Selenium: An Element of Life Essential for Thyroid Function
Abstract
:1. Introduction
2. Selenium in the Environment
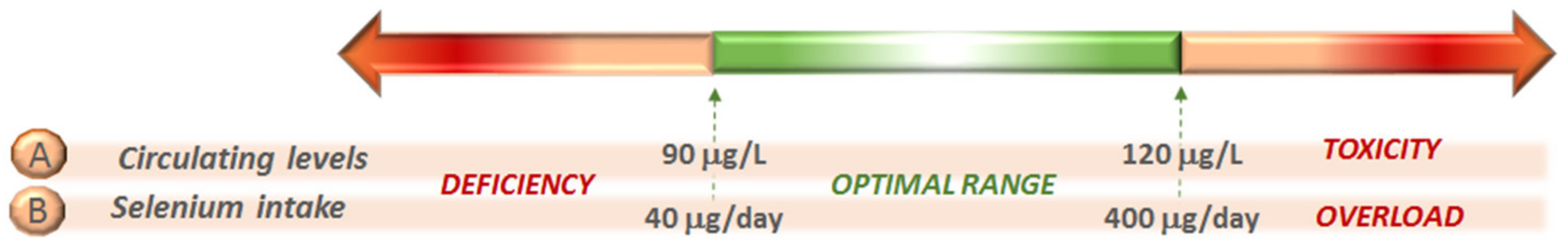
3. Selenium in Food and Intake
4. Selenium and Thyroid Hormone Deiodinases
5. Selenium and Autoimmune Thyroiditis (AIT)
5.1. Grave’s Disease (GD)
Graves’ Ophthalmopathy (GO)
5.2. Hashimoto’s Thyroiditis (HT)
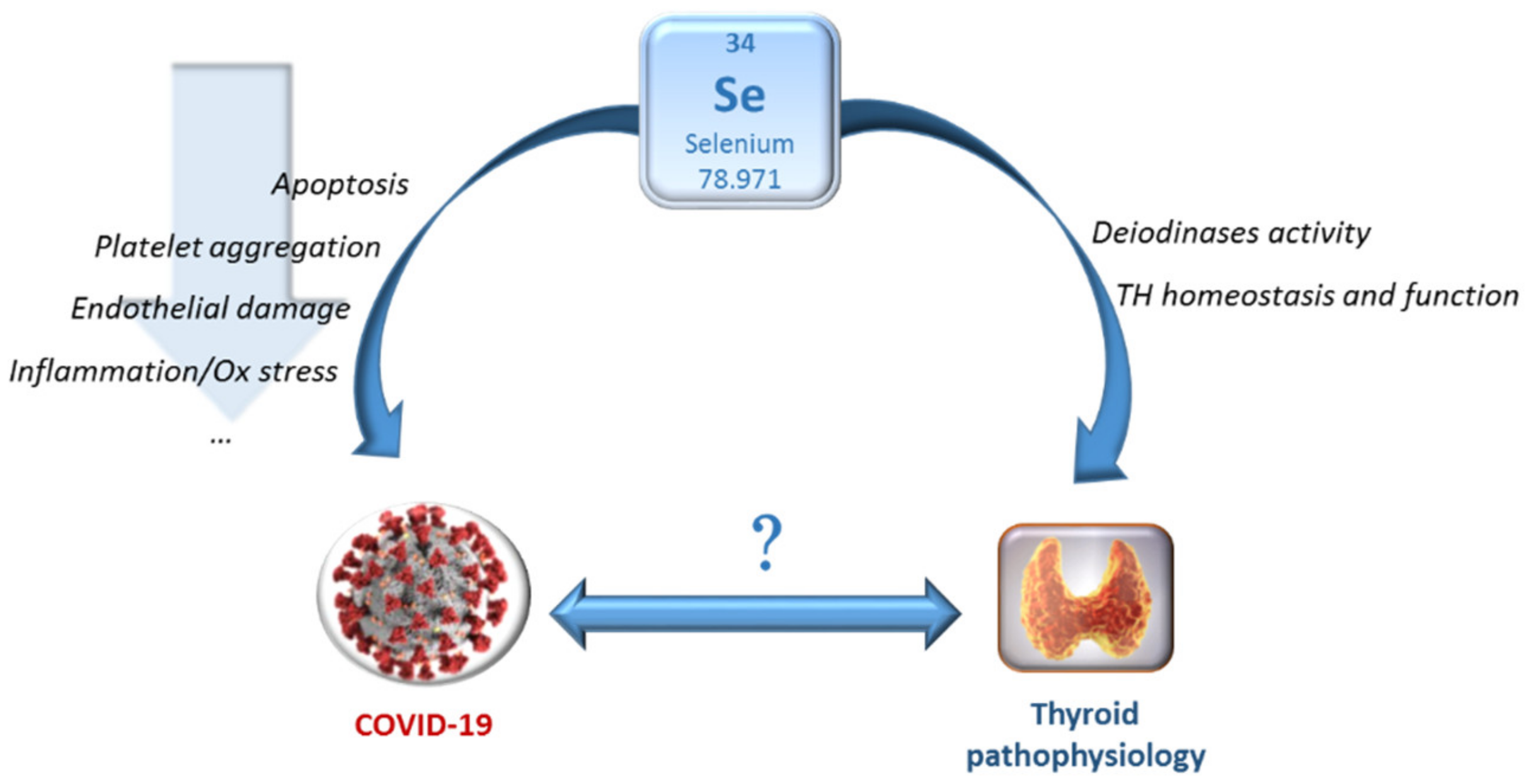
6. Other Pathophysiological Conditions beyond Intake Potentially Affecting Se Levels and the Relationship with Thyroid Function
7. Conclusions
- (1)
- The Se uptake by the thyroid gland appears to be independent of SEPP1-mediated selenium supply utilized by organs such as the kidney and testis. This is exemplified by the knockout of SEPP1, which does not affect thyroid function, suggesting that the thyroid gland may be able to effectively accumulate, retain and recycle selenium, even in the absence of SEPP1 [115].
- (2)
- Thyroidal Se content is typically not reflected by serum Se levels, as liver secreted SEPP1 is the main determinant of plasma Se levels [116]. Hence, thyroid tissue-specific markers of Se bioavailability and cellular action have yet to be identified.
- (3)
- Rare mutations in the “SeCys insertion sequence binding protein 2” gene (SBP2), a protein required for selenoprotein synthesis, induce a multisystem condition that includes abnormalities in thyroid biomarkers (elevated serum fT4 and rT3 levels, low to low-normal serum T3 levels and normal to slightly elevated serum TSH levels, in the presence of reduced blood Se levels) [117]. In particular, low circulating levels of Se in patients with SBP2 mutations result from impaired synthesis of SEPP1 and GPx3, major carriers of Se in serum [117]. These interesting and rare cases, in addition to the SBP2 mouse model, support the role of SBP2 as a critical limiting factor in thyroid selenoproteins synthesis and, therefore, thyroid function [118].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AIT | autoimmune thyroiditis |
| Dx | deiodinase |
| fT3 | free triiodothyronine |
| fT4 | free thyroxine |
| GD | Graves’ disease |
| GO | Graves’ ophthalmopathy |
| GPx | glutathione peroxidase |
| HT | Hashimoto’s thyroiditis |
| rT3 | reverse T3 |
| Se | selenium |
| SECIS | SeCys insertion sequence |
| SeCys | selenocysteine |
| SeMet | selenomethionine |
| SEPP1 | selenoprotein P |
| T2D | type 2 diabetes |
| T3 | triiodothyronine |
| T4 | thyroxine |
| TrxR | thyoredoxine reducatase |
| TSH | thyroid stimulating hormone |
| TRAb | thyroid stimulating hormone |
References
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef]
- Ventura, M.; Melo, M.; Carrilho, F. Selenium and Thyroid Disease: From Pathophysiology to Treatment. Int. J. Endocrinol. 2017, 2017, 1297658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schomburg, L. The other view: The trace element selenium as a micronutrient in thyroid disease, diabetes, and beyond. Hormones 2020, 19, 15–24. [Google Scholar] [CrossRef]
- Kiełczykowska, M.; Kocot, J.; Paździor, M.; Musik, I. Selenium––A fascinating antioxidant of protective properties. Adv. Clin. Exp. Med. 2018, 27, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Xu, X.; Ye, H.; Jin, L.; Zhang, X.; Zhu, Y. High levels of plasma selenium are associated with metabolic syndrome and elevated fasting plasma glucose in a Chinese population: A case-control study. J. Trace Elem. Med. Biol. 2015, 32, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, Y.; Hornick, J.L.; Istasse, L.; Dufrasne, I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef] [Green Version]
- National Research Council (US) Subcommittee on Selenium. Selenium in Nutrition: Revised Edition; National Academies Press: Washington, DC, USA, 1983. [Google Scholar]
- Alexander, J. Selenium. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 1175–1208. [Google Scholar]
- He, Y.; Xiang, Y.; Zhou, Y.; Yang, Y.; Zhang, J.; Huang, H.; Shang, C.; Luo, L.; Gao, J.; Tang, L. Selenium contamination, consequences and remediation techniques in water and soils: A review. Environ. Res. 2018, 164, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Winkel, L.H.; Vriens, B.; Jones, G.D.; Schneider, L.S.; Pilon-Smits, E.; Bañuelos, G.S. Selenium cycling across soil-plant-atmosphere interfaces: A critical review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zane Davis, T.; Hall, J.O. Selenium. In Reproductive and Developmental Toxicology, 2nd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 595–605. [Google Scholar]
- Weng, L.; Vega, F.A.; Supriatin, S.; Bussink, W.; Van Riemsdijk, W.H. Speciation of Se and DOC in soil solution and their relation to Se bioavailability. Environ. Sci. Technol. 2011, 45, 262–267. [Google Scholar] [CrossRef]
- Johnsson, L. Selenium uptake by plants as a function of soil type, organic matter content and pH. Plant Soil. 1991, 133, 57–64. [Google Scholar] [CrossRef]
- Renkema, H.; Koopmans, A.; Kersbergen, L.; Kikkert, J.; Hale, B.; Berkelaar, E. The effect of transpiration on selenium uptake and mobility in durum wheat and spring canola. Plant Soil. 2012, 354, 239–250. [Google Scholar] [CrossRef]
- Huang, Q.; Yu, Y.; Wang, Q.; Luo, Z.; Jiang, R.; Li, H. Uptake kinetics and translocation of selenite and selenate as affected by iron plaque on root surfaces of rice seedlings. Planta 2015, 241, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.L.; Zhang, L.H.; Marcus, M.A.; Fakra, S.; McGrath, S.P.; Pilon-Smits, E.A. Spatial imaging, speciation, and quantification of selenium in the hyperaccumulator plants Astragalus bisulcatus and Stanleya pinnata. Plant Physiol. 2006, 142, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Kikkert, J.; Berkelaar, E. Plant uptake and translocation of inorganic and organic forms of selenium. Arch. Environ. Contam. Toxicol. 2013, 65, 458–565. [Google Scholar] [CrossRef] [PubMed]
- Sors, T.G.; Ellis, D.R.; Salt, D.E. Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth. Res. 2005, 86, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinn, C.F.; Prins, C.N.; Freeman, J.L.; Gross, A.M.; Hantzis, L.J.; Reynolds, R.J.; Yang, S.; Covey, P.A.; Bañuelos, G.S.; Pickering, I.J.; et al. Selenium accumulation in flowers and its effects on pollination. New Phytol. 2011, 192, 727–737. [Google Scholar] [CrossRef]
- Wen, H.; Carignan, J. Reviews on atmospheric selenium: Emissions, speciation and fate. Atmos. Environ. 2007, 41, 7151–7165. [Google Scholar] [CrossRef]
- Tan, L.C.; Nancharaiah, Y.V.; van Hullebusch, E.D.; Lens, P.N.L. Selenium: Environmental significance, pollution, and biological treatment technologies. Biotechnol. Adv. 2016, 34, 886–907. [Google Scholar] [CrossRef]
- Santos, S.; Ungureanu, G.; Boaventura, R.; Botelho, C. Selenium contaminated waters: An overview of analytical methods, treatment options and recent advances in sorption methods. Sci. Total Environ. 2015, 521, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Winkel, L.H.; Johnson, C.A.; Lenz, M.; Grundl, T.; Leupin, O.X.; Amini, M.; Charlet, L. Environmental selenium research: From microscopic processes to global understanding. Environ. Sci. Technol. 2012, 46, 571–579. [Google Scholar] [CrossRef]
- Emmanuelle, B.; Virginie, M.; Fabienne, S.; Isabelle, I.; Martine, P.G.; Bernard, L.; Sylvie, R. Selenium exposure in subjects living in areas with high selenium concentrated drinking water: Results of a French integrated exposure assessment survey. Environ. Int. 2012, 40, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Gebreeyessus, G.D.; Zewge, F. A review on environmental selenium issues. SN Appl. Sci. 2019, 1, 55. [Google Scholar] [CrossRef]
- World Health Organization. Selenium in Drinking Water. 2011. Available online: https://www.who.int/water_sanitation_health/dwq/chemicals/selenium.pdf (accessed on 14 September 2021).
- European Commission, 1998. Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020L2184&from=ES (accessed on 14 September 2021).
- United States Environmental Protection Agency, 2021. National Primary Drinking Water Regulations, List of Primary Drinking Water Contaminants and their Maximum Contaminant Levels. Available online: https://www.epa.gov/sdwa/drinking-water-regulations-and-contaminants (accessed on 14 September 2021).
- World Health Organization, 2017. Guidelines for Drinking-water Quality. Fourth Edition. Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 11 September 2021).
- Vinceti, M.; Solovyev, N.; Mandrioli, J.; Crespi, C.M.; Bonvicini, F.; Arcolin, E.; Georgoulopoulou, E.; Michalke, B. Cerebrospinal fluid of newly diagnosed amyotrophic lateral sclerosis patients exhibits abnormal levels of selenium species including elevated selenite. Neurotoxicology. 2013, 38, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassil, J.; Naveau, A.; Bueno, M.; Razack, M.; Kazpard, V. Leaching behavior of selenium from the karst infillings of the Hydrogeological Experimental Site of Poitiers. Chem. Geol. 2018, 483, 141–150. [Google Scholar] [CrossRef]
- Vinceti, M.; Crespi, C.M.; Malagoli, C.; Del Giovane, C.; Krogh, V. Friend or foe? The current epidemiologic evidence on selenium and human cancer risk. J. Environ. Sci. Health Part C 2013, 31, 305–341. [Google Scholar] [CrossRef] [Green Version]
- Terry, N.; Zayed, A.M.; De Souza, M.P.; Tarun, A.S. Selenium in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 401–432. [Google Scholar] [CrossRef] [Green Version]
- Satia, J.A.; King, I.B.; Morris, J.S.; Stratton, K.; White, E. Toenail and plasma levels as biomarkers of selenium exposure. Ann. Epidemiol. 2006, 16, 53–58. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for selenium. EFSA J. 2014, 12, 3846. [Google Scholar] [CrossRef]
- Filippini, T.; Michalke, B.; Wise, L.A.; Malagoli, C.; Malavolti, M.; Vescovi, L.; Salvia, C.; Bargellini, A.; Sieri, S.; Krogh, V.; et al. Diet composition and serum levels of selenium species: A cross-sectional study. Food Chem. Toxicol. 2018, 115, 482–490. [Google Scholar] [CrossRef]
- Bodnar, M.; Szczyglowska, M.; Konieczka, P.; Namiesnik, J. Methods of Selenium Supplementation: Bioavailability and Determination of Selenium Compounds. Crit. Rev. Food Sci. Nutr. 2016, 56, 36–55. [Google Scholar] [CrossRef]
- Rayman, M.P.; Infante, H.G.; Sargent, M. Food-chain selenium and human health: Spotlight on speciation. Br. J. Nutr. 2008, 100, 238–253. [Google Scholar] [CrossRef] [Green Version]
- Plateau, P.; Saveanu, C.; Lestini, R.; Dauplais, M.; Decourty, L.; Jacquier, A.; Blanquet, S.; Lazard, M. Exposure to selenomethionine causes selenocysteine misincorporation and protein aggregation in Saccharomyces cerevisiae. Sci. Rep. 2017, 7, 44761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juniper, D.T.; Phipps, R.H.; Ramos-Morales, E.; Bertin, G. Effect of dietary supplementation with selenium-enriched yeast or sodium selenite on selenium tissue distribution and meat quality in beef cattle. J. Anim. Sci. 2008, 86, 3100–3109. [Google Scholar] [CrossRef] [PubMed]
- National Health Institute. Selenium. Fact Sheet for Health Professionals. 2021. Available online: https://ods.od.nih.gov/factsheets/selenium-HealthProfessional/#en6 (accessed on 15 September 2021).
- Rayman, M.P. Food-chain selenium and human health: Emphasis on intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinceti, M.; Filippini, T.; Wise, L.A. Environmental Selenium and Human Health: An Update. Curr. Environ. Health Rep. 2018, 5, 464–485. [Google Scholar] [CrossRef] [PubMed]
- Ashton, K.; Hooper, L.; Harvey, L.J.; Hurst, R.; Casgrain, A.; Fairweather-Tait, S.J. Methods of assessment of selenium status in humans: A systematic review. Am. J. Clin. Nutr. 2009, 89, 2025S–2039S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jablonska, E.; Vinceti, M. Selenium and Human Health: Witnessing a Copernican Revolution? J. Environ. Sci. Health, Part C. 2015, 33, 328–368. [Google Scholar] [CrossRef]
- World Health Organization. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Geneva, Switzerland, 2005; Available online: https://apps.who.int/iris/handle/10665/42716 (accessed on 15 September 2021).
- Nordic Council of Ministers, 2014. Nordic Nutrition Recommendations 2012. Integrating Nutrition and Physical Activity. 5th Edition, 627p. Available online: https://norden.diva-portal.org/smash/get/diva2:704251/FULLTEXT01.pdf (accessed on 15 September 2021).
- Kipp, A.P.; Strohm, D.; Brigelius-Flohé, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Heseker, H. German Nutrition Society (DGE). Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Zhao, C.; Hu, H.; Yin, S. Food Sources of Selenium and Its Relationship with Chronic Diseases. Nutrients. 2021, 13, 1739. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Pilon-Smits, E.A. Selenium Biofortification and Phytoremediation Phytotechnologies: A Review. J. Environ. Qual. 2017, 46, 10–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, J.; Gupta, D.S.; Kumar, S.; Gupta, S.; Singh, N.P. Current Knowledge on Genetic Biofortification in Lentil. J. Agric. Food Chem. 2016, 64, 6383–6396. [Google Scholar] [CrossRef]
- D’Amato, R.; Regni, L.; Falcinelli, B.; Mattioli, S.; Benincasa, P.; Dal Bosco, A.; Pacheco, P.; Proietti, P.; Troni, E.; Santi, C.; et al. Current Knowledge on Selenium Biofortification to Improve the Nutraceutical Profile of Food: A Comprehensive Review. J. Agric. Food Chem. 2020, 68, 4075–4097. [Google Scholar] [CrossRef]
- Berry, M.J.; Kieffer, J.D.; Harney, J.W.; Larsen, P.R. Selenocysteine confers the biochemical properties characteristic of the type I iodothyronine deiodinase. J. Biol. Chem. 1991, 266, 14155–14158. [Google Scholar] [CrossRef]
- Low, S.C.; Berry, M.J. Knowing when not to stop: Selenocysteine incorporation in eukaryotes. Trends Biochem. Sci. 1996, 21, 203–208. [Google Scholar] [CrossRef]
- Mix, H.; Lobanov, A.V.; Gladyshev, V.N. SECIS elements in the coding regions of selenoprotein transcripts are functional in higher eukaryotes. Nucleic Acids Res. 2007, 35, 414–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef] [Green Version]
- Callebaut, I.; Curcio-Morelli, C.; Mornon, J.P.; Gereben, B.; Buettner, C.; Huang, S.; Castro, B.; Fonseca, T.L.; Harney, J.W.; Larsen, P.R.; et al. The iodothyronine selenodeiodinases are thioredoxin-fold family proteins containing a glycoside hydrolase clan GH-A-like structure. J. Biol. Chem. 2003, 278, 36887–36896. [Google Scholar] [CrossRef] [Green Version]
- Salvatore, D.; Harney, J.W.; Larsen, P.R. Mutation of the Secys residue 266 in human type 2 selenodeiodinase alters 75Se incorporation without affecting its biochemical properties. Biochimie 1999, 81, 535–538. [Google Scholar] [CrossRef]
- Baqui, M.; Gereben, B.; Harney, J.W.; Larsen, P.R.; Bianco, A.C. Distinct subcellular localization of transiently expressed types 1 and 2 iodothyronine deiodinases as determined by immunofluorescence confocal microscopy. Endocrinology 2000, 141, 4309–4312. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.C.; da Conceição, R.R. The Deiodinase Trio and Thyroid Hormone Signaling. Thyroid Horm. Nucl. Recept. 2018, 1801, 67–83. [Google Scholar]
- Dentice, M.; Salvatore, D. Deiodinases: The balance of thyroid hormone: Local impact of thyroid hormone inactivation. J. Endocrinol. 2011, 209, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, F.; Fallahi, P.; Elia, G.; Gonnella, D.; Paparo, S.R.; Giusti, C.; Churilov, L.P.; Ferrari, S.M.; Antonelli, A. Hashimotos’ thyroiditis: Epidemiology, pathogenesis, clinic and therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101367. [Google Scholar] [CrossRef]
- Wu, Q.; Rayman, M.P.; Lv, H.; Schomburg, L.; Cui, B.; Gao, C.; Chen, P.; Zhuang, G.; Zhang, Z.; Peng, X.; et al. Low Population Selenium Status Is Associated With Increased Prevalence of Thyroid Disease. J. Clin. Endocrinol. Metab. 2015, 100, 4037–4047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, S.A.Z.; Kerkadi, A.; Agouni, A. Selenium and Health: An Update on the Situation in the Middle East and North Africa. Nutrients. 2019, 11, 1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schomburg, L. Selenium Deficiency Due to Diet, Pregnancy, Severe Illness, or COVID-19-A Preventable Trigger for Autoimmune Disease. Int. J. Mol. Sci. 2021, 22, 8532. [Google Scholar] [CrossRef]
- Pedersen, I.B.; Knudsen, N.; Carlé, A.; Schomburg, L.; Köhrle, J.; Jørgensen, T.; Rasmussen, L.B.; Ovesen, L.; Laurberg, P. Serum selenium is low in newly diagnosed Graves’ disease: A population-based study. Clin. Endocrinol. 2013, 79, 584–590. [Google Scholar] [CrossRef]
- Wertenbruch, T.; Willenberg, H.S.; Sagert, C.; Nguyen, T.B.; Bahlo, M.; Feldkamp, J.; Groeger, C.; Hermsen, D.; Scherbaum, W.A.; Schott, M. Serum selenium levels in patients with remission and relapse of graves’ disease. Med. Chem. 2007, 3, 281–284. [Google Scholar] [CrossRef]
- Krassas, G.E.; Pontikides, N.; Tziomalos, K.; Tzotzas, T.; Zosin, I.; Vlad, M.; Luger, A.; Gessl, A.; Marculescu, R.; Toscano, V.; et al. Selenium status in patients with autoimmune and non-autoimmune thyroid diseases from four European countries. Expert Rev. Endocrinol. Metab. 2014, 9, 685–692. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Chen, S.R.; Hou, X.; Wang, X.F.; Zhao, S.H.; Song, J.Q.; Wang, Y.G. Effect of Selenium Supplementation on Recurrent Hyperthyroidism Caused by Graves’ Disease: A Prospective Pilot Study. Horm. Metab. Res. 2016, 48, 559–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahaly, G.J.; Riedl, M.; König, J.; Diana, T.; Schomburg, L. Double-Blind.; Placebo-Controlled, Randomized Trial of Selenium in Graves Hyperthyroidism. J. Clin. Endocrinol. Metab. 2017, 102, 4333–4341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leo, M.; Bartalena, L.; Rotondo Dottore, G.; Piantanida, E.; Premoli, P.; Ionni, I.; Di Cera, M.; Masiello, E.; Sassi, L.; Tanda, M.L.; et al. Effects of selenium on short-term control of hyperthyroidism due to Graves’ disease treated with methimazole: Results of a randomized clinical trial. J. Endocrinol. Investig. 2017, 40, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Calissendorff, J.; Mikulski, E.; Larsen, E.H.; Möller, M. A Prospective Investigation of Graves’ Disease and Selenium: Thyroid Hormones, Auto-Antibodies and Self-Rated Symptoms. Eur. Thyroid. J. 2015, 4, 93–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, H.; Wei, J.; Wang, L.; Wang, Q.; Zhao, J.; Chen, S.; Wei, F. Effects of Selenium Supplementation on Graves’ Disease: A Systematic Review and Meta-Analysis. Evid. Based Complement. Alternat. Med. 2018, 2018, 3763565. [Google Scholar]
- Perros, P.; Hegedus, L.; Bartalena, L.; Marcocci, C.; Kahaly, G.J.; Baldeschi, L.; Salvi, M.; Lazarus, J.H.; Eckstein, A.; Pitz, S.; et al. Graves’ orbitopathy as a rare disease in Europe: A European Group on Graves’ Orbitopathy (EUGOGO) position statement. Orphanet J. Rare Dis. 2017, 12, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khong, J.J.; Goldstein, R.F.; Sanders, K.M.; Schneider, H.; Pope, J.; Burdon, K.P.; Craig, J.E.; Ebeling, P.R. Serum selenium status in Graves’ disease with and without orbitopathy: A case-control study. Clin. Endocrinol. 2014, 80, 905–910. [Google Scholar] [CrossRef]
- Lumyongsatien, M.; Bhaktikamala, U.; Thongtong, P.; Sintuwong, S.; Nimitwongsakul, O.; Kanokkantapong, J.; Pongpirul, K. Relative selenium insufficiency is a risk factor for developing severe Graves’ orbitopathy: A case-control study. BMJ Open Ophthalmol. 2021, 6, e000713. [Google Scholar] [CrossRef]
- Lanzolla, G.; Marinò, M.; Marcocci, C. Selenium in the Treatment of Graves’ Hyperthyroidism and Eye Disease. Front. Endocrinol. 2021, 11, 608428. [Google Scholar] [CrossRef] [PubMed]
- Dottore, G.R.; Leo, M.; Casini, G.; Latrofa, F.; Cestari, L.; Sellari-Franceschini, S.; Nardi, M.; Vitti, P.; Marcocci, C.; Marinò, M. Antioxidant Actions of Selenium in Orbital Fibroblasts: A Basis for the Effects of Selenium in Graves’ Orbitopathy. Thyroid. 2017, 27, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Marcocci, C.; Kahaly, G.J.; Krassa, G.E.; Bartalena, L.; Prummel, M.; Stahl, M.; Altea, M.A.; Nardi, M.; Pitz, S.; Boboridis, K.; et al. European Group on Graves’ Orbitopathy. Selenium and the course of mild Graves’ orbitopathy. N. Engl. J. Med. 2011, 364, 1920–1931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartalena, L.; Kahaly, G.J.; Baldeschi, L.; Dayan, C.M.; Eckstein, A.; Marcocci, C.; Marinò, M.; Vaidya, B.; Wiersinga, W.M.; EUGOGO. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur. J. Endocrinol. 2021, 185, G43–G67. [Google Scholar] [CrossRef] [PubMed]
- Rostami, R.; Nourooz-Zadeh, S.; Mohammadi, A.; Khalkhali, H.R.; Ferns, G.; Nourooz-Zadeh, J. Serum Selenium Status and Its Interrelationship with Serum Biomarkers of Thyroid Function and Antioxidant Defense in Hashimoto’s Thyroiditis. Antioxidants. 2020, 9, 1070. [Google Scholar] [CrossRef]
- Zuo, Y.; Li, Y.; Gu, X.; Lei, Z. The correlation between selenium levels and autoimmune thyroid disease: A systematic review and meta-analysis. Ann. Palliat. Med. 2021, 10, 4398–4408. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.B.; Schomburg, L.; Köhrle, J.; Pedersen, I.B.; Hollenbach, B.; Hög, A.; Ovesen, L.; Perrild, H.; Laurberg, P. Selenium status, thyroid volume, and multiple nodule formation in an area with mild iodine deficiency. Eur. J. Endocrinol. 2011, 164, 585–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turan, E.; Turksoy, V.A. Selenium, Zinc, and Copper Status in Euthyroid Nodular Goiter: A Cross-Sectional Study. Int. J. Prev. Med. 2021, 12, 46. [Google Scholar] [PubMed]
- Kryczyk-Kozioł, J.; Zagrodzki, P.; Prochownik, E.; Błażewska-Gruszczyk, A.; Słowiaczek, M.; Sun, Q.; Schomburg, L.; Ochab, E.; Bartyzel, M. Positive effects of selenium supplementation in women with newly diagnosed Hashimoto’s thyroiditis in an area with low selenium status. Int. J. Clin. Pract. 2021, 9, e14484. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Feng, W.; Chen, H.; Shi, H.; Jiang, L.; Zheng, X.; Liu, X.; Zhang, W.; Ge, Y.; Liu, Y.; et al. Effect of selenium on thyroid autoimmunity and regulatory T cells in patients with Hashimoto’s thyroiditis: A prospective randomized-controlled trial. Clin. Transl. Sci. 2021, 14, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- Pirola, I.; Rotondi, M.; Cristiano, A.; Maffezzoni, F.; Pasquali, D.; Marini, F.; Coperchini, F.; Paganelli, M.; Apostoli, P.; Chiovato, L.; et al. Selenium supplementation in patients with subclinical hypothyroidism affected by autoimmune thyroiditis: Results of the SETI study. Endocrinol. Diabetes Nut. 2020, 67, 28–35. [Google Scholar] [CrossRef]
- Wichman, J.; Winther, K.H.; Bonnema, S.J.; Hegedüs, L. Selenium Supplementation Significantly Reduces Thyroid Autoantibody Levels in Patients with Chronic Autoimmune Thyroiditis: A Systematic Review and Meta-Analysis. Thyroid 2016, 26, 1681–1692. [Google Scholar] [CrossRef]
- Qiu, Y.; Xing, Z.; Xiang, Q.; Yang, Q.; Zhu, J.; Su, A. Insufficient evidence to support the clinical efficacy of selenium supplementation for patients with chronic autoimmune thyroiditis. Endocrine 2021, 73, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Van Zuuren, E.J.; Albusta, A.Y.; Fedorowicz, Z.; Carter, B.; Pijl, H. Selenium supplementation for Hashimoto’s thyroiditis. Cochrane Database Syst. Rev. 2013, 6. [Google Scholar] [CrossRef]
- Winther, K.H.; Wichma, J.E.; Bonnema, S.J.; Hegedü, L. Insufficient documentation for clinical efficacy of selenium supplementation in chronic autoimmune thyroiditis, based on a systematic review and meta-analysis. Endocrine 2017, 55, 376–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duntas, L.H. Selenium and at-risk pregnancy: Challenges and controversies. Thyroid Res. 2020, 13, 16. [Google Scholar] [CrossRef]
- Negro, R.; Greco, G.; Mangieri, T.; Pezzarossa, A.; Dazzi, D.; Hassan, H. The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodies. J. Clin. Endocrinol. Metab. 2007, 92, 1263–1268. [Google Scholar] [CrossRef]
- Mantovani, G.; Isidori, A.M.; Moretti, C.; Di Dato, C.; Greco, E.; Ciolli, P.; Bonomi, M.; Petrone, L.; Fumarola, A.; Campagna, G.; et al. Selenium supplementation in the management of thyroid autoimmunity during pregnancy: Results of the “SERENA study”; a randomized, double-blind, placebo-controlled trial. Endocrine 2019, 66, 542–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiffler, L.; Rakotoambinina, B. Selenium and RNA Virus Interactions: Potential Implications for SARS-CoV-2 Infection (COVID-19). Front. Nutr. 2020, 7, 164. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Gowda, S.; Mundkur, L. An exploratory study of selenium status in healthy individuals and in patients with COVID-19 in a south Indian population: The case for adequate selenium status. Nutrition 2021, 82, 111053. [Google Scholar] [CrossRef]
- Younesian, O.; Khodabakhsh, B.; Abdolahi, N.; Norouzi, A.; Behnampour, N.; Hosseinzadeh, S.; Alarzi, S.S.H.; Joshaghani, H. Decreased Serum Selenium Levels of COVID-19 Patients in Comparison with Healthy Individuals. Biol. Trace Elem. Res. 2021, 7, 1–6. [Google Scholar]
- Jahromi, S.R.; Tabriz, H.M.; Togha, M.; Ariyanfar, S.; Ghorbani, Z.; Naeeni, S.; Haghighi, S.; Jazayeri, A.; Montazeri, M.; Talebpour, M.; et al. The correlation between serum selenium, zinc, and COVID-19 severity: An observational study. BMC Infect. Dis. 2021, 21, 899. [Google Scholar]
- Pour, O.B.; Yahyavi, Y.; Karimi, A.; Khamaneh, A.M.; Milani, M.; Khalili, M.; Sharifi, A. Serum trace elements levels and clinical outcomes among Iranian COVID-19 patients. Int. J. Infect. Dis. 2021, 111, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Rijntjes, E.; Wu, Q.; Lv, H.; Gao, C.; Shi, B.; Schomburg, L. Selenium deficiency is linearly associated with hypoglycemia in healthy adults. Redox Biol. 2020, 37, 101709. [Google Scholar] [CrossRef] [PubMed]
- Vavougios, G.D.; Ntoskas, K.T.; Doskas, T.K. Impairment in selenocysteine synthesis as a candidate mechanism of inducible coagulopathy in COVID-19 patients. Med. Hypotheses. 2021, 147, 110475. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.; Heller, R.A.; Sun, Q.; Seelig, J.; Cherkezov, A.; Seibert, L.; Hackler, J.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Selenium Deficiency Is Associated with Mortality Risk from COVID-19. Nutrients 2020, 12, 2098. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Zhang, A.R.; Lu, Q.B.; Zhang, X.A.; Zhang, Z.J.; Guan, X.G.; Che, T.L.; Yang, Y.; Li, H.; Liu, W.; et al. Association between fatality rate of COVID-19 and selenium deficiency in China. BMC Infect. Dis. 2021, 21, 452. [Google Scholar] [CrossRef]
- Im, J.H.; Je, Y.S.; Baek, J.; Chung, M.H.; Kwon, H.Y.; Lee, J.S. Nutritional status of patients with COVID-19. Int. J. Infect. Dis. 2020, 100, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhao, X.; Ma, J.; Mu, Y.; Wang, Y.; Yang, S.; Wu, Y.; Wu, F.; Zhou, Y. Selenium (Se) plays a key role in the biological effects of some viruses: Implications for COVID-19. Environ. Res. 2021, 196, 110984. [Google Scholar] [CrossRef]
- Zhang, J.; Taylor, E.W.; Bennett, K.; Saad, R.; Rayman, M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020, 111, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Notz, Q.; Herrmann, J.; Schlesinger, T.; Helmer, P.; Sudowe, S.; Sun, Q.; Hackler, J.; Roeder, D.; Lotz, C.; Meybohm, P.; et al. Clinical Significance of Micronutrient Supplementation in Critically Ill COVID-19 Patients with Severe ARDS. Nutrients 2021, 13, 2113. [Google Scholar] [CrossRef]
- Duntas, L.H.; Jonklaas, J. COVID-19 and Thyroid Diseases: A Bidirectional Impact. J. Endocr. Soc. 2021, 5, bvab076. [Google Scholar] [CrossRef] [PubMed]
- Gorini, F.; Bianchi, F.; Iervasi, G. COVID-19 and Thyroid: Progress and Prospects. Int. J. Environ. Res. Public Health 2020, 17, 6630. [Google Scholar] [CrossRef] [PubMed]
- Giovanella, L.; Ruggeri, R.M.; Ovčariček, P.P.; Campenni, A.; Treglia, G.; Deandreis, D. Prevalence of thyroid dysfunction in patients with COVID-19: A systematic review. Clin. Transl. Imaging 2021, 9, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.; Zaidi, S.M.J.; Waqar, A.U.; Khawaja, H.; Malik, A.; Ishaq, U.; Rana, A.S.; Awan, A.H. Association of hypothyroidism with acute COVID-19: A systematic review. Expert Rev. Endocrinol. Metab. 2021, 16, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, L.; Riese, C.; Michaelis, M.; Griebert, E.; Klein, M.O.; Sapin, R.; Schweizer, U.; Köhrle, J. Synthesis and metabolism of thyroid hormones is preferentially maintained in selenium-deficient transgenic mice. Endocrinology 2006, 147, 1306. [Google Scholar] [CrossRef] [Green Version]
- Schweizer, U.; Streckfuss, F.; Pelt, P.; Carlson, B.A.; Hatfield, D.L.; Köhrle, J.; Schomburg, L. Hepatically derived selenoprotein P is a key factor for kidney but not for brain selenium supply. Biochem. J. 2005, 386, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Visser, W.E.; van Mullem, A.A.; Visser, T.J.; Peeters, R.P. Different causes of reduced sensitivity to thyroid hormone: Diagnosis and clinical management. Clin. Endocrinol. 2013, 79, 595–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujisawa, H.; Korwutthikulrangsri, M.; Fu, J.; Liao, X.H.; Dumitrescu, A.M. Role of the Thyroid Gland in Expression of the Thyroid Phenotype of Sbp2-Deficient Mice. Endocrinology 2020, 161, bqz032. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorini, F.; Sabatino, L.; Pingitore, A.; Vassalle, C. Selenium: An Element of Life Essential for Thyroid Function. Molecules 2021, 26, 7084. https://doi.org/10.3390/molecules26237084
Gorini F, Sabatino L, Pingitore A, Vassalle C. Selenium: An Element of Life Essential for Thyroid Function. Molecules. 2021; 26(23):7084. https://doi.org/10.3390/molecules26237084
Chicago/Turabian StyleGorini, Francesca, Laura Sabatino, Alessandro Pingitore, and Cristina Vassalle. 2021. "Selenium: An Element of Life Essential for Thyroid Function" Molecules 26, no. 23: 7084. https://doi.org/10.3390/molecules26237084
APA StyleGorini, F., Sabatino, L., Pingitore, A., & Vassalle, C. (2021). Selenium: An Element of Life Essential for Thyroid Function. Molecules, 26(23), 7084. https://doi.org/10.3390/molecules26237084








