Evaluation of the Antioxidant and Antiradical Properties of Some Phyto and Mammalian Lignans
Abstract
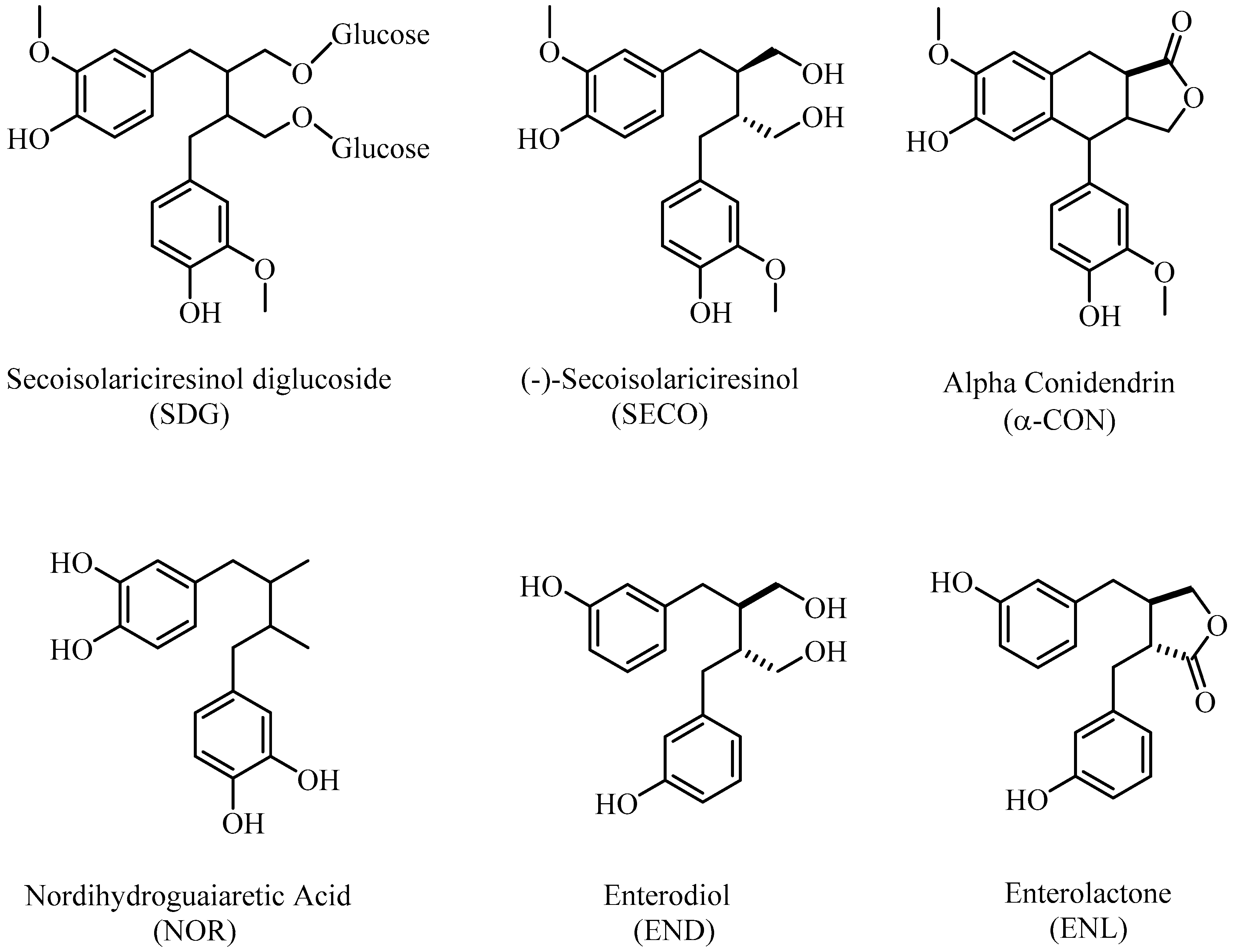
:1. Introduction
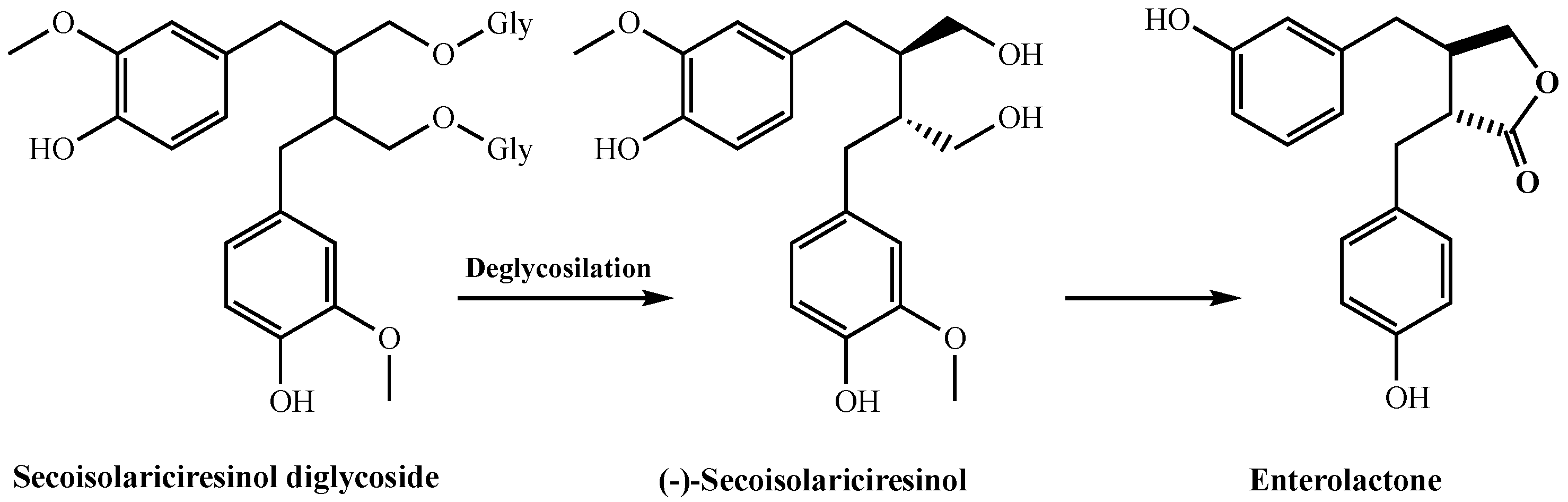
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Fe3+ Reducing Ability Assays
4.3. Cu2+ Reduction Ability
4.4. The FRAP Reduction Ability
4.5. DPPH Radical Scavenging Activities
4.6. ABTS Radical Scavenging Activities
4.7. IC50 Values Determination
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Slanina, J.; Glatz, Z. Separation procedures applicable to lignan analysis. J. Chromatogr. B 2004, 812, 215–229. [Google Scholar] [CrossRef]
- Polat Kose, L.; Gulcin, I. Inhibition effects of some lignans on carbonic anhydrase, acetylcholinesterase and butyrylcholinesterase enzymes. Rec. Nat. Prod. 2017, 11, 558–561. [Google Scholar] [CrossRef]
- Kızıltas, H.; Bingol, Z.; Goren, A.C.; Polat Kose, L.; Durmaz, L.; Topal, F.; Alwasel, S.H.; Gulcin, I. LC-HRMS profiling, antidiabetic, anticholinergic and anti-oxidant activities of aerial parts of kınkor (Ferulago stelleta). Molecules 2021, 26, 2469. [Google Scholar] [CrossRef] [PubMed]
- Ayres, D.; Loike, J.D. Chemistry and pharmacology of natural products. In Lignans: Chemical, Biological and Clinical Properties; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Kangas, L.; Saarinen, N.; Mutanen, M.; Ahotupa, M.; Hirsinummi, R.; Unkila, M.; Perala, M.; Soininen, P.; Laatikainen, R.; Korte, H.; et al. Antioxidant and antitumor effects of hydroxymatairesinol (HM-3000, HMR), a lignan isolated from the knots of spruce. Eur. J. Cancer Prev. 2002, 11, S48–S57. [Google Scholar] [PubMed]
- Ip, S.P.; Ko, K.M. The crucial antioxidant action of Schisandrin B in protecting against carbon tetrachloride hepatotoxicity in mice: A comparative study with butylated hydroxytoluene. Biochem. Pharmacol. 1996, 52, 1687–1693. [Google Scholar] [CrossRef]
- Borriello, S.P.; Setchell, K.D.; Axelson, M.; Lawson, A.M. Production and metabolism of lignans by the human faecal flora. J. Appl. Bacteriol. 1985, 58, 37–43. [Google Scholar] [CrossRef]
- Adlercreutz, H. Reproductive and Developmental Toxicology; Marcel Dekker: New York, NY, USA, 1998; p. 299. [Google Scholar]
- Gulcin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I. Antioxidants and antioxidant methods-An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Bursal, E.; Gulcin, I. Polyphenol contents and in vitro antioxidant activities of lyophilized aqueous extract of kiwifruit (Actinidia deliciosa). Food Res. Int. 2011, 44, 1482–1489. [Google Scholar] [CrossRef]
- Gulcin, I.; Topal, F.; Çakmakçı, R.; Gören, A.C.; Bilsel, M.; Erdoğan, U. Pomological features, nutritional quality, polyphenol content analysis and antioxidant properties of domesticated and three wild ecotype forms of raspberries (Rubus idaeus L.). J. Food Sci. 2011, 76, C585–C593. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I.; Alici, H.A.; Cesur, M. Determination of in vitro antioxidant and radical scavenging activities of propofol. Chem. Pharm. Bull. 2005, 53, 281–285. [Google Scholar] [CrossRef] [Green Version]
- Topal, M.; Gocer, H.; Topal, F.; Kalin, P.; Polat Köse, P.; Gulcin, I.; Çakmak, K.C.; Küçük, M.; Durmaz, L.; Gören, A.C.; et al. Antioxidant, antiradical and anticholinergic properties of cynarin purified from the illyrian thistle (Onopordum illyricum L.). J. Enzyme Inhib. Med. Chem. 2016, 31, 266–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiziltas, H.; Bingol, Z.; Goren, A.C.; Alwasel, S.H.; Gulcin, I. Anticholinergic, antidiabetic and antioxidant activities of Ferula orientalis L.—Analysis of its polyphenol contents by LC-HRMS. Rec. Nat. Prod. 2021, 15, 513–528. [Google Scholar] [CrossRef]
- Gulcin, I.; Bursal, E.; Sehitoglu, H.M.; Bilsel, M.; Goren, A.C. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem. Toxicol. 2010, 48, 2227–2238. [Google Scholar] [CrossRef]
- Bursal, E.; Taslimi, P.; Goren, A.; Gulcin, I. Assessments of anticholinergic, antidiabetic, antioxidant activities and phenolic content of Stachys annua. Biocat. Agric. Biotechnol. 2020, 28, 101711. [Google Scholar] [CrossRef]
- Koksal, E.; Gulcin, I. Antioxidant activity of cauliflower (Brassica oleracea L.). Turk. J. Agric. For. 2008, 32, 65–78. [Google Scholar]
- Taslimi, P.; Koksal, E.; Gören, A.C.; Bursal, E.; Aras, A.; Kılıç, O.; Alwasel, S.; Gulcin, I. Anti-Alzheimer, antidiabetic and antioxidant potential of Satureja cuneifolia and analysis of its phenolic contents by LC-MS/MS. Arab. J. Chem. 2020, 13, 4528–4537. [Google Scholar] [CrossRef]
- Tohma, H.; Altay, A.; Koksal, E.; Gören, A.C.; Gulcin, I. Measurement of anticancer, antidiabetic and anticholinergic properties of sumac (Rhus coriaria)—Analysis of its phenolic compounds by LC-MS/MS. J. Food Meas. Charact. 2019, 13, 1607–1619. [Google Scholar] [CrossRef]
- Artunç, T.; Menzek, A.; Taslimi, P.; Gulcin, I.; Kazaz, C.; Şahin, E. Synthesis and antioxidant activities of phenol derivatives from 1,6-bis(dimethoxyphenyl)hexane-1,6-dione. Bioorg. Chem. 2020, 100, 103884. [Google Scholar] [CrossRef]
- Gulçin, I.; Gören, A.C.; Taslimi, P.; Akyuz, B.; Tüzün, B. Anticholinergic, antidiabetic and antioxidant activities of Anatolian pennyroyal (Mentha pulegium)—Analysis of its polyphenol contents by LC-MS/MS. Biocat. Agric. Biotechnol. 2020, 23, 101441. [Google Scholar] [CrossRef]
- Turkan, F.; Atalar, M.N.; Aras, A.; Gulçin, I.; Bursal, E. ICP-MS and HPLC analyses, enzyme inhibition and antioxidant potential of Achillea schischkinii Sosn. Bioorg. Chem. 2020, 94, 103333. [Google Scholar] [CrossRef]
- Altay, A.; Tohma, H.; Durmaz, L.; Taslimi, P.; Korkmaz, M.; Gulcin, I.; Koksal, E. Preliminary phytochemical analysis and evaluation of in vitro antioxidant, antiproliferative, antidiabetic and anticholinergics effects of endemic Gypsophila taxa from Turkey. J. Food Biochem. 2019, 43, e12908. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I.; Huyut, Z.; Elmastas, M.; Aboul-Enein, H.Y. Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Gulcin, I.; Kirecci, E.; Akkemik, E.; Topal, F.; Hisar, O. Antioxidant and antimicrobial activities of an aquatic plant: Duckweed (Lemna minor L.). Turk. J. Biol. 2010, 34, 175–188. [Google Scholar]
- Polat Kose, L.; Gulcin, I.; Goren, A.C.; Namiesnik, J.; Martinez-Ayala, A.L.; Gorinstein, S. LC-MS/MS analysis, antioxidant and anticholinergic properties of galanga (Alpinia officinarum Hance) rhizomes. Ind. Crops Prod. 2015, 74, 712–721. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006, 78, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I.; Kaya, R.; Goren, A.C.; Akıncıoğlu, H.; Topal, M.; Bingol, Z.; Cetin Cakmak, K.; Ozturk Sarikaya, S.B.; Durmaz, L.; Alwasel, S. Anticholinergic, antidiabetic and antioxidant activities of cinnamon (Cinnamomum verum) bark extracts: Polyphenol contents analysis by LC-MS/MS. Int. J. Food Prop. 2019, 22, 1511–1526. [Google Scholar] [CrossRef] [Green Version]
- Cetin Cakmak, K.; Gulcin, I. Anticholinergic and antioxidant activities of usnic acid-An activity-structure insight. Toxicol. Rep. 2019, 6, 1273–1280. [Google Scholar] [CrossRef]
- Şerbetçi Tohma, H.; Gulcin, I. Antioxidant and radical scavenging activity of aerial parts and roots of Turkish liquorice (Glycyrrhiza glabra L.). Int. J. Food Prop. 2010, 13, 657–671. [Google Scholar] [CrossRef]
- Balaydın, H.T.; Gulcin, I.; Menzek, A.; Göksu, S.; Şahin, E. Synthesis and antioxidant properties of diphenylmethane derivative bromophenols including a natural product. J. Enzyme Inhib. Med. Chem. 2010, 25, 685–695. [Google Scholar] [CrossRef] [Green Version]
- Gulcin, I. Comparison of in vitro antioxidant and antiradical activities of L-tyrosine and L-Dopa. Amino Acids 2007, 32, 431–843. [Google Scholar] [CrossRef]
- Kalin, P.; Gulcin, I.; Goren, A.C. Antioxidant activity and polyphenol content of cranberries (Vaccinium macrocarpon). Rec. Nat. Prod. 2015, 9, 496–502. [Google Scholar]
- Aksu, K.; Ozgeris, B.; Taslimi, P.; Naderi, A.; Gulcin, I.; Goksu, S. Antioxidant activity, acetylcholinesterase and carbonic anhydrase inhibitory properties of novel ureas derived from phenethylamines. Arch. Pharm. 2016, 349, 944–954. [Google Scholar] [CrossRef]
- Gulcin, I.; Beydemir, S.; Sat, İ.G.; Küfrevioglu, Ö.İ. Evaluation of antioxidant activity of cornelian cherry (Cornus mas L.). Acta Aliment. 2005, 34, 193–202. [Google Scholar] [CrossRef]
- Tohma, H.; Gulçin, I.; Bursal, E.; Goren, A.C.; Alwasel, S.H.; Koksal, E. Antioxidant activity and phenolic compounds of ginger (Zingiber officinale Rosc.) determined by HPLC-MS/MS. J. Food Meas. Charact. 2017, 11, 556–566. [Google Scholar] [CrossRef]
- Cetinkaya, Y.; Gocer, H.; Menzek, A.; Gulcin, I. Synthesis and antioxidant properties of (3,4-dihydroxyphenyl) (2,3,4-trihydroxyphenyl)methanone and its derivatives. Arch. Pharm. 2012, 345, 323–334. [Google Scholar] [CrossRef]
- Gulcin, I.; Sat, I.G.; Beydemir, S.; Kufrevioglu, O.I. Evaluation of the in vitro antioxidant properties of extracts of broccoli (Brassica oleracea L.). Ital. J. Food Sci. 2004, 16, 17–30. [Google Scholar]
- Bursal, E.; Köksal, E.; Gulcin, I.; Bilsel, G.; Gören, A.C. Antioxidant activity and polyphenol content of cherry stem (Cerasus avium L.) determined by LC-MS/MS. Food Res. Int. 2013, 51, 66–74. [Google Scholar] [CrossRef]
- Polat Köse, L.; Bingöl, Z.; Kaya, R.; Gören, A.C.; Akincioğlu, H.; Durmaz, L.; Koksal, E.; Alwasel, S.; Gulcin, I. Anticholinergic and antioxidant activities of avocado (Folium perseae) leaves—Phytochemical content by LC-MS/MS analysis. Int. J. Food Prop. 2020, 23, 878–893. [Google Scholar] [CrossRef]
- Davies, K. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB 2000, 50, 279–289. [Google Scholar] [CrossRef]
- Puupponen-Pimia, R.; Aura, A.M.; Oksman-Caldentey, K.M.; Mylla Rinen, P.; Saarela, M.; Mattila-Sandholm, T.; Poutanen, K. Development of functional ingredients for gut health. Trends Food Sci. Technol. 2002, 13, 3–11. [Google Scholar] [CrossRef]
- Erdoğan, M.; Polat Kose, L.; Essiz, S.; Gulcin, I. Synthesis and biological evaluation of some 1-naphthol derivatives as antioxidants, acetylcholinesterase, carbonic anhydrase inhibitors. Arch. Pharm. 2021, 354, e2100113. [Google Scholar] [CrossRef] [PubMed]
- Oztaskin, N.; Kaya, R.; Maras, A.; Sahin, E.; Gulcin, I.; Goksu, S. Synthesis and characterization of novel bromophenols: Determination of their anticholinergic, antidiabetic and antioxidant activities. Bioorg. Chem. 2019, 87, 91–102. [Google Scholar] [CrossRef]
- Gulcin, I. Measurement of antioxidant ability of melatonin and serotonin by the DMPD and CUPRAC methods as trolox equivalent. J. Enzyme Inhib. Med. Chem. 2008, 23, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Lan, P.; Du, M.; Teng, Y.; Banwell, M.G.; Nie, H.; Reaney, M.J.T.; Wang, Y. Structural modifications of a flaxseed lignan in pursuit of higher liposolubility: Evaluation of the antioxidant and permeability properties of the resulting derivatives. J. Agric. Food Chem. 2019, 67, 14152–14159. [Google Scholar] [CrossRef] [PubMed]
- Ghedini, C.P.; Moura, D.C. Flaxseed meal feeding to dairy cows as a strategy to improve milk enterolactone concentration: A literature review. Nativa 2021, 9, 373–381. [Google Scholar] [CrossRef]
- Landete, J.M. Plant and mammalian lignans: A review of source, intake metabolism, intestinal bacteria and health. Food Res. Int. 2012, 46, 410–424. [Google Scholar] [CrossRef]
- Cortes, C.; Gagnon, N.; Benchaar, C.; Silva, D.; Santos, G.T.D.; Petit, H.V. In vitro metabolism of flax lignans by ruminal and faecal microbiota of dairy cows. J. Appl. Microbiol. 2008, 105, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Aras, A.; Bursal, E.; Turkan, F.; Tohma, H.; Kılıc, O.; Gulcin, I.; Koksal, E. Phytochemical content, antidiabetic, anticholinergic, and antioxidant activities of endemic Lecokia cretica extracts. Chem. Biodivers. 2019, 16, e1900341. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.D.; Yuan, Y.V.; Wijewickreme, A.N.; Thompson, L.U. Antioxidant activity of the flaxseed lignan secoisolariciresinol diglycoside and its mammalian lignan metabolites enterodiol and enterolactone. Mol. Cell. Biochem. 1999, 202, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Duh, P.D. Antioxidant activity of burdock (Arctium lappa L.): It’s scavenging effect on free radical and active oxygen. J. Am. Oil Chem. Soc. 1998, 75, 455–461. [Google Scholar] [CrossRef]
- Gulcin, I.; Mshvildadze, V.; Gepdiremen, A.; Elias, R. Antioxidant activity of a triterpenoid glycoside isolated from the berries of Hedera colchica: 3-O-(β-d-glucopyranosyl)-hederagenin. Phytother. Res. 2006, 20, 130–134. [Google Scholar] [CrossRef]
- Gulcin, I.; Elias, R.; Gepdiremen, A.; Taoubi, K.; Köksal, E. Antioxidant secoiridoids from fringe tree (Chionanthus virginicus L.). Wood Sci. Technol. 2009, 43, 195–212. [Google Scholar] [CrossRef]
- Elmastas, M.; Türkekul, İ.; Öztürk, L.; Gulcin, I.; Işıldak, Ö.; Aboul-Enein, H.Y. The antioxidant activity of two wild edible mushrooms (Morchella vulgaris and Morchella esculanta). Comb. Chem. High Throughput Screen. 2006, 9, 443–448. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant activity of eugenol—A structure and activity relationship study. J. Med. Food 2011, 14, 975–985. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant properties of resveratrol: A structure-activity insight. Innov. Food Sci. Emerg. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Topal, F.; Topal, M.; Gocer, H.; Kalın, P.; Koçyiğit, U.M.; Gulcin, I.; Alwasel, S.H. Antioxidant activity of taxifolin: An activity-structure relationship. J. Enzyme Inhib. Med. Chem. 2016, 31, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Taslimi, P.; Gulcin, I. Antioxidant and anticholinergic properties of olivetol. J. Food Biochem. 2018, 42, e12516. [Google Scholar] [CrossRef]
- Gulcin, I.; Tel, A.Z.; Gören, A.C.; Taslimi, P.; Alwasel, S. Sage (Salvia pilifera): Determination its polyphenol contents, anticholinergic, antidiabetic and antioxidant activities. J. Food Meas. Charact. 2019, 13, 2062–2074. [Google Scholar] [CrossRef]
- Ak, T.; Gulcin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Topal, F.; Gulcin, I.; Dastan, A.; Guney, M. Novel eugenol derivatives: Potent acetylcholinesterase and carbonic anhydrase inhibitors. Int. J. Biol. Macromol. 2017, 94, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Maharramova, G.; Taslimi, P.; Sujayev, A.; Farzaliyev, F.; Durmaz, L.; Gulcin, I. Synthesis, characterization, antioxidant, antidiabetic, anticholinergic, and antiepileptic properties of novel N-substituted tetrahydropyrimidines based on phenylthiourea. J. Biochem. Mol. Toxicol. 2018, 32, e22221. [Google Scholar] [CrossRef] [PubMed]
- Rezai, M.; Bayrak, Ç.; Taslimi, P.; Gulcin, I.; Menzek, A. The first synthesis, antioxidant and anticholinergic activities of 1-(4,5-dihydroxybenzyl)pyrrolidin-2-one derivative bromophenols including natural products. Turk. J. Chem. 2018, 42, 808–825. [Google Scholar]
- Elmastas, M.; Celik, S.M.; Genc, N.; Aksit, H.; Erenler, R.; Gulcin, I. Antioxidant activity of an Anatolian herbal tea-Origanum minutiflorum: Isolation and characterization of its secondary metabolites. Int. J. Food Prop. 2018, 21, 374–384. [Google Scholar] [CrossRef] [Green Version]
- Taslimi, P.; Sujayev, E.; Turkan, F.; Garibov, E.; Huyut, Z.; Farzaliyev, F.; Mamedova, S.; Gulcin, I. Synthesis and investigation of the conversion reactions of pyrimidine-thiones with nucleophilic reagent and evaluation of their acetylcholinesterase, carbonic anhydrase inhibition and antioxidant activities. J. Biochem. Mol. Toxicol. 2018, 32, e22019. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, A.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V.; Recanatini, M.; Melchiorre, C. Multi-target-directed ligands to combat neurodegenerative diseases. J. Med. Chem. 2008, 51, 347–372. [Google Scholar] [CrossRef]
- Oztaskin, N.; Cetinkaya, Y.; Taslimi, P.; Goksu, S.; Gulcin, I. Antioxidant and acetylcholinesterase inhibition properties of novel bromophenol derivatives. Bioorg. Chem. 2015, 60, 49–57. [Google Scholar] [CrossRef]
- Tohma, H.; Koksal, E.; Kılıc, O.; Alan, Y.; Yılmaz, M.A.; Gulcin, I.; Bursal, E.; Alwasel, S.H. RP-HPLC/MS/MS analysis of the phenolic compounds, antioxidant and antimicrobial activities of Salvia, L. species. Antioxidants 2016, 5, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamad, H.O.; Alma, M.H.; Gulcin, I.; Yılmaz, M.A.; Karaogul, E. Evaluation of phenolic contents and bioactivity of root and nutgall extracts from Iraqian Quercus infectoria Olivier. Rec. Nat. Prod. 2017, 11, 205–210. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 26, 1199–1200. [Google Scholar] [CrossRef]
- Koksal, E.; Bursal, E.; Gulcin, I.; Korkmaz, M.; Caglayan, C.; Goren, A.C.; Alwasel, S.H. Antioxidant activity and polyphenol content of Turkish thyme (Thymus vulgaris) monitored by LC-MS/MS. Int. J. Food Prop. 2017, 20, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Gulcin, I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I. Antioxidant activity of L-Adrenaline: An activity-structure insight. Chem. Biol. Interact. 2009, 179, 71–80. [Google Scholar] [CrossRef]
- Koksal, E.; Gulcin, I.; Ozturk Sarikaya, S.B.; Bursal, E. On the in vitro antioxidant activity of silymarin. J. Enzyme Inhib. Med. Chem. 2009, 24, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Lin, H.; Lin, M.; Lin, P.; Chen, J. Effects of thermal preparation and in vitro digestion on lignan profiles and antioxidant activity in defatted-sesame meal. Food Chem. Toxicol. 2019, 128, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Guo, X.; Brennan, C.S.; Li, T.; Fu, X.; Chen, G.; Liu, R.H. Effect of germination on lignan biosynthesis, and antioxidant and antiproliferative activities in flaxseed (Linum usitatissimum L.). Food Chem. 2016, 205, 170–177. [Google Scholar] [CrossRef]
- Zeng, X.L.; Fu, G.M.; Tian, K.; Sun, J.X.; Xiong, H.B.; Huang, X.Z.; Jiang, Z.Y. Acutissimanide, a new lignan with antioxidant activity isolated from the bark of Quercus acutissima Carruth. Nat. Prod. Res. 2014, 28, 1364–1370. [Google Scholar] [CrossRef]
- Huang, X.Z.; Cheng, C.M.; Dai, Y.; Fu, G.M.; Guo, J.M.; Liang, H.; Wang, C. A novel lignan glycoside with antioxidant activity from Tinospora sagittata var. yunnanensis. Nat. Prod. Res. 2012, 26, 1876–1880. [Google Scholar] [CrossRef]
- Hu, C.; Yuan, Y.V.; Kitts, D.D. Antioxidant activities of the flaxseed lignan secoisolariciresinol diglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone in vitro. Food Chem. Toxicol. 2007, 45, 2219–2227. [Google Scholar] [CrossRef]
- Sadhu, S.K.; Khatun, A.; Phattanawasin, P.; Ohtsuki, T.; Ishibashi, M. Lignan glycosides and flavonoids from Saraca asoca with antioxidant activity. J. Nat. Med. 2007, 61, 480–482. [Google Scholar] [CrossRef]
- Min, B.S.; Cui, H.S.; Lee, H.K.; Sok, D.E.; Kim, M.R. A New furofuran lignan with antioxidant and antiseizure activities from the leaves of Petasites japonicus. Arch. Pharm. Res. 2005, 28, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on product of browning reaction prepared from glucose amine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Han, H.; Yılmaz, H.; Gulcin, I. Antioxidant activity of flaxseed (Linum usitatissimum L.) and analysis of its polyphenol contents by LC-MS/MS. Rec. Nat. Prod. 2018, 12, 397–402. [Google Scholar] [CrossRef]
- Bulut, N.; Kocyigit, U.M.; Gecibesler, I.H.; Dastan, T.; Karci, H.; Taslimi, P.; Durna Dastan, S.; Gulcin, I.; Cetin, A. Synthesis of some novel pyridine compounds containing bis-1,2,4-triazole moiety and investigation of their antioxidant properties, carbonic anhydrase and acetylcholinesterase enzymes inhibition profiles. J. Biochem. Mol. Toxicol. 2018, 32, e22006. [Google Scholar] [CrossRef]
- Apak, R.; Guclu, K.; Ozyurek, M.; Karademir, S.E.; Erca, E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int. J. Food Sci. Nutr. 2006, 57, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Bingol, Z.; Kızıltas, H.; Goren, A.C.; Polat Kose, L.; Topal, M.; Durmaz, L.; Alwasel, S.H.; Gulcin, I. Antidiabetic, anticholinergic and antioxidant activities of aerial parts of shaggy bindweed (Convulvulus betonicifolia Miller subsp.)—Profiling of phenolic compounds by LC-HRMS. Heliyon 2021, 7, e06986. [Google Scholar] [CrossRef]
- Bursal, E.; Aras, A.; Kılıc, O.; Taslimi, P.; Goren, A.C.; Gulcin, I. Phytochemical content, antioxidant activity and enzyme inhibition effect of Salvia eriophora Boiss. & Kotschy against acetylcholinesterase, α-amylase, butyrylcholinesterase and α-glycosidase enzymes. J. Food Biochem. 2019, 43, e12776. [Google Scholar]
- Gulcin, I. The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. Int. J. Food Sci. Nutr. 2005, 56, 491–499. [Google Scholar] [CrossRef]
- Talaz, O.; Gulcin, I.; Goksu, S.; Saracoglu, N. Antioxidant activity of 5,10-dihydroindeno[1,2-b]indoles containing substituents on dihydroindeno part. Bioorg. Med. Chem. 2009, 17, 6583–6589. [Google Scholar] [CrossRef]
- Gulcin, I.; Dastan, A. Synthesis of dimeric phenol derivatives and determination of in vitro antioxidant and radical scavenging activities. J. Enzyme Inhib. Med. Chem. 2007, 22, 685–695. [Google Scholar] [CrossRef]
- Huyut, Z.; Beydemir, S.; Gulcin, I. Antioxidant and antiradical properties of some flavonoids and phenolic compounds. Biochem. Res. Int. 2017, 2017, 7616791. [Google Scholar] [CrossRef] [PubMed]
- Eruygur, N.; Atas, M.; Tekin, M.; Taslimi, P.; Kocyigit, U.M.; Gulcin, I. In vitro antioxidant, antimicrobial, anticholinesterase and antidiabetic activities of Turkish endemic Achillea cucullata (Asteraceae) from ethanol extract. S. Afr. J. Bot. 2019, 120, 141–145. [Google Scholar] [CrossRef]
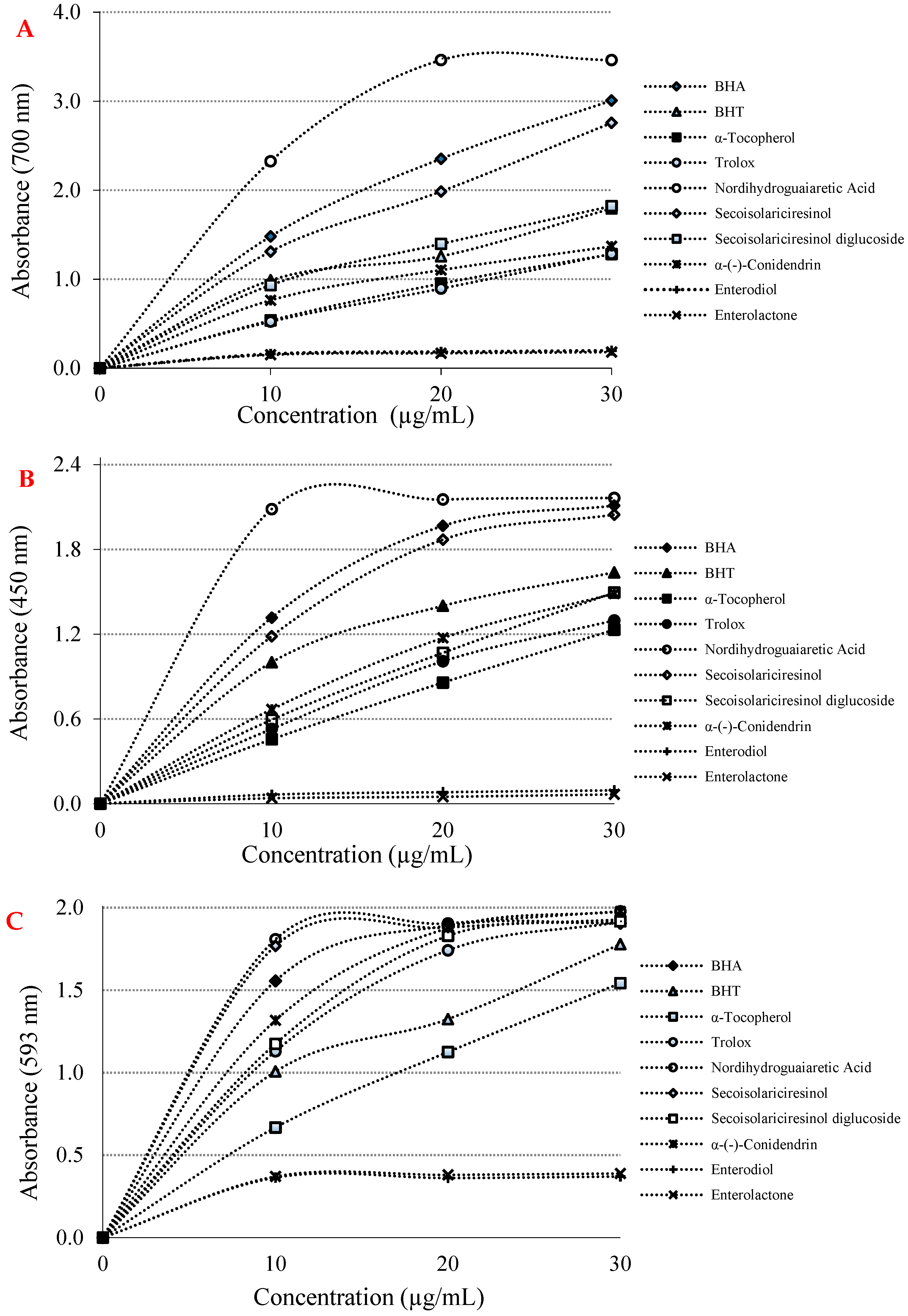
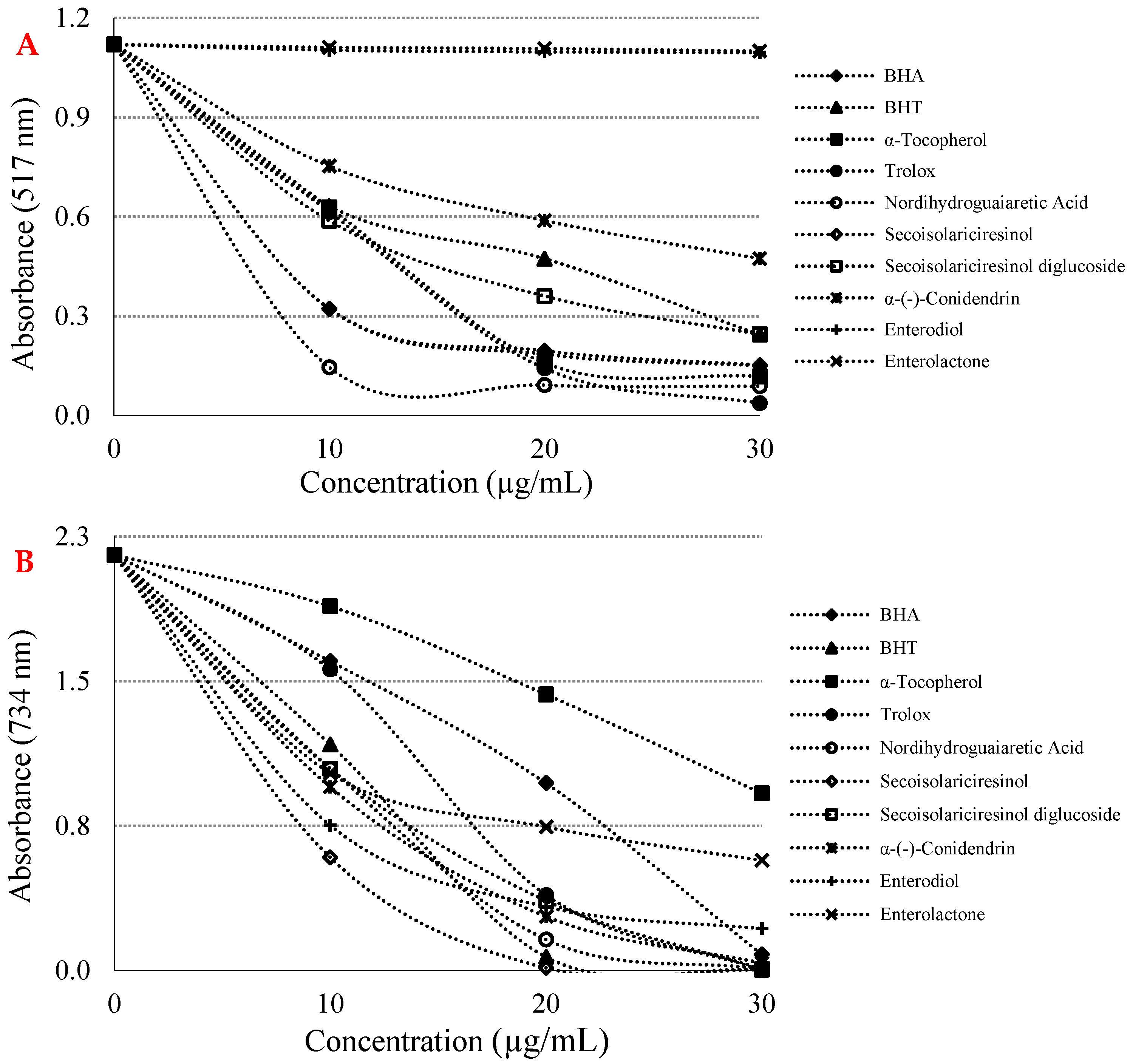
| Antioxidants | Fe3+ Reducing | Cu2+ Reducing | Fe3+-TPTZ Reducing | |||
|---|---|---|---|---|---|---|
| λ700 | r2 | λ450 | r2 | λ593 | r2 | |
| BHA | 1.480 ± 0.001 a | 0.9505 | 1.320 ± 0.002 a | 0.8290 | 1.560 ± 0.002 a | 0.6352 |
| BHT | 0.990 ± 0.00 a | 0.9198 | 1.000 ± 0.002 a | 0.8589 | 1.010 ± 0.002 a | 0.9085 |
| Trolox | 0.520 ± 0.002 a | 0.9907 | 0.530 ± 0.003 a | 0.9799 | 1.130 ± 0.003 a | 0.8600 |
| α-Tocopherol | 0.540 ± 0.003 a | 0.9841 | 0.460 ± 0.001 a | 0.9971 | 0.670 ± 0.001 a | 0.9815 |
| Nordihydroguaiaretic acid | 2.320 ± 0.003 a | 0.7704 | 2.090 ± 0.003 a | 0.4703 | 1.810 ± 0.003 a | 0.5289 |
| Secoisolariciresinol | 1.310 ± 0.001 a | 0.9655 | 1.180 ± 0.005 a | 0.8665 | 1.770 ± 0.003 a | 0.5248 |
| Secoisolariciresinol diglycoside | 0.930 ± 0.001 a | 0.9425 | 0.590 ± 0.002 a | 0.9917 | 1.170 ± 0.002 a | 0.8301 |
| α-(-)-Conidendrin | 0.760 ± 0.002 a | 0.9086 | 0.670 ± 0.002 a | 0.9666 | 1.320 ± 0.002 a | 0.7906 |
| Enterodiol | 0.160 ± 0.002 b | 0.6270 | 0.060 ± 0.002 b | 0.8867 | 0.360 ± 0.001 a | 0.4483 |
| Enterolactone | 0.150 ± 0.001 b | 0.6511 | 0.040 ± 0.001 b | 0.7938 | 0.370 ± 0.002 a | 0.4823 |
| Antioxidants | DPPH• Scavenging | ABTS•+ Scavenging | ||
|---|---|---|---|---|
| IC50 | r2 | IC50 | r2 | |
| BHA | 8.886 | 0.8406 | 16.552 | 0.9837 |
| BHT | 17.778 | 0.9397 | 13.007 | 0.9116 |
| Trolox | 6.478 | 0.9624 | 14.264 | 0.9660 |
| α-Tocopherol | 8.864 | 0.9437 | 27.829 | 0.9816 |
| Nordihydroguaiaretic acid | 6.601 | 0.8498 | 13.070 | 0.9174 |
| (-)-Secoisolariciresinol | 14.141 | 0.8426 | 12.252 | 0.8001 |
| Secoisolariciresinol diglycoside | 16.970 | 0.8998 | 13.547 | 0.9467 |
| α-(-)-Conidendrin | 23.295 | 0.9281 | 13.345 | 0.9140 |
| Enterodiol | 770.164 | 0.7746 | 13.878 | 0.8163 |
| Enterolactone | 932.167 | 0.9792 | 14.146 | 0.9070 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polat Kose, L.; Gulcin, İ. Evaluation of the Antioxidant and Antiradical Properties of Some Phyto and Mammalian Lignans. Molecules 2021, 26, 7099. https://doi.org/10.3390/molecules26237099
Polat Kose L, Gulcin İ. Evaluation of the Antioxidant and Antiradical Properties of Some Phyto and Mammalian Lignans. Molecules. 2021; 26(23):7099. https://doi.org/10.3390/molecules26237099
Chicago/Turabian StylePolat Kose, Leyla, and İlhami Gulcin. 2021. "Evaluation of the Antioxidant and Antiradical Properties of Some Phyto and Mammalian Lignans" Molecules 26, no. 23: 7099. https://doi.org/10.3390/molecules26237099
APA StylePolat Kose, L., & Gulcin, İ. (2021). Evaluation of the Antioxidant and Antiradical Properties of Some Phyto and Mammalian Lignans. Molecules, 26(23), 7099. https://doi.org/10.3390/molecules26237099







