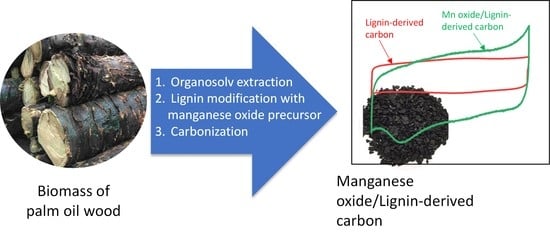
Mesoporous Manganese Oxide/Lignin-Derived Carbon for High Performance of Supercapacitor Electrodes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Lignin Extraction
2.2. A Kinetic Study of the Carbonization Process
2.3. Material Characterization
2.3.1. FTIR Spectra
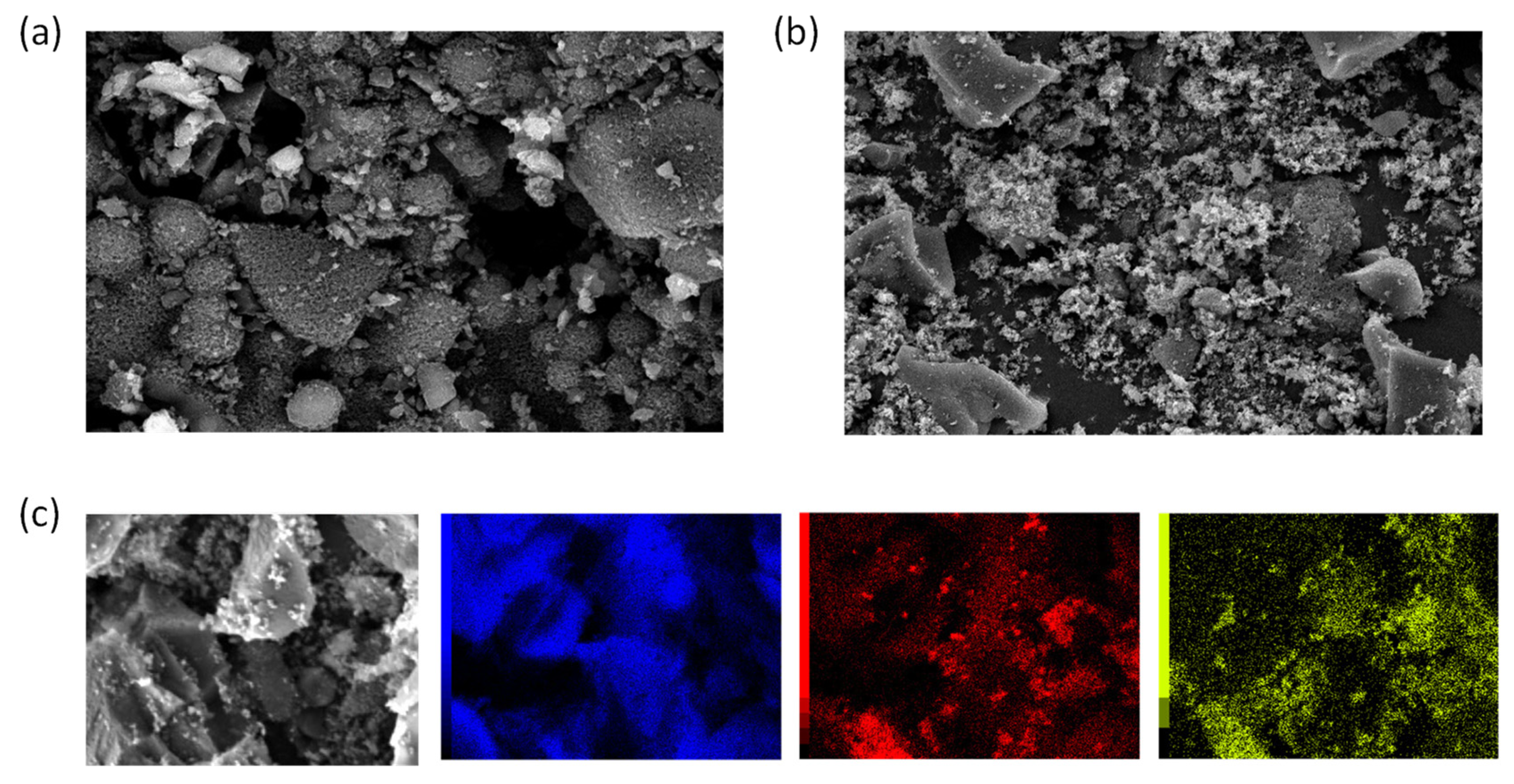
2.3.2. Surface Morphology and Elemental Contents
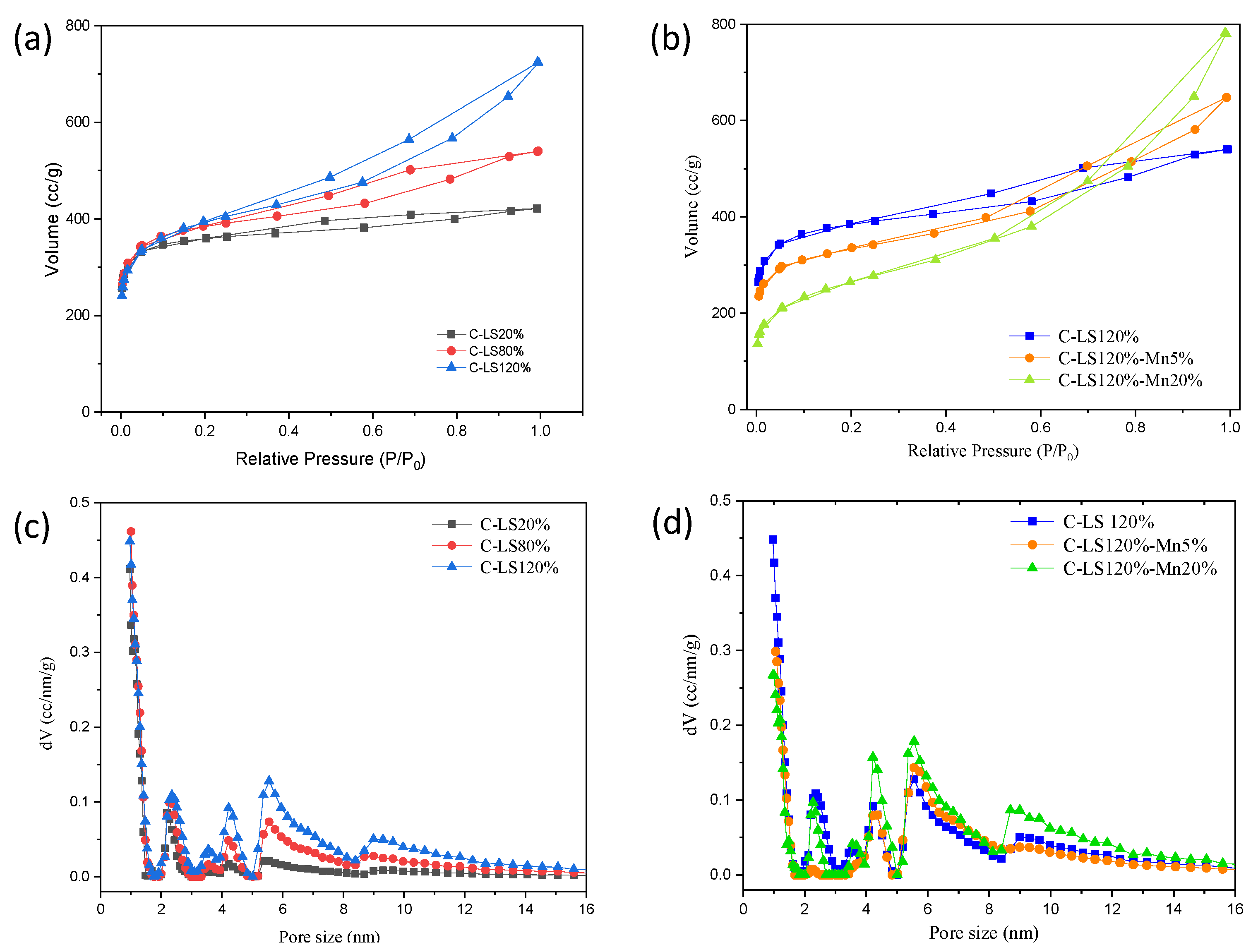
2.3.3. Pore Structures
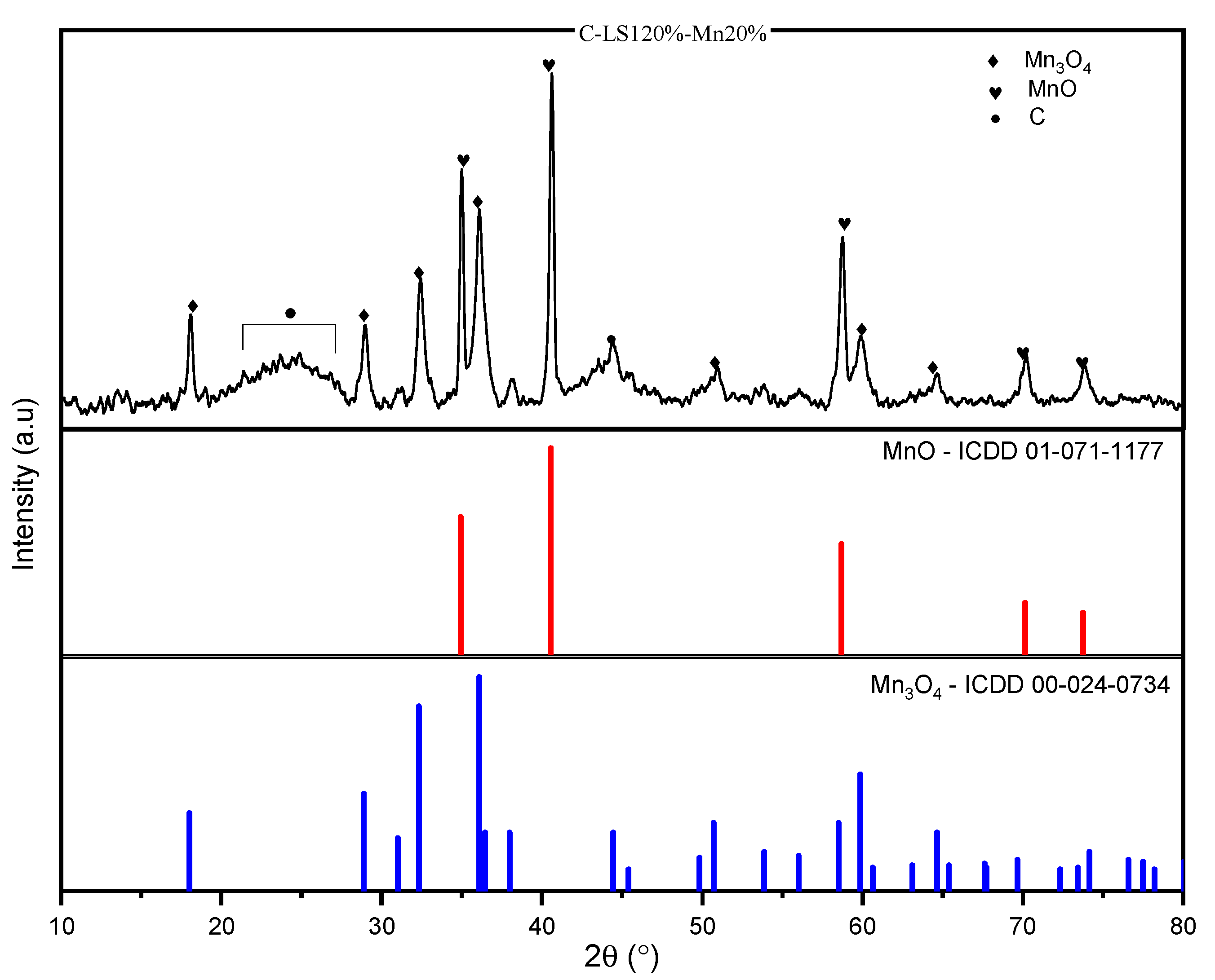
2.3.4. Crystal Phase
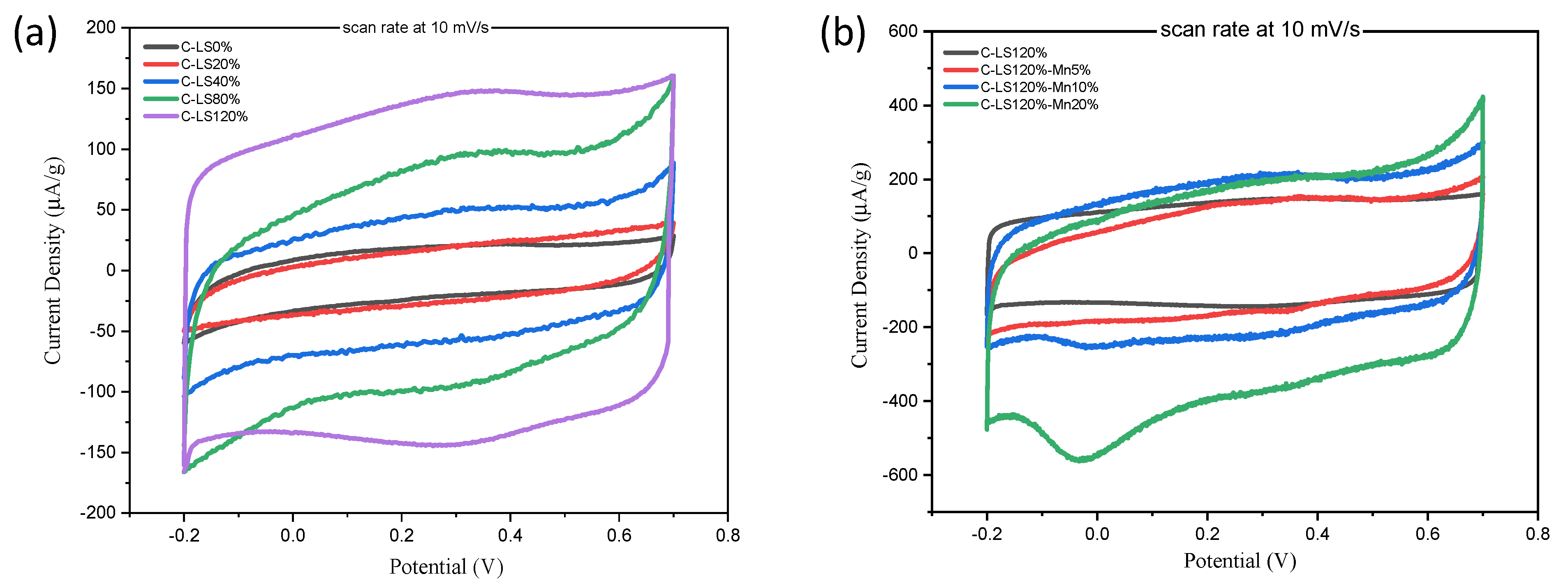
2.4. Electrochemical Properties
3. Materials and Methods
3.1. Material
3.2. Preparation
3.2.1. Lignin Extraction
3.2.2. Porous Carbon and Composite Synthesis
3.3. Kinetic Study
3.4. Material Characterization
3.5. Material Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhang, P.; Dong, S.J.; Ma, H.H.; Zhang, B.X.; Wang, Y.F.; Hu, X.M. Fractionation of corn stover into cellulose, hemicellulose and lignin using a series of ionic liquids. Ind. Crops Prod. 2015, 76, 688–696. [Google Scholar] [CrossRef]
- Prasetyo, I.; Permatasari, P.R.; Laksmana, W.T. Lignin Refinery Using Organosolv Process for Nanoporous Carbon Synthesis. Molecules 2020, 25, 3428. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Chandra, R.P.; Lee, J.S.; Lu, C.; Saddler, J.N. A comparison of various lignin-extraction methods to enhance the accessibility and ease of enzymatic hydrolysis of the cellulosic component of steam-pretreated poplar. Biotechnol. Biofuels 2017, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, H. Lignin pyrolysis reactions. J. Wood Sci. 2017, 63, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Zeiger, M.; Ariyanto, T.; Krüner, B.; Peter, N.J.; Fleischmann, S.; Etzold, B.J.M.; Presser, V. Vanadium pentoxide/carbide-derived carbon core-shell hybrid particles for high performance electrochemical energy storage. J. Mater. Chem. A 2016, 4, 18899–18909. [Google Scholar] [CrossRef] [Green Version]
- Ariyanto, T.; Masruroh, K.; Pambayun, G.Y.S.; Mukti, N.I.F.; Cahyono, R.B.; Prasetya, A.; Prasetyo, I. Improving the Separation of CO2/CH4 Using Impregnation of Deep Eutectic Solvents on Porous Carbon. ACS Omega 2021, 6, 19194–19201. [Google Scholar] [CrossRef]
- Hernando, A.; Ariyanto, T.; Prasetyo, I. Preserving climacteric fruits by ripening hormone oxidation using nano-KMnO4 confined within nanoporous carbon. ASEAN J. Chem. Eng. 2019, 19, 54–65. [Google Scholar] [CrossRef]
- Gläsel, J.; Diao, J.; Feng, Z.; Hilgart, M.; Wolker, T.; Su, D.S.; Etzold, B.J.M. Mesoporous and Graphitic Carbide-Derived Carbons as Selective and Stable Catalysts for the Dehydrogenation Reaction. Chem. Mater. 2015, 27, 5719–5725. [Google Scholar] [CrossRef]
- Konnerth, P.; Jung, D.; Straten, J.W.; Raffelt, K.; Kruse, A. Metal oxide-doped activated carbons from bakery waste and coffee grounds for application in supercapacitors. Mater. Sci. Energy Technol. 2021, 4, 69–80. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, K.; Wang, Y.; Li, H.; Jiang, H.; Chen, L. N,S co-doped carbon confined MnO/MnS heterostructures derived from a one-step pyrolysis of Mn-methionine frameworks for advanced lithium storage. J. Alloys Compd. 2021, 860, 158451. [Google Scholar] [CrossRef]
- Zang, X.-B.; Li, L.-T.; Sun, Z.-X.; Boukherroub, R.; Meng, J.-X.; Cai, K.-P.; Shao, Q.-G.; Cao, N. A simple physical mixing method for MnO2/MnO nanocomposites with superior Zn2+ storage performance. Trans. Nonferr. Met. Soc. China 2020, 30, 3347–3355. [Google Scholar] [CrossRef]
- Zhou, X.; Meng, T.; Yi, F.; Shu, D.; Li, Z.; Zeng, Q.; Gao, A.; Zhu, Z. Supramolecular assisted fabrication of Mn3O4 anchored nitrogen-doped reduced graphene oxide and its distinctive electrochemical activation process during supercapacitive study. Electrochim. Acta 2021, 370, 137739. [Google Scholar] [CrossRef]
- Zijlstra, D.S.; Lahive, C.W.; Analbers, C.A.; Figueirêdo, M.B.; Wang, Z.; Lancefield, C.S.; Deuss, P.J. Mild Organosolv lignin extraction with alcohols: The importance of benzylic alkoxylation. ACS Sustain. Chem. Eng. 2020, 8, 5119–5131. [Google Scholar] [CrossRef]
- Hosseinaei, O.; Harper, D.P.; Bozell, J.J.; Rials, T.G. Role of Physicochemical Structure of Organosolv Hardwood and Herbaceous Lignins on Carbon Fiber Performance. ACS Sustain. Chem. Eng. 2016, 4, 5785–5798. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, S.; Lucia, L.A.; Chu, J. A comparison of the pyrolysis behavior of selected Β-O-4 type lignin model compounds. J. Anal. Appl. Pyrolysis 2017, 125, 185–192. [Google Scholar] [CrossRef]
- Fernandez, A.; Saffe, A.; Pereyra, R.; Mazza, G.; Rodriguez, R. Kinetic study of regional agro-industrial wastes pyrolysis using non-isothermal TGA analysis. Appl. Therm. Eng. 2016, 106, 1157–1164. [Google Scholar] [CrossRef]
- Anca-Couce, A. Reaction mechanisms and multi-scale modelling of lignocellulosic biomass pyrolysis. Prog. Energy Combust. Sci. 2016, 53, 41–79. [Google Scholar] [CrossRef]
- Jang, B.; Yang, K.; Quan, B.; Piao, Y. Simple synthesis of thin-layered hollow carbon nanostructures by the direct pyrolysis of surfactants. Mater. Lett. 2013, 104, 68–71. [Google Scholar] [CrossRef]
- Chen, H.; Xu, G.; Xiao, C.; Bi, Y.; Hu, J. Fast Pyrolysis of Organosolv Lignin: Effect of Adding Stabilization Reagents to the Extraction Process. Energy Fuels 2019, 33, 8676–8682. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, Y.; Yang, H.; Xin, S.; Zhang, X.; Wang, X.; Chen, H. Effect of volatiles interaction during pyrolysis of cellulose, hemicellulose, and lignin at different temperatures. Fuel 2019, 248, 1–7. [Google Scholar] [CrossRef]
- Dai, G.; Zou, Q.; Wang, S.; Zhao, Y.; Zhu, L.; Huang, Q. Effect of Torrefaction on the Structure and Pyrolysis Behavior of Lignin. Energy Fuels 2018, 32, 4160–4166. [Google Scholar] [CrossRef]
- Bach, Q.V.; Trinh, T.N.; Tran, K.Q.; Thi, N.B.D. Pyrolysis characteristics and kinetics of biomass torrefied in various atmospheres. Energy Convers. Manag. 2017, 141, 72–78. [Google Scholar] [CrossRef]
- Slopiecka, K.; Bartocci, P.; Fantozzi, F. Thermogravimetric analysis and kinetic study of poplar wood pyrolysis. Appl. Energy 2012, 97, 491–497. [Google Scholar] [CrossRef]
- Asatryan, R.; Hudzik, J.M.; Bozzelli, J.W.; Khachatryan, L.; Ruckenstein, E. OH-Initiated Reactions of p-Coumaryl Alcohol Relevant to the Lignin Pyrolysis. Part I. Potential Energy Surface Analysis. J. Phys. Chem. A 2019, 123, 2570–2585. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, W.; Chang, J. Study on the product characteristics of pyrolysis lignin with calcium salt additives. Materials 2019, 12, 1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sayed, S.A.; Khairy, M. Effect of heating rate on the chemical kinetics of different biomass pyrolysis materials. Biofuels 2015, 6, 157–170. [Google Scholar] [CrossRef]
- Hu, G.; Cateto, C.; Pu, Y.; Samuel, R.; Ragauskas, A.J. Structural characterization of switchgrass lignin after ethanol organosolv pretreatment. Energy Fuels 2012, 26, 740–745. [Google Scholar] [CrossRef]
- Ponnuchamy, V.; Gordobil, O.; Diaz, R.H.; Sandak, A.; Sandak, J. Fractionation of lignin using organic solvents: A combined experimental and theoretical study. Int. J. Biol. Macromol. 2021, 168, 792–805. [Google Scholar] [CrossRef]
- Yáñez-S, M.; Matsuhiro, B.; Nuñez, C.; Pan, S.; Hubbell, C.A.; Sannigrahi, P.; Ragauskas, A.J. Physicochemical characterization of ethanol organosolv lignin (EOL) from Eucalyptus globulus: Effect of extraction conditions on the molecular structure. Polym. Degrad. Stab. 2014, 110, 184–194. [Google Scholar] [CrossRef]
- Sun, Y.C.; Wang, M.; Sun, R.C. Toward an understanding of inhomogeneities in structure of lignin in green solvents biorefinery. Part 1: Fractionation and characterization of lignin. ACS Sustain. Chem. Eng. 2015, 3, 2443–2451. [Google Scholar] [CrossRef]
- Sivasankarapillai, G.; Eslami, E.; Laborie, M.P. Potential of organosolv lignin based materials in pressure sensitive adhesive applications. ACS Sustain. Chem. Eng. 2019, 7, 12817–12824. [Google Scholar] [CrossRef]
- Tagami, A.; Gioia, C.; Lauberts, M.; Budnyak, T.; Moriana, R.; Lindström, M.E.; Sevastyanova, O. Solvent fractionation of softwood and hardwood kraft lignins for more efficient uses: Compositional, structural, thermal, antioxidant and adsorption properties. Ind. Crops Prod. 2018, 139, 123–134. [Google Scholar] [CrossRef]
- Günay, M.; Baykal, A.; Toprak, M.S.; Sözeri, H. A green chemical synthesis and characterization of Mn3O4 nanoparticles. J. Supercond. Nov. Magn. 2012, 25, 1535–1539. [Google Scholar] [CrossRef]
- Cui, W.G.; Zhuang, X.Y.; Li, Y.T.; Zhang, H.; Dai, J.J.; Zhou, L.; Hu, Z.; Hu, T.L. Engineering Co/MnO heterointerface inside porous graphitic carbon for boosting the low-temperature CO2methanation. Appl. Catal. B Environ. 2021, 287, 119959. [Google Scholar] [CrossRef]
- Vo, T.K.; Kim, J. Facile synthesis of mesoporous Cr2O3 microspheres by spray pyrolysis and their photocatalytic activity: Effects of surfactant and pyrolysis temperature. Korean J. Chem. Eng. 2020, 37, 571–575. [Google Scholar] [CrossRef]
- Salim, N.V.; Mateti, S.; Cizek, P.; Hameed, N.; Parameswaranpillai, J.; Fox, B. Large, Mesoporous Carbon Nanoparticles with Tunable Architectures for Energy Storage. ACS Appl. Nano Mater. 2019, 2, 1727–1736. [Google Scholar] [CrossRef]
- Ran, F.; Yang, X.; Xu, X.; Li, S.; Liu, Y.; Shao, L. Green Activation of Sustainable Resources to Synthesize Nitrogen-doped Oxygen-riched Porous Carbon Nanosheets towards High-performance Supercapacitor. Chem. Eng. J. 2021, 412, 128673. [Google Scholar] [CrossRef]
- Soneda, Y. Carbons for Supercapacitors, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780123854704. [Google Scholar]
- Fu, J.; Zhang, J.; Jin, C.; Wang, Z.; Wang, T.; Cheng, X.; Ma, C. Effects of temperature, oxygen and steam on pore structure characteristics of coconut husk activated carbon powders prepared by one-step rapid pyrolysis activation process. Bioresour. Technol. 2020, 310, 123413. [Google Scholar] [CrossRef]
- Saha, D.; Li, Y.; Bi, Z.; Chen, J.; Keum, J.K.; Hensley, D.K.; Grappe, H.A.; Meyer, H.M.; Dai, S.; Paranthaman, M.P.; et al. Studies on supercapacitor electrode material from activated lignin-derived mesoporous carbon. Langmuir 2014, 30, 900–910. [Google Scholar] [CrossRef]
- Byun, J.S.; Jeong, Y.C.; Kim, J.H.; Shin, M.C.; Park, J.Y.; Jin, H.J.; Park, C.R.; Kim, T.; Yang, S.J. Pseudo metal-organic coordination derived one-step carbonization of non-carbonizable carboxylate organic molecules toward functional mesostructured porous carbons. Carbon N. Y. 2021, 173, 637–645. [Google Scholar] [CrossRef]
- Olcese, R.N.; Francois, J.; Bettahar, M.M.; Petitjean, D.; Dufour, A. Hydrodeoxygenation of guaiacol, a surrogate of lignin pyrolysis vapors, over iron based catalysts: Kinetics and modeling of the lignin to aromatics integrated process. Energy Fuels 2013, 27, 975–984. [Google Scholar] [CrossRef]
- Navarro-Suárez, A.M.; Saurel, D.; Sánchez-Fontecoba, P.; Castillo-Martínez, E.; Carretero-González, J.; Rojo, T. Temperature effect on the synthesis of lignin-derived carbons for electrochemical energy storage applications. J. Power Sources 2018, 397, 296–306. [Google Scholar] [CrossRef]
- Li, L.-W.; Wang, L.-P.; Zhang, M.-Y.; Huang, Q.-Z.; He, K.-J.; Wu, F.-X. Enhancement of lithium storage capacity and rate performance of Se-modified MnO/Mn3O4 hybrid anode material via pseudocapacitive behavior. Trans. Nonferr. Met. Soc. China 2020, 30, 1904–1915. [Google Scholar] [CrossRef]
- Tian, Z.Y.; Mountapmbeme Kouotou, P.; Bahlawane, N.; Tchoua Ngamou, P.H. Synthesis of the catalytically active Mn3O4 spinel and its thermal properties. J. Phys. Chem. C 2013, 117, 6218–6224. [Google Scholar] [CrossRef]
- Li, G.; Li, Z.; Hou, Z.; Liu, Y.; Jiao, S. Unraveling superior lithium storage performance of MnO by a three-dimensional structure-memory anode. Electrochim. Acta 2020, 363, 137184. [Google Scholar] [CrossRef]
- Zhao, N.; Deng, L.; Luo, D.; Zhang, P. One-step fabrication of biomass-derived hierarchically porous carbon/MnO nanosheets composites for symmetric hybrid supercapacitor. Appl. Surf. Sci. 2020, 526, 146696. [Google Scholar] [CrossRef]
- Gao, M.; Dong, X.; Wang, K.; Duan, W.; Sun, X.; Zhu, C.; Wang, W. Laser direct preparation and processing of graphene/MnO nanocomposite electrodes for microsupercapacitors. J. Energy Storage 2021, 33, 102162. [Google Scholar] [CrossRef]
- Dessie, Y.; Tadesse, S.; Eswaramoorthy, R.; Abebe, B. Recent developments in manganese oxide based nanomaterials with oxygen reduction reaction functionalities for energy conversion and storage applications: A review. J. Sci. Adv. Mater. Devices 2019, 4, 353–369. [Google Scholar] [CrossRef]
- Dey, S.; Praveen Kumar, V.V. The performance of highly active manganese oxide catalysts for ambient conditions carbon monoxide oxidation. Curr. Res. Green Sustain. Chem. 2020, 3, 100012. [Google Scholar] [CrossRef]
- Nam, K.M.; Kim, Y.I.; Jo, Y.; Lee, S.M.; Kim, B.G.; Choi, R.; Choi, S.I.; Song, H.; Park, J.T. New crystal structure: Synthesis and characterization of hexagonal wurtzite MnO. J. Am. Chem. Soc. 2012, 134, 8392–8395. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Y.; Li, X.; Lv, Z.; Wang, X.; Liu, Z.; Huang, X. Capacitive mechanism of oxygen functional groups on carbon surface in supercapacitors. Electrochim. Acta 2018, 282, 618–625. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.Q.; Lu, A.H.; Sun, Q.; Yu, X.F.; Chen, J.Y.; Li, W.C. Unconventional Synthesis of Large Pore Ordered Mesoporous Carbon Nanospheres for Ionic Liquid-Based Supercapacitors. ACS Appl. Energy Mater. 2018, 1, 5999–6005. [Google Scholar] [CrossRef]
- Tian, J.; Wu, S.; Yin, X.; Wu, W. Novel preparation of hydrophilic graphene/graphene oxide nanosheets for supercapacitor electrode. Appl. Surf. Sci. 2019, 496, 143696. [Google Scholar] [CrossRef]
- Andreas, H.A.; Black, J.M.; Oickle, A.A. Self-discharge in manganese oxide electrochemical capacitor electrodes in aqueous electrolytes with comparisons to faradaic and charge redistribution models. Electrochim. Acta 2014, 140, 116–124. [Google Scholar] [CrossRef]
- Xu, W.; Liu, L.; Weng, W. High-performance supercapacitor based on MnO/carbon nanofiber composite in extended potential windows. Electrochim. Acta 2021, 370, 137713. [Google Scholar] [CrossRef]
- Xu, S.; Wang, T.H.; Wang, C.F.; Chen, C.W.; Dong, C.D.; Huang, C.P. The effect of crystal phase of manganese oxide on the capacitive deionization of simple electrolytes. Sci. Total Environ. 2019, 675, 31–40. [Google Scholar] [CrossRef]
- Ubale, A.U.; Waghmare, M.A.; Iqbal, K.S.; Pathan, H.M. Manganese oxides: Promising electrode materials for Li-ion batteries and supercapacitors. J. Mater. Sci. Mater. Electron. 2020, 31, 14003–14021. [Google Scholar] [CrossRef]
- Zhang, S.W.; Chen, G.Z. Manganese oxide based materials for supercapacitors. Energy Mater. Mater. Sci. Eng. Energy Syst. 2008, 3, 186–200. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, H.; Li, W.; Zhang, X. Resorcinol-formaldehyde based porous carbon as an electrode material for supercapacitors. Carbon N. Y. 2007, 45, 160–165. [Google Scholar] [CrossRef]
- Nikitina, V.A. Charge transfer processes in the course of metal-ion electrochemical intercalation. Curr. Opin. Electrochem. 2020, 19, 71–77. [Google Scholar] [CrossRef]
- Du, W.; Wang, X.; Zhan, J.; Sun, X.; Kang, L.; Jiang, F.; Zhang, X.; Shao, Q.; Dong, M.; Liu, H.; et al. Biological cell template synthesis of nitrogen-doped porous hollow carbon spheres/MnO2 composites for high-performance asymmetric supercapacitors. Electrochim. Acta 2019, 296, 907–915. [Google Scholar] [CrossRef]
- Xie, X.B.; Zhang, B.; Wang, Q.; Zhao, X.; Wu, D.; Wu, H.; Sun, X.; Hou, C.; Yang, X.; Yu, R.; et al. Efficient microwave absorber and supercapacitors derived from puffed-rice-based biomass carbon: Effects of activating temperature. J. Colloid Interface Sci. 2021, 594, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, H.D. Modifikasi Karbon dari Lignin Menggunakan Oksida Mangan untuk Meningkatkan Performa Elektroda Superkapasitor. Master’s Thesis, Universitas Gadjah Mada, Yogyakarta, Indonesia, 2021. [Google Scholar]
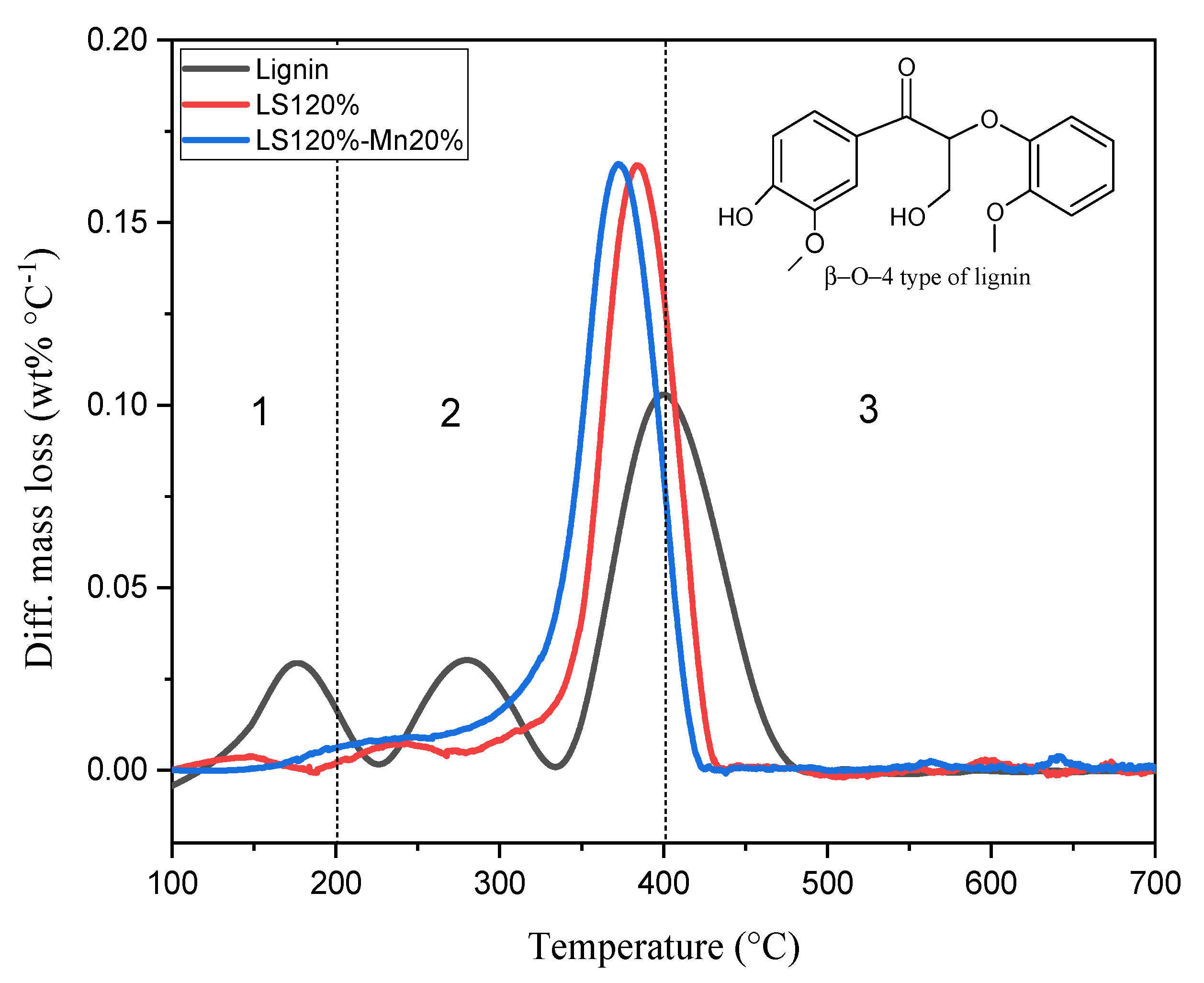
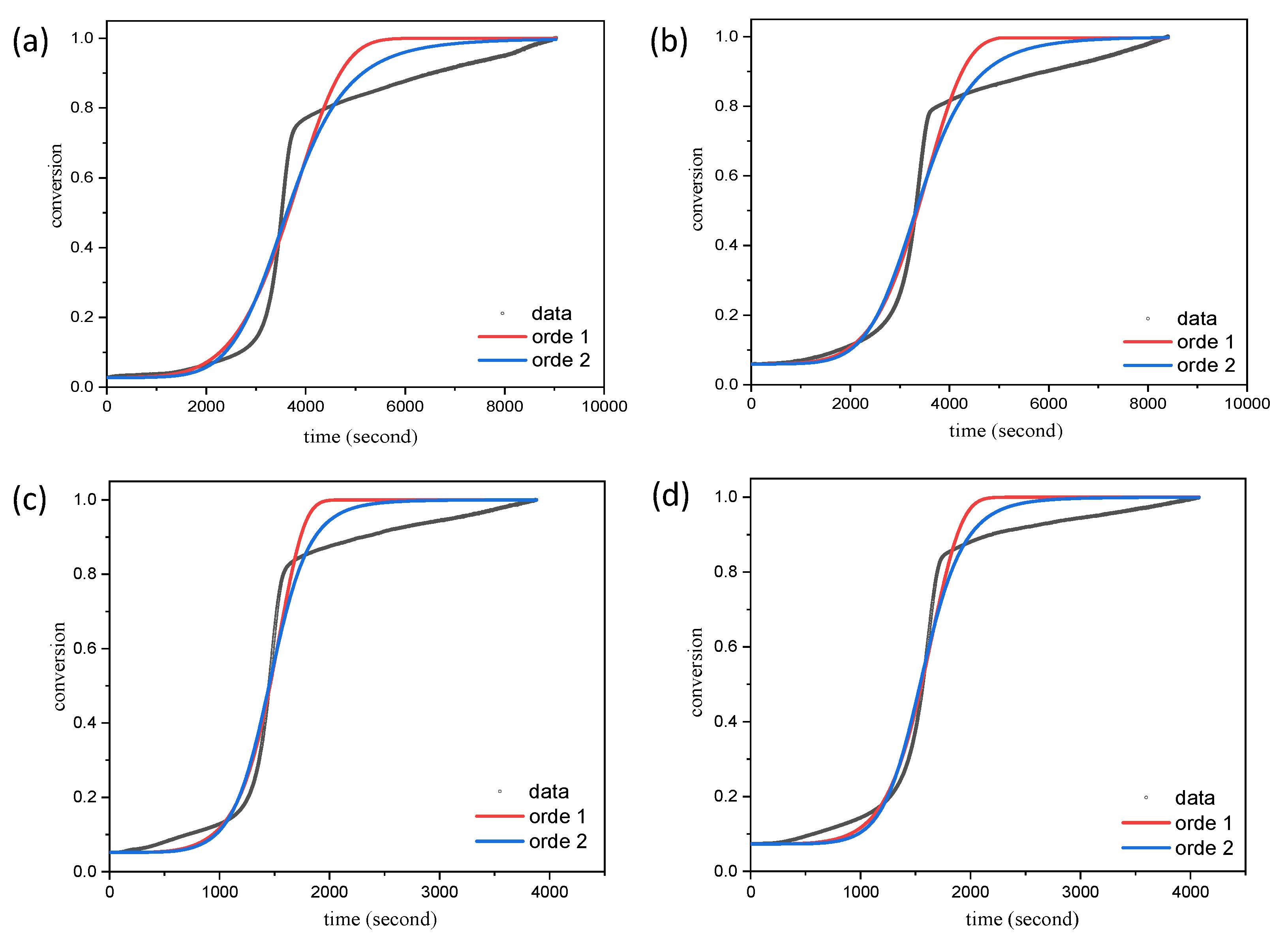
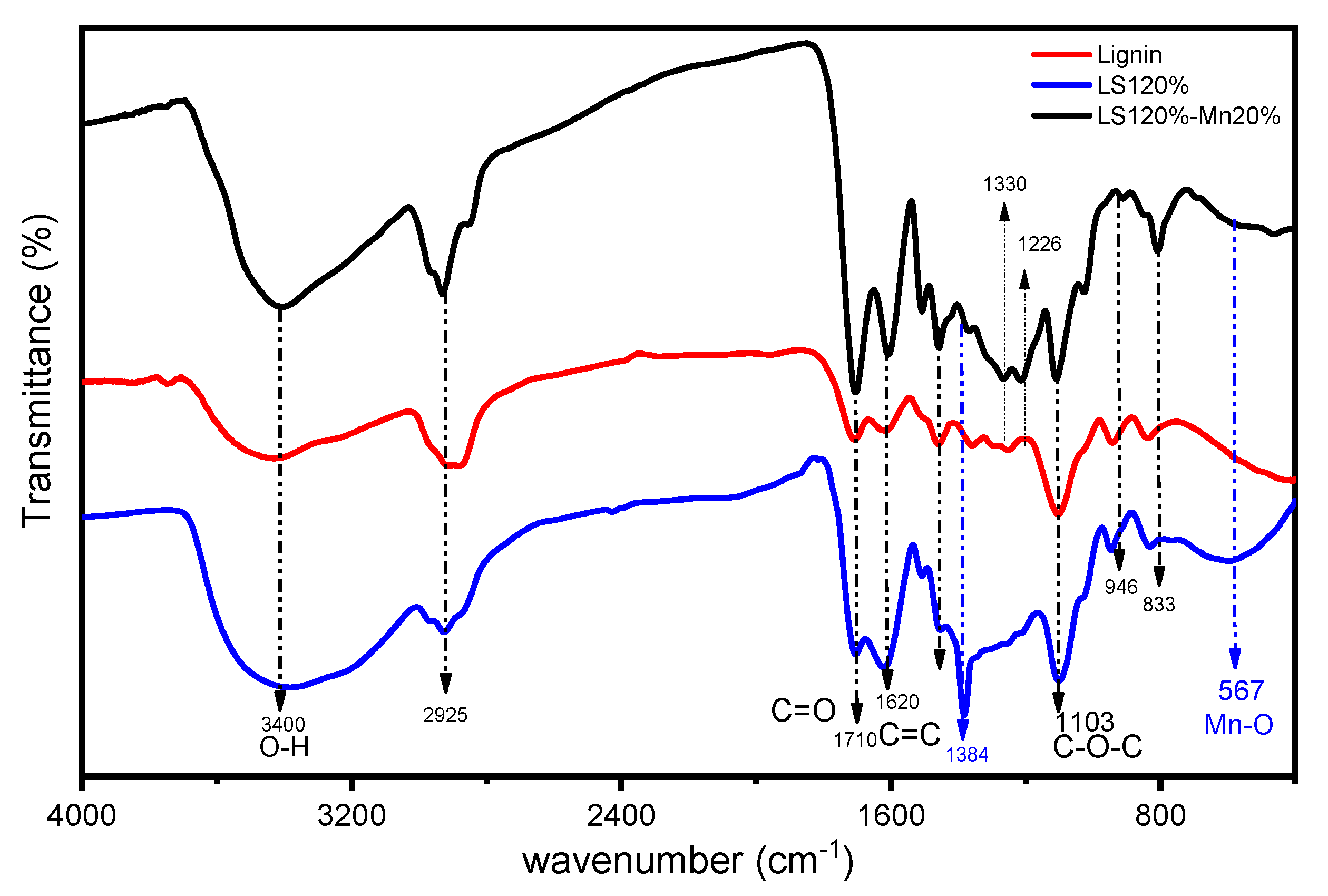
| Parameters | Ramp Rate (°C/min) | Order | A (/s) | Ea (kJ/mol) | R2 |
|---|---|---|---|---|---|
| LS120% | 5 | 1 | 0.33 ± 0.06 | 5.00 ± 0.09 | 0.9730 |
| 2 | 13.99 ± 2.31 | 6.70 ± 0.87 | 0.9832 | ||
| 10 | 1 | (4.45 ± 1.98) × 104 | 10.02 ± 0.02 | 0.9835 | |
| 2 | (5.22 ± 2.25) × 106 | 100.25 ± 0.02 | 0.9878 | ||
| LS120%-Mn20% | 5 | 1 | 1.83 ± 0.35 | 5.59 ± 0.09 | 0.9786 |
| 2 | 42.68 ± 7.14 | 6.95 ± 0.08 | 0.9866 | ||
| 10 | 1 | (1.54 ± 0.47) × 103 | 8.143 ± 0.15 | 0.9887 | |
| 2 | (3.40 ± 1.09) × 105 | 10.07 ± 0.02 | 0.9915 |
| Material | Elements (%) | ||
|---|---|---|---|
| C | O | Mn | |
| C-LS120%-Mn20% | 74.61 | 9.25 | 16.14 |
| Materials | Addition of Surfactant/Mn (%.wt) | Pore Characteristics | ||
|---|---|---|---|---|
| SSA * (m2/gr) | Vmic ** (%) | Dave *** (nm) | ||
| C-LS | 20 | 1085 | 79.26 | 1.89 |
| 80 | 1411 | 50.38 | 2.36 | |
| 120 | 1425 | 38.06 | 3.14 | |
| C-LS120%-Mn | 5 | 1210 | 37.26 | 3.31 |
| 20 | 922 | 12.67 | 5.23 | |
| Material | Specific Capacitance (F/gr) | Reference |
|---|---|---|
| C-LS0% | 22.0 | This research |
| C-LS20% | 14.1 | |
| C-LS40% | 33.6 | |
| C-LS80% | 81.7 | |
| C-LS120% | 140.9 | |
| C-LS120%-Mn5% | 128.3 | |
| C-LS120%-Mn10% | 223.8 | |
| C-LS120%-Mn20% | 345.3 | |
| Lignin-derived mesoporous carbon | 91.7 | [40] |
| MnO/carbon nanofiber | 301.8 | [56] |
| Porous carbon/MnO nanosheet | 162.7 | [47] |
| Nitrogen-doped porous hollow carbon spheres/MnO2 | 255 | [62] |
| Puffedrice-based biomass carbon | 117.2 | [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusuma, H.D.; Rochmadi; Prasetyo, I.; Ariyanto, T. Mesoporous Manganese Oxide/Lignin-Derived Carbon for High Performance of Supercapacitor Electrodes. Molecules 2021, 26, 7104. https://doi.org/10.3390/molecules26237104
Kusuma HD, Rochmadi, Prasetyo I, Ariyanto T. Mesoporous Manganese Oxide/Lignin-Derived Carbon for High Performance of Supercapacitor Electrodes. Molecules. 2021; 26(23):7104. https://doi.org/10.3390/molecules26237104
Chicago/Turabian StyleKusuma, Hersandy Dayu, Rochmadi, Imam Prasetyo, and Teguh Ariyanto. 2021. "Mesoporous Manganese Oxide/Lignin-Derived Carbon for High Performance of Supercapacitor Electrodes" Molecules 26, no. 23: 7104. https://doi.org/10.3390/molecules26237104
APA StyleKusuma, H. D., Rochmadi, Prasetyo, I., & Ariyanto, T. (2021). Mesoporous Manganese Oxide/Lignin-Derived Carbon for High Performance of Supercapacitor Electrodes. Molecules, 26(23), 7104. https://doi.org/10.3390/molecules26237104