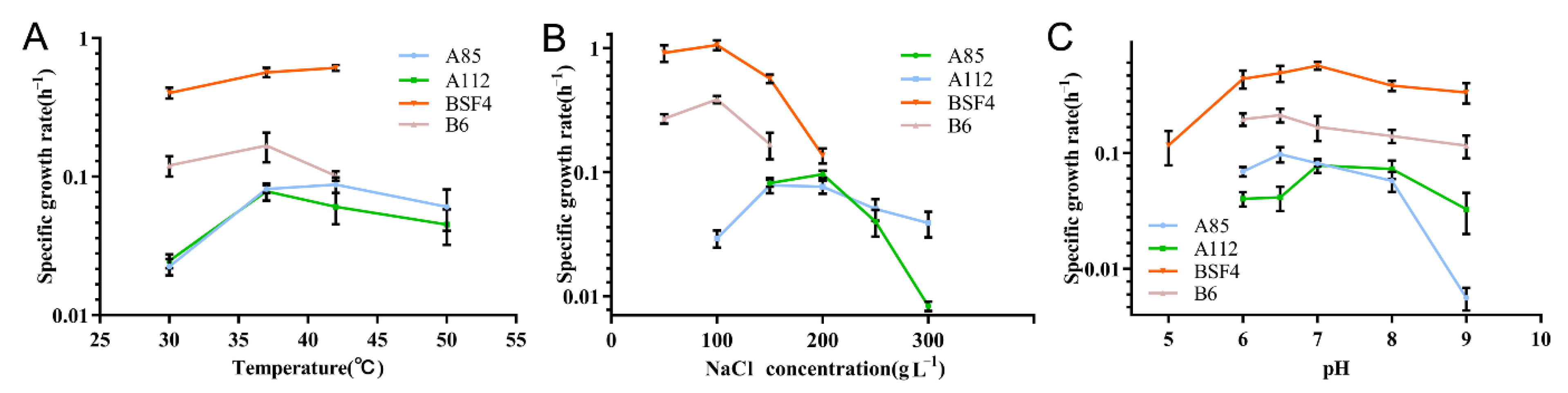
2.3. Effects of Temperature, Salinity, and pH on the Growth of PHA-Producing Strains
2.4. Utilization of Silkworm Excrement by Strains
2.5. Feasibility Study of the Open Fermentation Process
3. Discussion
4. Materials and Methods
4.1. Pretreatment of Silkworm Excrement and Culture Mediums
4.2. Isolation and Identification of Endogenous Microorganisms in Silkworm Excrement
4.3. Detection of PHA Production Using a Microscopy Approach and Gas Chromatography
4.4. Optimization of Culture Conditions
4.5. Feasibility Study on the Open Fermentation Process
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chae, Y.; An, Y.J. Current research trends on plastic pollution and ecological impacts on the soil ecosystem: A review. Environ. Pollut. 2018, 240, 387–395. [Google Scholar] [CrossRef]
- Lee, S.Y. Bacterial polyhydroxyalkanoates. Biotechnol. Bioeng. 1996, 49, 1–14. [Google Scholar] [CrossRef]
- Polyak, P.; Dohovits, E.; Nagy, G.N.; Vertessy, B.G.; Voros, G.; Pukanszky, B. Enzymatic degradation of poly-[(R)-3-hydroxybutyrate]: Mechanism, kinetics, consequences. Int. J. Biol. Macromol. 2018, 112, 156–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zonari, A.; Novikoff, S.; Electo, N.R.; Breyner, N.M.; Gomes, D.A.; Martins, A.; Neves, N.M.; Reis, R.L.; Goes, A.M. Endothelial differentiation of human stem cells seeded onto electrospun polyhydroxybutyrate/polyhydroxybutyrate-co-hydroxyvalerate fiber mesh. PLoS ONE 2012, 7, e35422. [Google Scholar] [CrossRef] [Green Version]
- Koller, M.; Bona, R.; Braunegg, G.; Hermann, C.; Horvat, P.; Kroutil, M.; Martinz, J.; Neto, J.; Pereira, L.; Varila, P. Production of polyhydroxyalkanoates from agricultural waste and surplus materials. Biomacromolecules 2005, 6, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.T.; Li, J.C.; Ali, A.; Shen, P.K. Using silkworm excrement and spent lead paste to prepare additives for improving the cycle life of lead-acid batteries. J. Energy Storage 2021, 41, 102785. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, D.G.; Yeon, S.W.; Kwon, H.S.; Ko, J.H.; Shin, D.J.; Park, H.S.; Kim, Y.S.; Bang, M.H.; Baek, N.I. Isolation of Megastigmane Sesquiterpenes from the Silkworm (Bombyx mori L.) Droppings and Their Promotion Activity on HO-1 and SIRT1. Arch. Pharm. Res. 2011, 34, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Jiang, S.; Yan, X.; Qin, Z.; Jia, C.; Li, Z.; Zhang, J.; Huang, R. The mobility of cadmium and lead in the soil-mulberry-silkworm system. Chemosphere 2020, 242, 125179. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y.; Ma, J.; Wu, Y.; Hu, Y.; Ni, L.; Li, Y. Simultaneous aqueous two-phase extraction and saponification reaction of chlorophyll from silkworm excrement. Sep. Purif. Technol. 2013, 115, 51–56. [Google Scholar] [CrossRef]
- Edbeib, M.F.; Wahab, R.A.; Huyop, F. Halophiles: Biology, adaptation, and their role in decontamination of hypersaline environments. World J. Microb. Biot. 2016, 32, 135. [Google Scholar] [CrossRef]
- Kucera, D.; Pernicova, I.; Kovalcik, A.; Koller, M.; Mullerova, L.; Sedlacek, P.; Mravec, F.; Nebesarova, J.; Kalina, M.; Marova, I. Characterization of the promising poly(3-hydroxybutyrate) producing halophilic bacterium Halomonas halophila. Bioresour. Technol. 2018, 256, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Di Donato, P.; Abbamondi, G.R.; Nicolaus, B. Synthesis, production, and biotechnological applications of exopolysaccharides and polyhydroxyalkanoates by archaea. Archaea 2011, 2011, 693253. [Google Scholar] [CrossRef] [Green Version]
- Ben Abdallah, M.; Karray, F.; Sayadi, S. Production of Polyhydroxyalkanoates by Two Halophilic Archaeal Isolates from Chott El Jerid Using Inexpensive Carbon Sources. Biomolecules 2020, 10, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, A.L.; Jin, H.L.; Huang, L.J.; Ye, H.M.; Chen, J.C.; Wu, Q.; Chen, G.Q. Biosynthesis and characterization of poly(3-hydroxydodecanoate) by beta-oxidation inhibited mutant of Pseudomonas entomophila L48. Biomacromolecules 2011, 12, 3559–3566. [Google Scholar] [CrossRef]
- Chen, G.Q. A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem. Soc. Rev. 2009, 38, 2434–2446. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wu, L.P.; Hou, J.; Zhao, D.; Xiang, H. Biosynthesis, characterization, and hemostasis potential of tailor-made poly(3-hydroxybutyrate-co-3-hydroxyvalerate) produced by Haloferax mediterranei. Biomacromolecules 2015, 16, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Alsafadi, D.; Al-Mashaqbeh, O. A one-stage cultivation process for the production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) from olive mill wastewater by Haloferax mediterranei. New Biotechnol. 2017, 34, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Saha, J.; Haldar, S.; Bhowmic, A.; Mukhopadhyay, U.K.; Mukherjee, J. Production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) by Haloferax mediterranei using rice-based ethanol stillage with simultaneous recovery and re-use of medium salts. Extremophiles 2014, 18, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yin, J.; Chen, G.Q. Polyhydroxyalkanoates, challenges and opportunities. Curr. Opin. Biotechnol. 2014, 30, 59–65. [Google Scholar] [CrossRef]
- Munir, S.; Jamil, N. Polyhydroxyalkanoate (PHA) production in open mixed cultures using waste activated sludge as biomass. Arch. Microbiol. 2020, 202, 1907–1913. [Google Scholar] [CrossRef]
- Tan, D.; Xue, Y.S.; Aibaidula, G.; Chen, G.Q. Unsterile and continuous production of polyhydroxybutyrate by Halomonas TD01. Bioresour. Technol. 2011, 102, 8130–8136. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Ling, C.; Yang, T.; Chen, X.; Chen, Y.; Deng, H.; Wu, Q.; Chen, J.; Chen, G.Q. A seawater-based open and continuous process for polyhydroxyalkanoates production by recombinant Halomonas campaniensis LS21 grown in mixed substrates. Biotechnol. Biofuels 2014, 7, 108. [Google Scholar] [CrossRef] [Green Version]
- Israni, N.; Shivakumar, S. Polyhydroxyalkanoate (PHA) biosynthesis from directly valorized ragi husk and sesame oil cake by Bacillus megaterium strain Ti3: Statistical optimization and characterization. Int. J. Biol. Macromol. 2020, 148, 20–30. [Google Scholar] [CrossRef]
- Xu, G.D.; Cai, L.; Ni, Y.S.; Tian, S.Y.; Lu, Y.Q.; Wang, L.N.; Wu, Y.R.; Jia, H.Z. The Utilization of Waste Excrementas of Bombyx mori using promised PHA-producing halophiles. 2021. manuscript in preparation. [Google Scholar]
- Hou, J.; Feng, B.; Han, J.; Liu, H.; Zhao, D.; Zhou, J.; Xiang, H. Haloarchaeal-Type beta-Ketothiolases Involved in Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Synthesis in Haloferax mediterranei. Appl. Environ. Microb. 2013, 79, 5104–5111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Q.; Han, J.; Zhou, L.; Zhou, J.; Xiang, H. Genetic and biochemical characterization of the poly(3-hydroxybutyrate-co-3-hydroxyvalerate) synthase in Haloferax mediterranei. J. Bacteriol. 2008, 190, 4173–4180. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Huang, G. The antioxidant activities of carboxymethylated garlic polysaccharide and its derivatives. Int. J. Biol. Macromol. 2019, 140, 1054–1063. [Google Scholar] [CrossRef]
- Laurens, L.M.L.; Olstad, J.L.; Templeton, D.W. Total Protein Content Determination of Microalgal Biomass by Elemental Nitrogen Analysis and a Dedicated Nitrogen-to-Protein Conversion Factor. Biofuels Algae 2018, 1980, 233–242. [Google Scholar]
- Cai, S.; Cai, L.; Liu, H.; Liu, X.; Han, J.; Zhou, J.; Xiang, H. Identification of the Haloarchaeal Phasin (PhaP) That Functions in Polyhydroxyalkanoate Accumulation and Granule Formation in Haloferax mediterranei. Appl. Environ. Microb. 2012, 78, 1946–1952. [Google Scholar] [CrossRef] [Green Version]
- Salgaonkar, B.B.; Mani, K.; Braganca, J.M. Characterization of polyhydroxyalkanoates accumulated by a moderately halophilic salt pan isolate Bacillus megaterium strain H16. J. Appl. Microbiol. 2013, 114, 1347–1356. [Google Scholar] [CrossRef]
- Han, J.; Lu, Q.; Zhou, L.; Zhou, J.; Xiang, H. Molecular characterization of the phaECHm genes, required for biosynthesis of poly(3-hydroxybutyrate) in the extremely halophilic archaeon Haloarcula marismortui. Appl. Environ. Microbiol. 2007, 73, 6058–6065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NCBI BLAST Service. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 24 October 2021).




| Strains | Identity | Strains | Identity | ||
|---|---|---|---|---|---|
| BSF4 | Halomonas salina | 100.00% | BZ7 | Staphylococcus epidermidis | 100.00% |
| B6 | Halomonas janggokensis | 98.89% | BZ8 | Gracilibacillus orientalis | 100.00% |
| BZ2 | Oceanobacillus kimchii | 100.00% | BZ9 | Bacillus oryzaecorticis | 100.00% |
| BZ3 | Halobacillus hunanensis | 100.00% | A11 | Halorubrum saccharovorum | 100.00% |
| BZ4 | Alkalibacillus halophilus | 100.00% | A28 | Halorubrum aidingense | 99.88% |
| BZ5 | Marinococcus halotolerans | 100.00% | A85 | Haloarcula hispanica | 99.72% |
| BZ6 | Brachybacterium paraconglomeratum | 99.72% | A112 | Natrinema altunense | 100.00% |
| Strains | CDW (g/L) | PHAs Concentration (g/L) | PHAs Content (%) |
|---|---|---|---|
| A85 | 4.68 ± 0.13 | 0.68 ± 0.05 | 14.63 ± 1.51 |
| A112 | 6.08 ± 0.58 | 0.69 ± 0.04 | 11.29 ± 0.46 |
| BSF4 | 2.19 ± 0.09 | 0.72 ± 0.02 | 32.97 ± 2.31 |
| B6 | 3.06 ± 0.11 | 0.15 ± 0.03 | 4.75 ± 0.78 |
| SE | MGL | SM | |||||
|---|---|---|---|---|---|---|---|
| Strains | Hours (h) | PHAs Conc. (g/L) | HV Fraction (%) | PHAs Conc. (g/L) | HV Fraction (%) | PHAs Conc. (g/L) | HV Fraction (%) |
| A85 | 72 | 0.13 ± 0.01 | n.d. | 0.59 ± 0.05 | 7.12 ± 0.08 | 0.43 ± 0.01 | 5.41 ± 0.20 |
| 96 | 0.23 ± 0.02 | n.d. | 0.96 ± 0.06 | 6.65 ± 0.18 | 0.72 ± 0.03 | 5.22 ± 0.22 | |
| 120 | 0.20 ± 0.02 | n.d. | 0.88 ± 0.03 | 6.61 ± 0.20 | 0.65 ± 0.04 | 4.97 ± 0.07 | |
| A112 | 96 | 0.06 ± 0.01 | n.d. | 0.37 ± 0.02 | 15.26 ± 1.01 | 0.24 ± 0.03 | 14.45 ± 0.07 |
| 120 | 0.08 ± 0.01 | n.d. | 0.71 ± 0.02 | 15.00 ± 0.18 | 0.46 ± 0.05 | 14.31 ± 0.98 | |
| 144 | 0.07 ± 0.01 | n.d. | 0.64 ± 0.06 | 14.17 ± 0.35 | 0.41 ± 0.03 | 13.77 ± 0.73 | |
| SE | SM | ||||
|---|---|---|---|---|---|
| Strains | Hours (h) | PHAs Conc. (g/L) | HV Fraction (%) | PHAs Conc. (g/L) | HV Fraction (%) |
| A85 | 72 | 0.16 ± 0.01 | n.d. | 0.49 ± 0.03 | 4.52 ± 0.13 |
| 96 | 0.31 ± 0.01 | n.d. | 0.81 ± 0.05 | 4.49 ± 0.04 | |
| 120 | 0.29 ± 0.02 | n.d. | 0.76 ± 0.06 | 4.05 ± 0.14 | |
| A112 | 96 | 0.08 ± 0.01 | n.d. | 0.30 ± 0.02 | 14.59 ± 0.30 |
| 120 | 0.12 ± 0.02 | n.d. | 0.58 ± 0.04 | 13.06 ± 0.03 | |
| 144 | 0.11 ± 0.01 | n.d. | 0.50 ± 0.03 | 13.50 ± 0.17 | |
| control | n.d. | n.d. | n.d. | n.d. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, S.; Wu, Y.; Li, Y.; Yang, S.; Liu, Z.; Ma, Y.; Lv, J.; Shao, Y.; Jia, H.; Zhao, Y.; et al. Production of Polyhydroxyalkanoates in Unsterilized Hyper-Saline Medium by Halophiles Using Waste Silkworm Excrement as Carbon Source. Molecules 2021, 26, 7122. https://doi.org/10.3390/molecules26237122
Cai S, Wu Y, Li Y, Yang S, Liu Z, Ma Y, Lv J, Shao Y, Jia H, Zhao Y, et al. Production of Polyhydroxyalkanoates in Unsterilized Hyper-Saline Medium by Halophiles Using Waste Silkworm Excrement as Carbon Source. Molecules. 2021; 26(23):7122. https://doi.org/10.3390/molecules26237122
Chicago/Turabian StyleCai, Shuangfeng, Yaran Wu, Yanan Li, Shuying Yang, Zhi Liu, Yuwen Ma, Jianqiang Lv, Yujia Shao, Hongzhe Jia, Yan Zhao, and et al. 2021. "Production of Polyhydroxyalkanoates in Unsterilized Hyper-Saline Medium by Halophiles Using Waste Silkworm Excrement as Carbon Source" Molecules 26, no. 23: 7122. https://doi.org/10.3390/molecules26237122
APA StyleCai, S., Wu, Y., Li, Y., Yang, S., Liu, Z., Ma, Y., Lv, J., Shao, Y., Jia, H., Zhao, Y., & Cai, L. (2021). Production of Polyhydroxyalkanoates in Unsterilized Hyper-Saline Medium by Halophiles Using Waste Silkworm Excrement as Carbon Source. Molecules, 26(23), 7122. https://doi.org/10.3390/molecules26237122





